Abstract
Mutations in the unc-82 locus of Caenorhabditis elegans were previously identified by screening for disrupted muscle cytoskeleton in otherwise apparently normal mutagenized animals. Here we demonstrate that the locus encodes a serine/threonine kinase orthologous to human ARK5/SNARK (NUAK1/NUAK2) and related to the PAR-1 and SNF1/AMP-Activated kinase (AMPK) families. The predicted 1600-amino-acid polypeptide contains an N-terminal catalytic domain and noncomplex repetitive sequence in the remainder of the molecule. Phenotypic analyses indicate that unc-82 is required for maintaining the organization of myosin filaments and internal components of the M-line during cell-shape changes. Mutants exhibit normal patterning of cytoskeletal elements during early embryogenesis. Defects in localization of thick filament and M-line components arise during embryonic elongation and become progressively more severe as development proceeds. The phenotype is independent of contractile activity, consistent with unc-82 mutations preventing proper cytoskeletal reorganization during growth, rather than undermining structural integrity of the M-line. This is the first report establishing a role for the UNC-82/ARK5/SNARK kinases in normal development. We propose that activation of UNC-82 kinase during cell elongation regulates thick filament attachment or growth, perhaps through phosphorylation of myosin and paramyosin. We speculate that regulation of myosin is an ancestral characteristic of kinases in this region of the kinome.
THE contractile apparatus of striated muscle is a highly ordered cytoskeletal structure (Figure 1) composed of actin and myosin filaments, the filament anchoring structures, and a host of regulatory proteins. During Caenorhabditis elegans embryogenesis, the body-wall muscle cells polarize and assemble their cytoskeletons in response to contact with the epidermal cells, to which they attach through focal-adhesion-like structures. The epidermal cells respond in a similar fashion and assemble attachment structures and fibrous organelles at the sites of muscle-cell contact (reviewed in Moerman and Williams 2006). The coordination of the cytoskeletons of the two tissue types provides the physical attachment that transmits the force of muscle-cell contraction to the epidermis and its secreted cuticle and allows the worm to locomote through its environment. The patterning of the contractile apparatus occurs through integrin-mediated signaling at the plasma membrane where muscle cells contact the epidermis. The assembly of more interior (membrane-distal) components of the contractile apparatus follows and requires the membrane-proximal events (Hresko et al. 1994). Failure to assemble functional epidermal–muscle-cell contacts or failure to make contractile muscle cells prevents elongation of the embryo from an egg shape into a long tube. Many genes required for these early patterning events, as well as those essential for muscle contraction, have been identified by screening for embryonic lethal mutations that produce the Pat phenotype (paralyzed, arrested elongation at two-fold) (Williams and Waterston 1994).
Figure 1.—
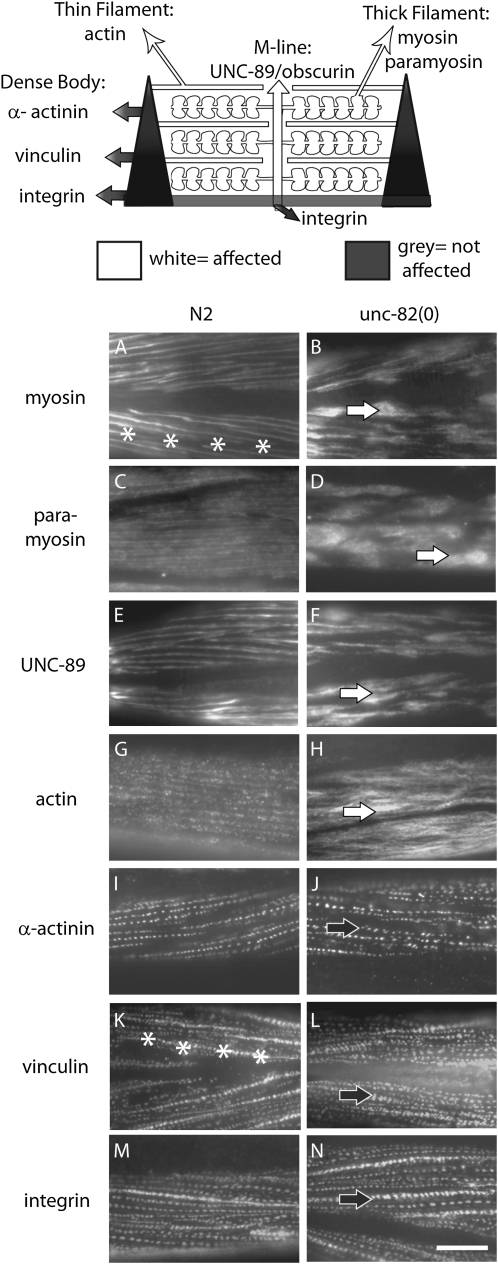
unc-82 mutants show dramatic defects in localization of thick-filament and M-line components, but normal patterning of membrane and dense-body proteins. A diagram of the sarcomere (top) is highlighted to indicate those components affected in unc-82 mutants. Structures represented include the actin filaments anchored to the dense body (the Z-line analog) and myosin-containing thick filaments associated with the M-line. The components represented in white exhibit abnormal staining patterns in unc-82 mutants; those represented in gray are relatively unaffected. (A–N) Adult fragments from wild-type (left column) and unc-82 mutant worms (right column) were stained with antibodies specific for components of the contractile apparatus. Thick-filament proteins myosin A (A and B) and paramyosin (C and D) are grossly mislocalized in unc-82 mutants, as is the M-line component UNC-89/obscurin (E and F). White arrows (B, D, F, and H) indicate abnormal accumulations of thick-filament and M-line proteins, and asterisks (A and K) mark a cell border. Actin staining (G and H) is mildly disrupted, but does not appear in large clumps. The distribution of α-actinin, vinculin, and integrin (I–N) (organized lines of puncta, solid arrows) is similar in mutant and wild type. Antibodies: myosin A, 5.6; paramyosin, 5.23; UNC-89, EU30; actin, C4; α-actinin, MH35; vinculin, MH24; integrin MH25. Bar, 10 μm.
However, proteins that act subsequent to the early patterning events or are not essential for contraction would not have been identified in the Pat screens. Mutations in the unc-82 gene were isolated by screening apparently normal animals for muscle-cell disorganization using polarized light microscopy (Waterston et al. 1980). Animals homozygous for unc-82 mutations exhibit patchy, bright birefringence rather than the uniform bright bands of signal that mark the areas of organized myosin-containing thick filaments in wild-type worms. To define the mechanisms underlying filament organization within the contractile apparatus, we undertook molecular and phenotypic analyses of unc-82 mutants. Our data suggest that UNC-82 is a kinase, orthologous to human ARK5 and SNARK, that is required specifically for myosin filament reorganization during cellular elongation in normal development.
MATERIALS AND METHODS
Nematode strains:
We used the following nematode strains: CB1220 unc-82(e1220) IV; CB1323 unc-82(e1323) IV; RW3536 unc-82(e1323) unc-24(e138) IV; RW1350 unc-44(e362) unc-82(e1323)/stDf7 IV; PZ51 unc-54(s95) I; unc-82(e1323) IV, PZ52 unc-54(s95) I; unc-82(e1220) IV; CB4856 Hawaiian; transgenic lines of unc-82(e1323) rescued by cosmid B0496, RW3918, RW3919, RW3920, RW3921; and transgenic line expressing UNC-82∷GFP, PZ73 unc-82(e1220) IV; phEx22.
