Abstract
Isogenic strains of Agrobacterium tumefaciens carrying pTiC58, pAtC58, or both were constructed and assayed semiquantitatively and quantitatively for virulence and vir gene expression to study the effect of the large 542-kb accessory plasmid, pAtC58, on virulence. Earlier studies indicate that the att (attachment) genes of A. tumefaciens are crucial in the ability of this soil phytopathogen to infect susceptible host plants. Mutations in many att genes, notably attR and attD, rendered the strain avirulent. These genes are located on pAtC58. Previous work also has shown that derivatives of the wild-type strain C58 cured of pAtC58 are virulent as determined by qualitative virulence assays and, hence, pAtC58 was described as nonessential for virulence. We show here that the absence of pAtC58 in pTiC58-containing strains results in reduced virulence but that disruption of the attR gene does not result in avirulence or a reduction in virulence. Our studies indicate that pAtC58 has a positive effect on vir gene induction as revealed by immunoblot analysis of Vir proteins and expression of a PvirB::lacZ fusion.
Agrobacterium tumefaciens is a soil phytopathogen that incites tumors on susceptible plants by transferring the T-DNA, a portion of its tumor-inducing plasmid (pTi), into plant cells (Van Larebeke et al., 1974; Zhu et al., 2000; Zupan et al., 2000; Gelvin, 2003). This T-DNA is translocated to the nucleus, integrated into plant chromosomal DNA, and expressed. In strain C58, the products of approximately 20 vir genes arranged in six operons (virA, virB, virC, virD, virE, and virG) on pTiC58, as well as several chromosomal genes, act in a concerted fashion to induce tumor formation on host plants. Two critical early steps in this process are the attachment of the bacterium to the plant cell and the activation of virulence genes. Subsequently, a DNA-protein intermediate is formed and crosses the bacterium-plant interface, followed by the transport of this intermediate into the plant cell nucleus, integration of the T-DNA into the plant chromosome and expression of the T-DNA (Zupan et al., 2000; Christie, 2001; Tzfira and Citovsky, 2002; Gelvin, 2003). In nature, this results in the formation of continuously proliferating tumors that produce novel metabolites (opines) that can be utilized by the inciting bacterium but not by the plant (Dessaux et al., 1998).
Chromosomal and Ti plasmid genes are involved in the recognition of a plant environment and activation of the virulence machinery. The Ti-encoded VirA/VirG two-component regulatory system activates vir gene expression in response to the integrated effects of phenols, sugars, and low pH that are found at the plant wound site (Stachel and Zambryski, 1986; Shimoda et al., 1990; Turk et al., 1991; Winans, 1991; Winans et al., 1994; Lee et al., 1995). chvE is a chromosomal virulence gene encoding a periplasmic sugar-binding protein that interacts with the periplasmic domain of VirA, resulting in optimal expression of vir genes (Cangelosi et al., 1990; Huang et al., 1990; Shimoda et al., 1993; Doty et al., 1996; Peng et al., 1998). ChvD, an ABC transporter, also contributes to optimal vir gene expression through effects on VirG expression or activity (Winans et al., 1988; Liu et al., 2001). ChvI and ChvG are essential for virulence and have been implicated in global control of gene expression in A. tumefaciens (Mantis and Winans, 1993; Li et al., 2002). Thus, the control of vir gene expression is a complex set of events to which both the chromosome and Ti plasmid contributes.
A. tumefaciens recognition and attachment to host cells is an early and essential step of the infection process (Lippincott and Lippincott, 1969) and possibly the least understood. Both non-Ti and Ti-carrying strains attach to host cells, and, although this process is saturable (Neff and Binns, 1985) and various plant cell components have been proposed as attachment sites (Tzfira and Citovsky, 2002), the fundamental nature of the plant-bacterial interaction is not known. Genetic studies have demonstrated that a variety of chromosomal genes (chvA, chvB, and pscA), are required for attachment (Douglas et al., 1985b; Kado, 1998). The ChvA and ChvB proteins are involved in β-1,2 glucan synthesis (Zorreguieta et al., 1988; Cangelosi et al., 1989; de Iannino and Ugalde, 1989). Mutants in these are deficient in their ability to attach to and infect plants (Douglas et al., 1985a, 1985b). A recent report suggests that these mutants are temperature sensitive (Bash and Matthysse, 2002). The exoC (pscA) gene (Cangelosi et al., 1987; Thomashow et al., 1987; Uttaro et al., 1990) and cel genes (Matthysse et al., 1995), also chromosomally encoded, are required for the synthesis of exopolysaccharides and cellulose, respectively. Mutations at the cel loci (located in two operons) result in an altered attachment phenotype compared with the wild type (Matthysse, 1983). However, no studies have identified either the plant or bacterial components that take part in the saturable attachment process.