Antibody staining:
Embryos were fixed with paraformaldehyde and methanol and stained using the methods of Hresko et al. (1994). Adults used in the z-series analysis (Figure 5, D–F) were processed using peroxide tube fixation (Duerr 2006). Images were collected over a depth of 3–4 μm at 0.2- to 0.3-μm intervals using a Leica DM5500 microscope and ImagePro 6.0 software. All other images show adults fragmented with a French press cell, extracted with detergent, fixed with methanol, and stained (Francis and Waterston 1985).
Figure 5.—
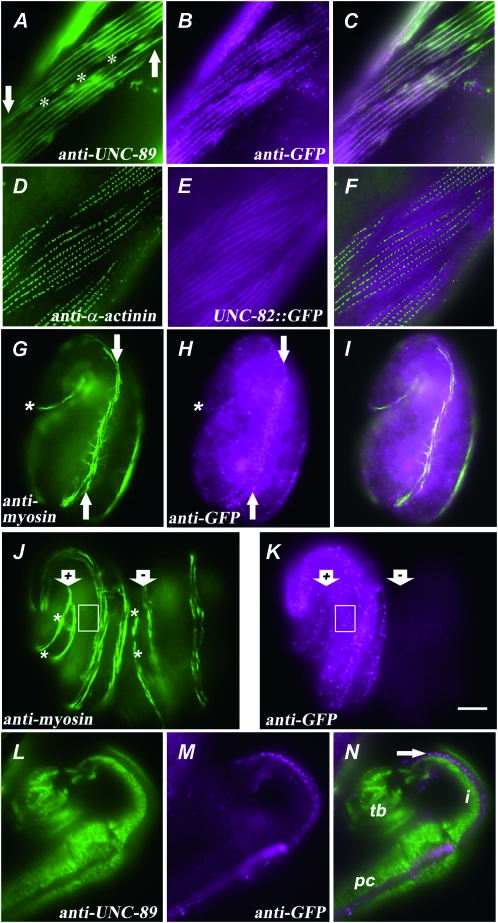
A rescuing UNC-82∷GFP fusion protein is localized near the M-line in body-wall muscle. (A–C) Portions of two body-wall muscle cells from an adult unc-82(e1323) homozygote expressing UNC-82∷GFP are shown stained with antibodies against (A) UNC-89/obscurin and (B) GFP. The signals coincide in the merged image (C). The asterisks (A) mark the boundary between the two muscle cells, and the arrows indicate one end of each muscle cell. (D–F) One plane from a z-series in which UNC-82∷GFP was detected throughout the depth of the myofilament lattice, as marked by α-actinin (Francis and Waterston 1985; see Figure 1). (G–I) A 1.5-fold embryo, positioned as in Figure 2A, exhibits punctate UNC-82∷GFP concentrated near myosin in a single muscle quadrant (arrows). Asterisks mark the posterior tip of the embryo. (J and K) The e1323 embryo on the left (arrow marked “+”) expresses UNC-82∷GFP, whereas the embryo on the right (arrow marked “−”) has lost the unstable transgene. (J) The myosin staining (between asterisks) appears broken and patchy in the mutant without the transgene (−). Anti-GFP signal (K) outside the body-wall muscle quadrants (box in J and K) in only the rescued embryo (+) suggests fusion protein expression in nonmuscle cells. An adult pharynx (L–N) exhibits no overlap between the UNC-89 and UNC-82∷GFP signals. (L) UNC-89 signal marks the regions of the single-sarcomere pharyngeal muscle cells that contain thick filaments. The UNC-82∷GFP signal (M, arrow in N) appears localized to the marginal cells or to a region of the muscle cell that does not contain thick filaments. (N) The pharynx shown is bent back in the isthmus (i) such that the procorpus (pc) lies alongside the terminal bulb (tb). The antibodies used were anti-UNC-89, MH42; rabbit anti-GFP, Abcam; antimyosin A, 5.6; anti-α-actinin, MH35. Bar, 5 μm.
Time-lapse video recording:
Embryos were mounted on slides and recorded using a modified version of the method described in Williams and Waterston (1994). Gravid adult hermaphrodites were cut in half with a razor blade in M9 buffer. The eggs in a small amount of buffer were transferred to a 2% agarose pad on a microscope slide and then covered and gently flattened with a Vaseline-lined coverslip. The developing embryos were videotaped overnight using Nomarski optics and a time-lapse VHS recorder.
Single nucleotide polymorphism mapping:
Using the single nucleotide polymorphism (SNP) method of Jakubowski and Kornfeld (1999), N2 Bristol animals homozygous for the linked mutations unc-82(e1323) and unc-24(e138) were crossed to males of the Hawaiian strain CB4856, and single F1 hermaphrodite cross progeny were picked to individual plates. F2 recombinant animals that were homozygous for either unc-82 or unc-24, but not for both, were picked singly, and F3 worms homozygous for the recombinant chromosome were isolated. SNPs within this interval were chosen from those identified and described by Wicks et al. (2001): F38A5-19715, D2024-25027, T12B3-3235, C48A7-11713, B0496-27999, F55G1-23412, and T09A12-14845. PCR fragments from 400 to 800 bp in length were amplifed from each recombinant strain and analyzed by restriction digest.
RNA interference:
Double-stranded RNA (dsRNA) fragments ∼500–1000 bp in length were made using the Megascript kit (Ambion). The dsRNA was injected into the gonads of L4 hermaphrodites at 1 μg/ml. Injected animals were maintained at 20° or 25°and transferred daily to fresh plates. Progeny were scored by polarized light microscopy. Primers used were aataatacgactcactatagggagaTACTCTAGCGGTGGAGAATT, aaatttaggtgacactatagaagagagCAGACTTCATCTCTTCCG, aataatacgactcactatagggagaACGGGCTGAAAGAGATGCTG, and aataatacgactcactatagggagaTCGAACTCCATTGCTTG. Lowercase letters denote residues that do not match C. elegans wild-type sequence.
DNA constructs and transgenic worm strains:
Cosmid rescue was obtained by injecting unc-82(e1323) animals with a 210 ng/μl DNA cocktail containing pPHgfp1 (Hoppe and Waterston 2000), Bluescript, and B0496 in a 5:15:1 ratio. Transgenic lines were marked by GFP expression in the hypodermis and scored for rescue using polarized light microscopy.
A full-length UNC-82∷GFP fusion construct was generated by recombination in vivo between a 17-kb PCR fragment and a plasmid encoding the C terminus of UNC-82 fused to GFP following the method of Yuan et al. (2000). Oligonucleotides GTCTCTGCTAAACAGCAATCG and GTTTGTGTACTTGTTGTGTGTG were used with cosmid template B0496 and the Expand Long Template PCR System (Roche) to amplify a genomic fragment beginning 2.6 kb upstream of the UNC-82 initiator methionine and terminating within intron 27. To fuse the C terminus of UNC-82 to GFP, primers gacacaagctTCGTTTCCGTCCAACTGCTCG and ccccggatccccATAAATATTTGGATCATCAT were used to amplify a 2-kb genomic fragment spanning exons 24–30, which was cut with HindIII and BamHI and cloned into pPD95_67. Prior to injection, the plasmid was cut with HindIII, extracted with phenol/chloroform, and ethanol precipitated.
Sequencing alleles:
Genomic DNA was isolated from worms homozygous for each unc-82 mutant allele, and two overlapping PCR fragments spanning the locus were amplified using the Expand Long Template PCR system (Roche). A library was generated from each of the four PCR fragments using a protocol from the Washington University Genome Sequencing Center (http://genome.wustl.edu/tools/protocols/). Briefly, the PCR fragments were sonicated and treated with mung bean nuclease. The smaller fragments were ligated into pZERO-2 vector and transformed into DH10b cells. The sequence was assembled and analyzed using the Phred/Phrap program.
cDNA analysis:
The following cDNA clones were obtained from Yuji Kohara for sequencing: yk76b5, yk159d1, yk286b1, yk360b4, yk896c08, yk1121c09, yk1232g01, yk1305a10, yk315d2, yk47c5, yk356a4, yk405f4, and yk8c7. Inserts from phage clones were amplified using the Expand Long Template PCR System (Roche) with vector primers GGTTTTCCCAGTCACGACGTTG and CAGGAAACAGCTATGACCATGATT. The PCR products were gel purified, and the DNA was recovered by using either a phenol extraction protocol or GenElute Minus EtBr Spin Columns (Sigma-Aldrich). DNA from plasmid clones was isolated using Wizard miniprep kits (Promega).
A cDNA fragment containing the 5′-end of the unc-82 mRNA was generated from total adult C. elegans RNA (a gift from Ziva Misulovin) using the gene-specific primer exon6RevBam CTGTGGATCCAGACTTCATCTCTTCCG and SuperScript II RT (Invitrogen) for first-strand synthesis and primers exon6RevBam and SL1HindDIII ACAGGAAGC TTCGGTTTAATTACCCAAGTTTGAG for PCR using Biolase DNA Polymerase (Bioline). The fragment was gel purified prior to DNA sequencing with ABI Big Dye Terminators version 3.1 and run on an ABI Prism 3100 Genetic Analyzer.
Sequence comparisons:
The pairwise alignment of the genomic DNA sequences from C. elegans and C. briggsae was generated and annotated using BioEdit Sequence Alignment Editor. Homologous protein sequences were identified by BLAST and aligned using CLUSTALX. Repetitive elements in UNC-82 and its orthologs were detected using Radar (http://www.ebi.ac.uk/Radar/) and DotPlot.
RESULTS
unc-82 is required for thick filament organization during embryonic elongation:
To further characterize the role of unc-82 in muscle, the phenotype of e1323 mutant adults was examined using antibodies specific for various components of the contractile apparatus. The staining experiments revealed profound defects in the distribution of the thick filament proteins myosin and paramyosin (Figure 1, A–D), consistent with the structural defects observed by transmission electron microscopy (TEM; Waterston et al. 1980). In addition, the morphology of the thick-filament-attachment structures, assessed by staining for the M-line component UNC-89/obscurin (Benian et al. 1996; Small et al. 2004), was severely disrupted (Figure 1, E and F). In contrast, vinculin and α-actinin, which are components of the thin-filament-attachment structure called the dense body, are comparatively unaffected, as is the distribution of integrin, which is found in the muscle-cell membrane at the base of both M-lines and dense bodies (Figure 1, I–N). These results suggest that unc-82 activity is required to organize internal proteins of the M-line and of the thick filament, but is not involved in overall patterning of the contractile apparatus, which occurs at the membrane. The distribution of actin staining is also notably altered in unc-82 adults (Figure 1H), despite the relatively normal positioning of the structures anchoring the actin filaments. Given the physical interaction between actin and myosin, the actin phenotype is likely a secondary consequence of thick filament disorganization.
To distinguish between a role in the initial organization of the affected proteins and a role in the maintenance of organization during growth, wild-type and mutant embryos at various stages of development were examined to determine when abnormalites first appear in unc-82 mutants. For all proteins examined, the antibody staining pattern in mutant embryos was indistinguishable from that of wild type up through the 1.5-fold stage (Figure 2, A–G), the time at which muscle contractions first occur. Furthermore, time-lapse video microscopy showed that the onset of muscle twitching (1.5-fold stage) and the progression to coordinated body movement (2-fold stage) is normal in unc-82 mutant embryos. These data argue that unc-82 is not required for the signaling events between the muscle and epidermal cells that establish the earliest pattern of the contractile apparatus.
Figure 2.—
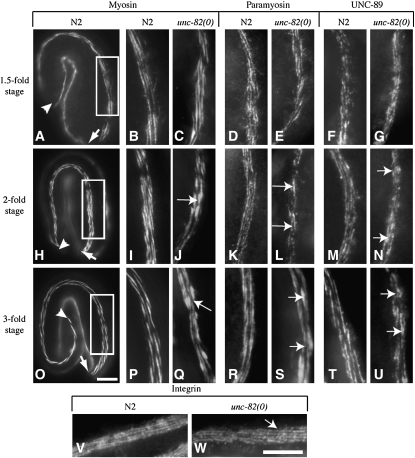
Defects in localization of thick-filament and M-line proteins appear during embryonic elongation in unc-82 mutants. Each row of micrographs shows a different stage in embryonic development. The first micrograph in each row shows a wild-type embryo at the stage and in the position and orientation of all embryos in that row. Arrowheads indicate the end of the elongating tail, and arrows mark the anterior tip of the head (A, H, and O). A single muscle quadrant is in focus. The boxed area (A, H, and O) indicates the portion of a dorsal muscle quadrant shown at higher magnification in the second column. Subsequent columns show an equivalent area of muscle from different animals. The staining pattern for each antibody is shown in wild-type and in unc-82(e1323) embryos. The unc-82 embryos show normal localization of all proteins at the 1.5-fold stage (B–G). The staining patterns of thick-filament and M-line proteins become abnormal by the twofold stage of development (H–N), exhibiting aberrant blotches of protein (arrows in J, L, N, Q, S, U) that are larger and more numerous in 3-fold embryos (O–U). Integrin appears normal in unc-82 at the 3-fold stage (V and W). Antibodies: myosin A, 5.6; paramyosin, 5.23; UNC-89, MH42; integrin, MH25. Bars, 10 μm.
Subsequent to the 1.5-fold stage, C. elegans embryos rapidly develop coordinated movement (Williams and Waterston 1994) and undergo body elongation. During this time, unc-82 mutants begin to exhibit defects in localization of thick filament and M-line components. The first detectable defects, aberrant accumulations and gaps in myosin and paramyosin staining, are consistently seen at the 2-fold stage (Figure 2, H–N). By the 3-fold stage, animals exhibit more numerous and severe defects in thick-filament and M-line protein localization throughout the length of the muscle quadrants. The staining pattern of integrin, which marks the base of the M-lines and dense bodies, remains normal through at least the 3-fold stage (Figure 2W). These results suggest that unc-82 activity is required to maintain proper thick filament and M-line organization during the stages when muscle cells are rapidly elongating and vigorously contracting.