Another series of genes proposed to be involved in the attachment process were identified as residing on a 29-kb locus of the A. tumefaciens genome (Matthysse, 1987; Matthysse et al., 2000). Mutations in many of these genes resulted in strains that were deficient in attachment to the host cell. These att (attachment) genes are organized into at least nine operons, and attachment deficiencies caused by mutations at this locus are broadly classified into two groups: those that could be complemented by wild-type C58-conditioned medium and those that could not (Matthysse, 1994; Matthysse et al., 1996). The former group suggests that their gene products (or metabolites synthesized by them) may be secreted into the medium by the wild-type bacterial cells, and these products are somehow involved in the attachment process. This group contains a variety of genes, with homology to ABC transporters, a transcriptional regulator, an acetolactate synthase, and an ATP-binding protein, among others. The second group contains mutations in genes that encode two possible transcriptional regulators, one ATPase, and a number of biosynthetic proteins including a zinc-dependent hydrolase and an acetyl transferase (AttR; Matthysse et al., 2000). AttR was reported to be involved in the synthesis of surface molecules and was proposed as a possible candidate in the actual attachment of the bacteria to the plant cell surface (Reuhs et al., 1997; Matthysse and McMahan, 1998, 2001; Matthysse et al., 2000).
The genome of A. tumefaciens strain C58 has four distinct replicons: a circular chromosome, a linear chromosome, and the plasmids pAtC58 and pTiC58 (Van Montagu and Schell, 1979; Allardet-Servent et al., 1993; Goodner et al., 2001; Wood et al., 2001). The selective advantage of the large accessory plasmid, pAtC58, is not well defined, although published reports demonstrate that it is self-conjugal (Van Montagu and Schell, 1979), carries its own type IV secretory system (Chen et al., 2002), and its transfer system may substitute for some of the transfer (trb) gene functions of the Type IV transfer apparatus responsible for conjugal transfer of pTiC58 (Li et al., 1999). Genes on pAtC58 also encode proteins involved in the catabolism of the Amadori compound deoxyfructosyl glucosamine (Vaudequin-Dransart et al., 1995; Kim et al., 1996a; Baek et al., 2003) and, thus, may contribute metabolic flexibility to the bacterium.
Surprisingly, the recently published genome sequence of C58 revealed that the att genes described above mapped between bases 130,826 and 159,059 in pAtC58 (Goodner et al., 2001). This finding was unexpected because independent reports by three groups (Hooykaas et al., 1977; Rosenberg and Huguet, 1984; Hynes et al., 1985) had earlier established that this plasmid was not essential for virulence, as revealed by Kalanchoë diagremontiana virulence assays with pAtC58-free C58 and LBA275 (a rifampicin-resistant A. tumefaciens strain that carries pAtC58 and pTiC58) derivatives: The absence of this plasmid did not result in loss of virulence. In addition, Ogawa and Mii (Ogawa et al., 2000; Ogawa and Mii, 2001) observed that super-virulent A. tumefaciens strain CN15 cured of pAtCN15c and pAtCN15d, two of the four cryptic plasmids carried by this strain besides its Ti plasmid, displayed attenuated tumorigenicity.
The reports that mutations in the att genes render strains avirulent are in apparent conflict with reports that pAtC58 is not required for virulence. Because the virulence assays used in earlier studies that demonstrated the nonessentiality of pAtC58 were not quantitative and because the nopaline (NOP) plasmid pTiC58 encodes enzymes for trans-zeatin synthesis by the bacterium at the infection site (Akiyoshi et al., 1985, 1987; Powell et al., 1988; Krall et al., 2002; Veselov et al., 2003), effects on tumorigenesis by pAtC58 may have been masked. These observations led us to revisit the possibility that this accessory plasmid plays a role in the virulence phenotype of A. tumefaciens C58. Toward this end, pAtC58 and/or pTiC58 were transferred into strain UIA5 (Kim et al., 1996a), a plasmid-less strain that carries the C58 linear and circular chromosomes, thereby creating an isogenic series for use in an analysis of virulence. The results of these studies indicate that pAtC58 is not required for virulence in pTiC58 containing strains but is required along with pTiC58 for maximal virulence. A disruption in attR, however, does not affect the capacity of pAtC58 to influence tumorigenesis. Rather, the basis for the effect of pAtC58 on tumor formation appears to be the maximization of vir gene expression from pTiC58.