To test whether the force of contraction is responsible for causing the observed cytoskeletal defects, contractile forces were diminished in an unc-82 mutant background by constructing double-mutant strains homozygous for either unc-82(e1323) or unc-82(e1220) and unc-54(s95). The s95 allele, a point mutation in the motor domain of the major body-wall muscle myosin (Dibb et al. 1985), greatly reduces muscle contractility but does not alter sarcomere structure (Moerman et al. 1982). Examination of double-mutant animals by polarized light microscopy (Figure 3) revealed that reducing contraction altered the muscle-cell phenotype but did not restore wild-type structure. Like unc-82 single mutants, double-mutant animals lacked organized striations and contained brightly birefringent material at the ends of the cells. However, the double mutants exhibited more uniform signal in the body of the muscle cell.
Figure 3.—
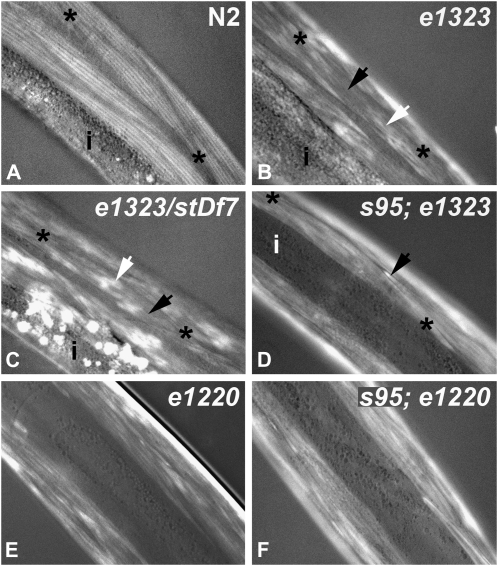
Muscle-cell organization in young adult worms was assessed by polarized light microscopy. Asterisks mark ends of spindle-shaped muscle cells, and “i” marks the intestine. (A) In wild type, longitudinal bright birefringent bands mark the positions of highly ordered thick filaments. (B) Animals homozygous for the presumptive null allele e1323 exhibit bright amorphous patches (white arrow) and regions of little signal (black arrow) within muscle cells. (C) The muscle-cell phenotype of e1323/stDf7 hemizygotes is similar to that of the e1323 homozgyote. There is an increase of birefringent material in the intestine. Compared to single mutants (B and E), double mutants homozygous for unc-82 and the myosin mutation unc-54(s95) (D and F) exhibit relatively uniform signal in the muscle-cell body and brightly birefringent needles at the ends of cells (black arrow in D).
unc-82 gene encodes a predicted serine/threonine kinase:
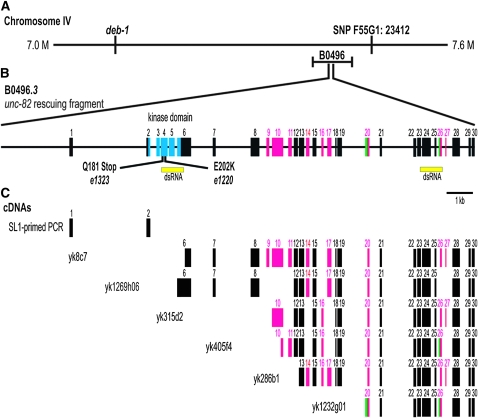
Previous three-factor mapping and deficiency complementation data had placed unc-82 on chromosome IV to the right of deb-1 and within nDf41 (Waddle 1993). Using SNP mapping, we defined an ∼400-kb region containing the unc-82 gene (Figure 4A). Rescue of unc-82(e1323) was obtained with B0496, a cosmid within this interval, and subsequently with an ∼18-kb PCR fragment containing genomic sequences that spanned the single predicted ORF called B0496.3 in Wormbase (http://www.wormbase.org/). The predicted protein product from this locus contains ∼1600 amino acids. The single conserved motif is a predicted serine/threonine kinase catalytic domain near the N terminus.
Figure 4.—
The unc-82 gene encodes a predicted serine/threonine kinase and produces multiple alternatively spliced messages. (A) Schematic of the 600-kb region containing unc-82 as defined by SNP mapping, which placed unc-82 to the left of marker F55G1:23412. Within this interval, both (A) the cosmid B0496 and (B) an 18-kb PCR fragment containing B0496.3 rescue unc-82(e1323). (B) The solid black line represents the introns and 2.6 kb of upstream sequence. Yellow rectangles indicate regions targeted in RNA interference experiments. Point mutations found in e1323 and e1220 are shown. (B and C) Rectangles represent exons: magenta denotes alternatively spliced exons, green marks exons with alternatively spliced borders, and blue shows the kinase domain. (C) The B0496.3 gene structure was determined by sequencing cDNAs and a 5′ PCR product. unc-82 has at least nine alternatively included exons that can exist in numerous combinations. Bar, 1 kb.
Several approaches were used to confirm the molecular identity of the unc-82 gene. To test whether the ∼18-kb rescuing fragment was likely to represent a single transcription unit, we used RNA interference to target sequences within either the 5′ or 3′ region of the predicted mRNA (Figure 4B). Injection of dsRNA derived from either region into wild-type worms phenocopied the cytoskeletal defects in unc-82 mutants, suggesting that both targeted sequences were part of the unc-82 gene. DNA sequencing revealed a molecular lesion within the predicted kinase domain in unc-82 mutant strains. The unc-82(e1220) mutation is a missense allele that changes a glutamic acid to a lysine. This charge reversal occurs within the catalytic loop, which is involved in substrate binding (Hanks and Hunter 1995). The unc-82(e1323) mutation changes a glutamine to a stop codon, which terminates translation within the catalytic domain. Animals hemizygous for the e1323 mutation over the chromosomal deficiency stDf7, which removes the unc-82 gene, had a muscle phenotype similar to that of e1323 homozygotes as young adult animals (Figure 3). However, older adult e1323/stDf7 hemizygotes may contain larger, brighter patches of signal (not shown). Further, the hemizygotes are often sterile and have a distinct polarized light phenotype in the intestine (Figure 3).
The exon structure of the unc-82 transcription unit was determined by sequencing several cDNA clones obtained from Yuji Kohara, as well as a 5′ PCR fragment. These data identified two previously undetected exons, including the true first exon, as well as a number of alternative splicing events. The unc-82 gene contains 30 exons (Figure 4B), with the potential to produce a full-length mRNA containing 4803 coding bases. Alternatively spliced cDNAs (Figure 4C) suggest that several protein isoforms between 1300 and 1600 amino acids in length are generated. Because none of the cDNAs are full length, we do not know the precise exon content of any single isoform. Isolation of a single SL1-primed PCR product from cDNA using a reverse primer in exon 6 indicates that isoforms that contain exon 6 also contain the N-terminal kinase domain. No attempt was made to use reverse primers in other exons to identify alternative 5′-ends. Comparison of the C. elegans genomic sequence to that of C. briggsae revealed that the unc-82 gene structure, including alternative exon borders, is conserved between the two species (see supporting information, Figure S1).
UNC-82 is located at or near the M-line:
To determine the subcellular location of the UNC-82 protein in vivo, a full-length C-terminal GFP fusion construct (see materials and methods) was injected into unc-82mutant worms, and transgenic lines exhibiting improved motility and muscle-cell structure were isolated. Because the final exon is found in all known cDNAs (Figure 4C), we expected the inserted GFP tag to label all protein products from the locus. Expression of the fusion protein in unc-82 mutants restored the wild-type pattern of UNC-89 (Figure 5A), demonstrating function in vivo. Further, UNC-82∷GFP is localized near the M-line and present throughout the depth of the myofilament lattice (Figure 5, A–F). The distribution of UNC-82∷GFP appears punctate by antibody staining in fragmented adults (Figure 5B), but as continuous lines when viewed by endogenous GFP fluorescence in living or fixed adults (Figure 5E). At the 1.5-fold stage, the antibody signal in transgenic embryos is punctate and concentrated in the region of the contractile apparatus (Figure 5, G–I). As development continues, the stain becomes organized into longitudinal lines of dots that roughly coincide with the striped myosin pattern (Figure 5, J and K).
Transgene expression is not limited to body-wall muscle. In embryos, anti-GFP produces signal in areas outside the muscle quadrants (Figure 5K). Interestingly, UNC-82∷GFP is expressed in the pharynx but not detected in the regions occupied by thick filaments (Figure 5, L–N). No readily apparent defects in pharyngeal morphology or function were revealed by light microscopy, consistent with earlier observations (Waterston et al. 1980).
UNC-82 homologs are found in vertebrates and other invertebrates:
BLAST searches using either the full-length UNC-82 protein sequence or only the kinase catalytic domain identified the same small set of high-scoring proteins, which includes the human proteins ARK5 and SNARK (NUAK1 and NUAK2), as well as anonymous proteins in many organisms. All proteins within the group contain a kinase domain near the N terminus, with no detectable conserved domains in the remainder of the protein. The sequences C-terminal to the kinase domain are noncomplex and repetitive and lack detectable homology among worms, flies, and humans. This 1225-amino-acid region of UNC-82 includes 33% charged residues (amino acids D, E, K, and R, or DEKR) and 12% serine. The C-terminal regions of the human orthologs have similar sequence composition: ARK5, 27% DEKR, 16% serine; SNARK, 26% DEKR, 12% serine. Comparison of the UNC-82 sequence to itself (see materials and methods) revealed a variety of repeated elements ranging from 10 to 91 residues in length that are positioned throughout the C-terminal domain. None of these repeats match any repetitive elements detected in ARK5 and SNARK. The secondary structure prediction programs DSC (King and Sternberg 1996) and GGR (Garnier et al. 1996) (http://workbench.sdsc.edu/) suggest that 20–34% of C-terminal domain residues form an α-helix and 9–17% form β-strands.
BLASTp identified the two highest-scoring C. elegans UNC-82 paralogs as the products of the aak-2 and par-1 genes. AAK-2 is a member of the Snf1/AMPK family, members of which have been studied for their roles in energy metabolism and the stress response. In contrast, the par-1 gene was originally identified in screens for mutations affecting the anterior–posterior polarity of the earliest cell divisions in the C. elegans embryo (Kemphues et al. 1988). To examine the relationship of UNC-82 to Snf1/AMPK and PAR-1 kinases, a protein alignment of the catalytic domains of representatives from each of these families in C. elegans, Drosophila, and humans was constructed (Figure 6). Comparison of all sequences reveals 30% identity overall, whereas the percentage of identical residues is much higher among sequences within a given family. These data support the proposed orthology of the UNC-82/ARK5/SNARK group of kinases and suggest that this group diverged from the Snf1/AMPK and PAR-1 families prior to the divergence of worms, flies, and humans.
Figure 6.—
Alignment of the 256-residue kinase domain protein sequences from representatives of the UNC-82, PAR-1, and Snf1 families from worm (Ce), fly (Dm), and human (Hs) reveals that sequence divergence between families has occurred throughout the catalytic domain. Amino acid identity among all included sequences, indicated by black backgrounds, is 30%. Sequence identity is higher within families: UNC-82, 56%; Snf1, 74%; PAR-1, 81%. Positions that are identical within the UNC-82 family (top three lines), but are not identical in all nine sequences, are indicated by a magenta background. Similarly, such family identities are in yellow backgrounds for the Snf1 sequences and in blue backgrounds for the PAR-1 group. The unc-82 mutant alleles are represented in green.
UNC-82 lies in a branch of the calcium/calmodulin-regulated kinases tree:
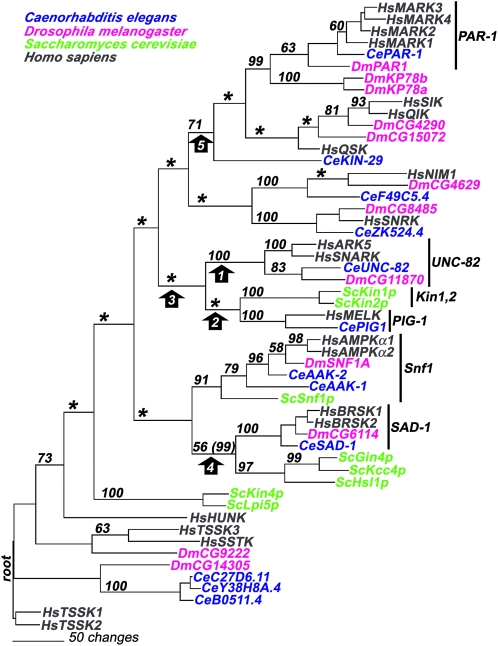
To establish the evolutionary relationship of UNC-82 and its apparent orthologs to related kinases, a phylogenetic tree (Figure 7) was estimated. The analysis (see Figure S2 and materials and methods) included all available kinase sequences within a small branch of the calcium/calmodulin-regulated kinases (CAMK) in the human kinome tree (Manning et al. 2002; http://www.kinase.com) and all related kinases in worm, Drosophila, and yeast, which were identified by BLAST. Both parsimony and neighbor-joining methods produced a single unique tree; these trees are very similar to each other and consistent with the relevant branch of the published human tree. Both methods support the proposed orthology of the UNC-82/ARK5/SNARK group of proteins suggested by BLAST analysis. Similarly, the orthologs within the well-characterized kinase families, such as PAR-1 and Snf1, form groups that have strong statistical support (Figure 7).
Figure 7.—
The parsimony tree presents a model of the descent of UNC-82 and related kinases from a common ancestral sequence. The tree was rooted to the HsTSSK sequences as shown in the human kinome tree (Manning et al. 2002; http://www.kinase.com). Bootstrap values (out of 100) are indicated on each branch; asterisks denote nodes that did not receive support >50. The proposed orthology of the UNC-82/ARK5/SNARK proteins is well supported (bootstrap value 100) for the node that separates this group from all other sequences (arrow 1). In contrast, neither the node connecting UNC-82 to the Kin1 and PIG-1 groups (arrow 2) nor the node that separates the UNC-82, Kin1, and PIG-1 groups from all other groups (arrow 3) is supported by bootstrap analysis. Two groupings that receive moderate bootstrap support in this parsimony analysis (arrows 4 and 5) receive higher support (in parentheses) using neighbor-joining techniques: the node grouping the SAD-1 family with the trio of yeast kinases Gin4/Kcc4/Hsl1 and the node grouping the PAR-1 family with HsSIK, HsQSK, and CeKIN-29.
In the parsimony tree (Figure 7), UNC-82 appears most closely related to the Kin1/Kin2 and PIG-1 groups, but these relationships are not statistically significant. Further, the grouping of UNC-82 with Kin1/Kin2 and PIG-1 does not occur in the tree constructed using neighbor-joining techniques (not shown). Therefore, the data do not allow us to discern whether UNC-82 is more closely related to any one of these conserved families: Kin1/Kin2, PIG-1, PAR-1, Snf1/AMPK, or SAD-1.
DISCUSSION
UNC-82 is a serine/threonine kinase:
Our combined molecular and genetic approaches have unambiguously identified the unc-82 transcription unit (Figure 4). The single conspicuous feature of the predicted ORF is a serine/threonine kinase catalytic domain. Both unc-82 alleles contain a mutation in the kinase domain (Figure 6). The missense allele e1220, in which a widely conserved glutamic acid is replaced by a lysine, is a likely kinase-dead mutation. In a scanning alanine mutagenesis of a yeast kinase, mutation of the homologous glutamic acid reduced catalytic activity to 1.7% of wild type (Gibbs and Zoller 1991). This confirms the importance of the kinase domain for UNC-82 function and argues against the presumptive catalytic domain having a different, unknown function, as has been suggested by mutational analysis of the ILK kinase (Mackinnon et al. 2002). The e1323 allele contains a premature stop within the kinase domain and is therefore a candidate for a molecular and genetic null mutation. Consistent with this possibility, the polarized light phenotype of e1323/stDf7 hemizygotes is similar to that of e1323 homozygotes at the young adult stage (Figure 3). However, the muscle phenotype of e1323/stDf7 animals becomes more severe in older adults, and the hemizygous animals exhibit additional phenotypes, such as sterility. These differences may reflect residual activity in the e1323 allele. A more likely alternative is that changes in phenotype are due to the deficit of proteins, such as vinculin, that are encoded by other genes deleted in stDf7.
Sequences outside the kinase domain in UNC-82 and its orthologs are noncomplex and repetitive and show no homology among the worm, fly, and human orthologs. If the sequences outside the UNC-82 catalytic domain are responsible for subcellular localization of the protein, the lack of homology or identifiable motifs in these regions suggests two extreme possibilities: (1) The C-terminal sequences have different ancestral origins in worms, flies, and humans and are therefore likely to be functionally different; and (2) the C-terminal sequences have a common origin and function, such as binding to an M-line protein, but are rapidly evolving and/or diverged from related kinases at a very ancient branch. Distinguishing between these will require comparison of the subcellular localization of the orthologs in the different species and identification of the protein region required for correct localization.
Role of UNC-82 in thick filament and M-line organization:
The first detectable defects in unc-82 mutant embryos appear during body elongation (Figure 2). Therefore, loss of unc-82 activity did not have a discernible effect on the early assembly events (Epstein et al. 1993; Hresko et al. 1994) such as protein localization to the muscle-cell membrane adjacent to the hypodermis, patterning of attachments at the membrane, or construction of functional contractile units. Vigorous contraction is not the primary cause of the M-line and thick filament defects in unc-82 mutants, since greatly decreasing myosin activity using the s95 allele did not rescue the muscle phenotype: double-mutant strains did not contain ordered bands of myosin filaments (Figure 3). However, the birefringent signal in the double-mutant lines appeared more uniform compared to that of the single mutants, suggesting that the more prominent patches of signal in the single mutants result from contractile forces acting on an already disorganized lattice. Previously, a myosin mutation was used to demonstrate that UNC-87/calponin is required to maintain sarcomere integrity during vigorous contraction (Goetinck and Waterston 1994).
Because thick filament disorganization in unc-82 mutants is not dependent on contractile activity, we propose that unc-82 is required for regulating some aspect of thick filament organization during changes in muscle-cell length. During the embryonic stages in which defects appear, the number of muscle cells remains constant as the length of the animal grows from 1.5 to approximately four times egg length. As cells elongate, thick and thin filament attachment sites move farther apart (Moerman and Williams 2006) so that the interdigitated filaments (see Figure 1), which are oriented longitudinally in the cell, must become longer and slide past each other. The UNC-82 kinase may play a role in one of these processes. The normal patterning of integrin in unc-82 mutants argues that, as cells elongate, M-lines are properly spaced at the membrane, but thick filament components and some M-line components become unevenly distributed within the cell (Figure 2). Since the central portion of the bipolar thick filament is positioned at the M-line (see Figure 1), as M-lines move apart, filament centers must also move. Possible roles for UNC-82 in filament translocation include proper attachment of thick filaments to the M-line or regulation of the ability of the interdigitated filaments to slide past each other. In these cases, the bright patches of staining in unc-82 mutants may correspond to abnormally wide regions of filament lattice that reflect a failure in filament placement. Alternatively, it is possible that unc-82 is required for the addition of protein components to the lengthening thick filament and that the abnormal accumulations of myosin and paramyosin represent unincorporated or misincorporated protein.
The localization of UNC-82∷GFP near the M-line (Figure 5) suggests that this kinase targets proteins in the central portion of the bipolar thick filament or the M-line. The punctate localization of UNC-82 in embryos and some adult preparations (Figure 5, B and H) does not resemble the distribution of any known protein or structure. Because the endogenous GFP signal from UNC-82∷GFP in living and fixed animals (Figure 5E) appears in unbroken lines, as in the anti-UNC-89 stain, the puncta may represent a portion of the UNC-82 protein pool that is more accessible to antibodies. The colocalization of UNC-82 and UNC-89/obscurin and the disorganization of UNC-89 in unc-82 mutants (Figure 1) suggest that the two proteins may directly interact or at least be members of a single signaling pathway. UNC-89/obscurin is a giant protein that contains two potential kinase domains and many Ig domains, which are hypothesized to mediate thick filament attachment to the M-line (Benian et al. 1996; Small et al. 2004). It is possible that UNC-82 targets UNC-89/obscurin to regulate thick filament attachment or some other process that requires transmission of a signal from membrane-proximal components of the M-line to the thick filaments.
Myosin and paramyosin are the earliest affected proteins in unc-82 mutants (Figure 2) and are therefore candidate targets of the enzyme as well. Previous ultrastructural analyses of single- and double-mutant adult worms revealed that unc-82 mutations resulted in thick filaments that lacked paramyosin and aberrant filaments that were likely composed of paramyosin. This led to the suggestion that the unc-82 gene product probably affects thick filament assembly through its actions on paramyosin (Waterston et al. 1980). Paramyosin is a coiled-coil protein homologous to the C-terminal two-thirds of the myosin heavy chain rod and may be considered a “headless myosin” (Kagawa et al. 1989). The small N-terminal nonhelical “headpiece” region of paramyosin, but not the coiled-coil domain, is phosphorylated on serine residues by an endogenous kinase (Schriefer and Waterston 1989). The nonhelical headpiece contains multiple copies of the proposed phosphorylation motif, S_S_A. The more acidic isoelectric species of paramyosin are absent from extracts of unc-82 mutant worms (unpublished data cited in Schriefer and Waterston 1989), suggesting that the headpiece is a direct or indirect target of UNC-82.
The S_S_A motif is also present in multiple copies in the nonhelical C-terminal tailpieces of both myosin heavy chains expressed in body-wall muscle (Schriefer and Waterston 1989), suggesting that myosin and paramyosin may be targeted by the same kinase. Abnormal thick-filament structures found in unc-82 mutants contain both myosin and paramyosin (Epstein et al. 1987). The possibility that myosin is a target of UNC-82 is supported by the observation that the myosin phenotype observed in unc-82 mutant embryos is similar to that caused by removal of the phosphorylation motifs contained in the myosin nonhelical tailpiece: early patterning of myosin is normal, but aberrant patchy distribution appears as elongation proceeds (Hoppe et al. 2003). While the mechanisms guiding thick filament elongation and placement during growth are not well understood in striated muscle, many prior studies have examined the regulation of assembly and disassembly of nonmuscle and smooth muscle myosins by phosphorylation (Castellani and Cohen 1987; Castellani et al. 1988; reviews: Moussavi et al. 1993; Brzeska and Korn 1996; Redowicz 2001; Bosgraafa and van Haastert 2006). In C. elegans striated muscle, both myosin and paramyosin are phosphorylated in an assembly-dependent manner (Dey et al. 1992). UNC-82∷GFP is present at or near the M-line and therefore distant from the ends of the thick filament where much of the subunit addition presumably occurs. Its location may imply that, if UNC-82 regulates these proteins, UNC-82 directly phosphorylates only a subpopulation of myosin and paramyosin molecules or that it serves as an intermediate in a kinase cascade that targets these proteins.
unc-82 mutations identify a role for this conserved kinase family in normal development:
The UNC-82 orthologs ARK5 and SNARK (NUAK-1 and NUAK-2) (Figure 7) are named for their similarity to the Snf1/AMPK kinases, which are thought to be activated by increased levels of the nucleotide AMP during metabolic or other stress conditions. In humans, ARK5 (cDNA KIAA0537) is strongly expressed in both skeletal and cardiac muscle (http://www.kazusa.or.jp/huge/) and may therefore play a role similar to that of UNC-82 in developing striated muscle. Tests of the in vivo function of ARK5 in vertebrate muscle (Fisher et al. 2005; Niesler et al. 2007) have been limited to its putative role as an AMP-activated kinase.
The mechanism of UNC-82 activation during muscle development is unknown. Despite its similarity to AMPK, UNC-82 functions in cells of healthy developing embryos where it is unlikely to be exposed to the elevated levels of AMP or other activating signals important in the stress response. Further, although the UNC-82/ARK5/SNARK family lies within the so-called CAMK portion of the kinome tree (Manning et al. 2002; http://www.kinase.com), this designation is applied to a large region of the kinome, including many proteins whose specific mode of regulation has not been established experimentally.
If expression of UNC-82∷GFP outside body-wall muscle (Figure 5) represents distribution of the native protein, it is likely that UNC-82 has unidentified functions in other tissue types. Similarly, ARK5 (http://www.kazusa.or.jp/huge/; Fisher et al. 2005) and SNARK (Lefebvre and Rosen 2005) are expressed in many nonmuscle tissues and organs. As putative AMP-activated enzymes, both have been studied for roles in the cellular stress response in tissue culture cells and cancer cell lines. ARK5 is induced in response to glucose starvation (Suzuki et al. 2003) and can confer resistance to apoptosis induced by stress (Suzuki et al. 2005) or apoptotic signals (Suzuki et al. 2004). The response of SNARK activity to different stresses varies greatly depending upon the cell type tested (Lefebvre and Rosen 2005). The role of UNC-82, ARK5, and SNARK in normal development or physiology in nonmuscle tissues is unknown. A role for the human kinases in the regulation of the cytoskeleton, such as that proposed for UNC-82 in muscle, might be implied by changes observed in tumor cells when kinase activity is altered. For example, increased SNARK activity is associated with increased motility and invasiveness (Legembre et al. 2004) and with cell detachment in culture (Suzuki et al. 2003). ARK5 activity is also correlated with metastatic invasiveness in tumors derived from several tissue types (Kusakai et al. 2004a,b; Suzuki et al. 2004, 2005).
UNC-82 lies in a branch of the kinome tree rich in cytoskeletal regulators:
Our phylogenetic analyses support a close evolutionary relationship between UNC-82 and the AMPK, PAR-1, SAD-1, PIG-1, and Kin1/2 protein families. Several of the kinases within these families have been shown by mutation to play a role in normal development and in particular are involved in the regulation of the cytoskeleton and cell polarity. We propose that UNC-82 is an additional member of this superfamily of developmentally important kinases whose salient feature is the regulation of cellular organization rather than any presumed mode of activation.
In light of the disorganized myosin phenotype in unc-82 mutants, it is intriguing to note that other kinases within the related kinase families interact with myosin in nonmuscle cells. The C. elegans nonmuscle myosin NMY-2 was identified through its interaction with the PAR-1 protein and is required for establishing embryonic polarity (Guo and Kemphues 1996). Drosophila SNF1/AMPK is required during embryogenesis for establishment of epithelial cell polarity and acts through phosphorylation of the myosin regulatory light chain (Lee et al. 2007). The presence of various myosin-associated kinases in this region of the kinome suggests that myosin was a substrate of an ancestral kinase prior to the divergence of the UNC-82, AMPK, and PAR-1 groups.
Acknowledgments
We thank Mike Becker of the Genome Sequencing Center for instructions and assistance in library production and sequencing, Yuji Kohara for cDNA clones, Ziva Misulovin for RNA and expert advice, Guy Benian for antibodies and for suggesting that we analyze the protein sequence for internal repeats, and Xhirong Bao and Todd Barkman for help with our phylogenetic analysis. We thank Tim Schedl and his lab members for use of microscopes and scientific input and Doug Coulter for critical reading of the manuscript. Some strains were obtained from the C. elegans Genetic Center. This material is based upon work supported by the National Science Foundation under grant no. MCB 0454737.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.110189/DC1.
References
- Benian, G. M., T. L. Tinley, X. Tang and M. Borodovsky, 1996. The Caenorhabditis elegans gene unc-89, required for muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J. Cell Biol. 132 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraafa, L., and P. J. M. van Haastert, 2006. The regulation of myosin II in Dictyostelium. Eur. J. Cell Biol. 85 969–979. [DOI] [PubMed] [Google Scholar]
- Brzeska, H., and E. D. Korn, 1996. Regulation of class I and class II myosins by heavy chain phosphorylation. J. Biol. Chem. 271 16983–16986. [DOI] [PubMed] [Google Scholar]
- Castellani, L., and C. Cohen, 1987. Rod phosphorylation favors folding in a catch muscle myosin. Proc. Natl. Acad. Sci. USA 84 4058–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani, L., B.W. Elliott, Jr. and C. Cohen, 1988. Phosphorylatable serine residues are located in a non-helical tailpiece of a catch muscle myosin. J. Muscle Res. Cell Motil. 9 533–540. [DOI] [PubMed] [Google Scholar]
- Dey, C. S., P. R. Deitiker and H. F. Epstein, 1992. Assembly-dependent phosphorylation of myosin and paramyosin of native thick filaments in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 14 1528–1532. [DOI] [PubMed] [Google Scholar]
- Dibb, N. J., D. M. Brown, J. Karn, D. G. Moerman, S. L. Bolten et al., 1985. Sequence analysis of mutations that affect the synthesis, assembly and enzymatic activity of the unc-54 myosin heavy chain of Caenorhabditis elegans. J. Mol. Biol. 183 543–551. [DOI] [PubMed] [Google Scholar]
- Duerr, J. S., 2006. Immunohistochemistry, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org.
- Epstein, H. F., I. Ortiz and G. C. Berliner, 1987. Assemblages of multiple thick filaments in nematode mutants. J. Muscle Res. Cell Motil. 8 527–536. [DOI] [PubMed] [Google Scholar]
- Epstein, H. F., D. L. Casey and I. Ortiz, 1993. Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J. Cell Biol. 122 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, J. S., J. S. Ju, P. J. Oppelt, J. L. Smith, A. Suzuki et al., 2005. Muscle contractions, AICAR, and insulin cause phosphorylation of an AMPK-related kinase. Am. J. Physiol. Endocrinol. Metab. 289 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, G. R., and R. H. Waterston, 1985. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J. Cell Biol. 101 1532–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier, J., J. F. Gibrat and B. Robson, 1996. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 266 540–553. [DOI] [PubMed] [Google Scholar]
- Gibbs, C. S., and M. J. Zoller, 1991. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J. Biol. Chem. 266 8923–8931. [PubMed] [Google Scholar]
- Goetinck, S., and R. H. Waterston, 1994. The Caenorhabditis elegans UNC-87 protein is essential for maintenance, but not assembly, of bodywall muscle. J. Cell Biol. 127 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S., and K. J. Kemphues, 1996. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature 382 455–458. [DOI] [PubMed] [Google Scholar]
- Hanks, S. K., and T. Hunter, 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase catalytic domain structure and classification. FASEB J. 9 576–596. [PubMed] [Google Scholar]
- Hoppe, P. E., and R. H. Waterston, 2000. A region of the myosin rod important for interaction with paramyosin in Caenorhabditis elegans striated muscle. Genetics 156 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, P. E., R. C. Andrews and P. D. Parikh, 2003. Differential requirement for the nonhelical tailpiece and the C-terminus of the myosin rod in C. elegans muscle. Mol. Biol. Cell 14 1677–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko, M. C., B. D. Williams and R. H. Waterston, 1994. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J. Cell Biol. 124 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski, J., and K. Kornfeld, 1999. A local, high-density, single-nucleotide polymorphism map used to clone Caenorhabditis elegans cdf-1. Genetics 153 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa, H., K. Gengyo, A. D. McLachlan, S. Brenner and J. Karn, 1989. Paramyosin gene (unc-15) of Caenorhabditis elegans: molecular cloning, nucleotide sequence and models for thick filament structure. J. Mol. Biol. 207 311–333. [DOI] [PubMed] [Google Scholar]
- Kemphues, K. J., J. R. Priess, D. G. Morton and N. S. Cheng, 1988. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52 311–320. [DOI] [PubMed] [Google Scholar]
- King, R. D., and M. J. Sternberg, 1996. Identification and application of the concepts important for accurate and reliable protein secondary structure prediction. Protein Sci. 5 2298–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakai, G., A. Suzuki, T. Ogura, M. Kaminishi and H. Esumi, 2004. a Strong association of ARK5 with tumor invasion and metastasis. J. Exp. Clin. Cancer Res. 23 263–268. [PubMed] [Google Scholar]
- Kusakai, G., A. Suzuki, T. Ogura, S. Miyamoto, A. Ochiai et al., 2004. b ARK5 expression in colorectal cancer and its implications for tumor progression. Am. J. Pathol. 164 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H., H. Koh, M. Kim, Y. Kim, S. Y. Lee et al., 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447 1017–1020. [DOI] [PubMed] [Google Scholar]
- Lefebvre, D. L., and C. F. Rosen, 2005. Regulation of SNARK activity in response to cellular stresses. Biochim. Biophys. Acta 1724 71–85. [DOI] [PubMed] [Google Scholar]
- Legembre, P., R. R. Schickel, B. C. Barnhart and M. E. Peter, 2004. Identification of SNF1/AMP kinase-related kinase as an NF-kappaB-regulated anti-apoptotic kinase involved in CD95-induced motility and invasiveness. J. Biol. Chem. 279 46742–46747. [DOI] [PubMed] [Google Scholar]
- Mackinnon, A. C., H. Qadota, K. R. Norman, D. G. Moerman and B. D. Williams, 2002. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12 787–797. [DOI] [PubMed] [Google Scholar]
- Manning, G., D. B. Whyte, R. Martinez, T. Hunter and S. Sudarsanam, 2002. The protein kinase complement of the human genome. Science 298 1912–1934. [DOI] [PubMed] [Google Scholar]
- Moerman, D. G., and B. D. Williams, 2006. Sarcomere assembly in C. elegans muscle, in WormBook, edited by The C. elegans Research Community. Wormbook, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Moerman, D. G., S. Plurad, R. H. Waterston and D. L. Baillie, 1982. Mutations in the unc-54 myosin heavy chain gene of Caenorhabditis elegans that alter contractility but not muscle structure. Cell 29 773–781. [DOI] [PubMed] [Google Scholar]
- Moussavi, R. S., C. A. Kelley and R. S. Adelstein, 1993. Phosphorylation of vertebrate nonmuscle and smooth muscle myosin heavy chains and light chains. Mol. Cell. Biochem. 128 219–227. [DOI] [PubMed] [Google Scholar]
- Niesler, C. U., K. H. Myburgh and F. Moore, 2007. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp. Physiol. 92 207–217. [DOI] [PubMed] [Google Scholar]
- Redowicz, M. J., 2001. Regulation of nonmuscle myosins by heavy chain phosphorylation. J. Muscle Res. Cell Motil. 22 163–173. [DOI] [PubMed] [Google Scholar]
- Schriefer, L., and R. H. Waterston, 1989. Phosphorylation of the N-terminal region of paramyosin in Caenorhabditis elegans. J. Mol. Biol. 207 451–454. [DOI] [PubMed] [Google Scholar]
- Small, T. M., K. M. Gernert, D. B. Flaherty, K. B. Mercer, M. Borodovsky et al., 2004. Three new isoforms of Caenorhabditis elegans UNC-89 containing MLCK-like protein kinase domains. J. Mol. Biol. 342 91–108. [DOI] [PubMed] [Google Scholar]
- Suzuki, A., G. Kusakai, A. Kishimoto, J. Lu, T. Ogura et al., 2003. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 278 48–53. [DOI] [PubMed] [Google Scholar]
- Suzuki, A., J. Lu, G. Kusakai, A. Kishimoto, T. Ogura et al., 2004. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol. Cell. Biol. 24 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A., S. Iida, M. Kato-Uranishi, E. Tajima, F. Zhan et al., 2005. ARK5 is transcriptionally regulated by the Large-MAF family and mediates IGF-1-induced cell invasion in multiple myeloma: ARK5 as a new molecular determinant of malignant multiple myeloma. Oncogene. 24 6936–6944. [DOI] [PubMed] [Google Scholar]
- Waddle, J. A., 1993. Molecular analysis of actin capping protein function in Caenorhabditis elegans. Ph.D. Thesis, Washington University, St. Louis.
- Waterston, R. H., J. N. Thomson and S. Brenner, 1980. Mutants with altered muscle structure of Caenorhabditis elegans. Dev. Biol. 77 271–302. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28 160–164. [DOI] [PubMed] [Google Scholar]
- Williams, B. D., and R. H. Waterston, 1994. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutation. J. Cell Biol. 124 475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, A., M. Dourado, A. Butler, N. Walton, A. Wei et al., 2000. SLO-2, a K+ channel with an unusual Cl- dependence. Nat. Neurosci. 3 771–779. [DOI] [PubMed] [Google Scholar]