RESULTS
Construction of Isogenic Strains with Different Combinations of Plasmids
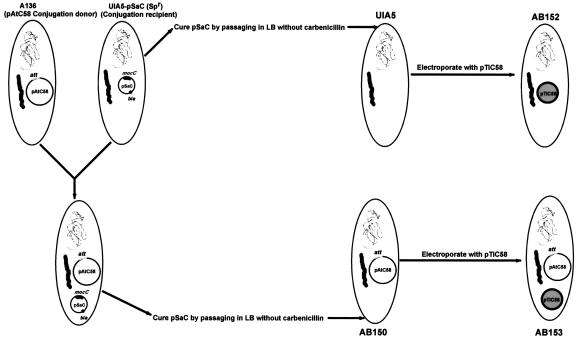
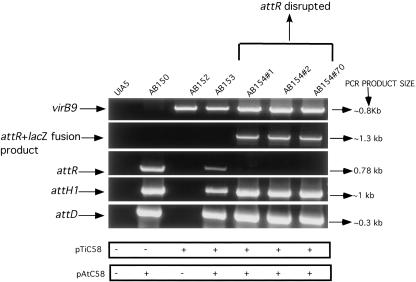
The construction of four isogenic strains carrying different combinations of plasmids was required for these studies (Fig. 1) and is detailed in “Materials and Methods.” In brief, strain UIA5 (pSa-C) that lacks pTiC58 and pAtC58 but contains the plasmid pSa-C (Table I) was used as a recipient for pAtC58 in matings with strain A136. MocC (an oxidoreductase) of pSa-C converts mannopine (MOP) to santhopine (Kim et al., 1996a). Because pAtC58 has genes that encode MOP uptake and santhopine utilization (Kim et al., 1996a), it was possible to select for the conjugal transfer of pAtC58 from strain A136 into UIA5 (pSa-C) by plating putative transconjugants onto minimal media with MOP as the sole carbon source. This scheme resulted in the isolation of strain AB150 (pSa-C). After both UIA5 (pSa-C) and AB150 (pSa-C) were cured of pSa-C, they were electroporated with pTiC58, and transformants were selected on minimal media containing NOP and Arg as the sole carbon and nitrogen source (von Bodman et al., 1989). The presence of pAtC58 and pTiC58 in our strains was verified using PCR with primers (listed in Table II) specific to these plasmids (Fig. 2). For example, strains carrying pAtC58 (AB150 and AB153) gave positive results with the attH1, attR, and attD primers, whereas strains carrying pTiC58 (AB152, AB153, and AB154) gave a positive result with virB9 primers. UIA5 does not carry either plasmid and, hence, was negative for all PCR analyses. Growth of all strains on rich or minimal Agrobacterium (AB) media was similar as observed by spectrophotometric measurements (data not shown). In addition, an insertional disruption of attR was constructed in strain AB153. A 659-bp internal fragment of the attR gene was cloned into pVIK112, an R6K-derived suicide vector (Kalogeraki and Winans, 1997) for A. tumefaciens, yielding pGN8. Recombination of this vector at the attR locus results in a 39-amino acid truncation of the 260-amino acid protein AttR and a transcriptional fusion to lacZ (Kalogeraki and Winans, 1997). Three different isolates of the strain AB154 that carry a disruption in the attR ORF did not yield PCR product when the primers homologous to the extreme 5′ and 3′ ends of the gene were used. However, when the attR 5′ primer was used in conjunction with the lacZ 3′ primer, a product of approximately 1.3 kb was observed. This is the expected product assuming the insertion of pGN8 had taken place in the attR gene by Campbell-type integration to produce an attR-lacZ transcriptional fusion. PCR analysis of these attR mutant strains also indicated that the other att genes tested were intact and that they carried pTiC58 (Fig. 2). Colonies of AB154 were blue when grown on AB minimal media (pH 5.5), containing 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (x-gal) and l-arabinose (data not shown) as a carbon source, indicating that the attR gene is normally expressed under these conditions.
Figure 1.
Construction of isogenic strains. See text for details.
Table I.
Strains and plasmids used in this study
| Strain/Plasmid | Relevant Features | Reference or Source |
|---|---|---|
| A. tumefaciens | ||
| C58 | Wild-type A. tumefaciens strain. Carries pTiC58 and pAtC58. | Gift from Stephen K. Farrand (University of Illinois, Urbana Champaign) |
| UIA5 (pSaC) | GMI9017 (Rosenberg and Huguet, 1984) heat cured of pTiC58, pAtC58, and pTiC58 free. Contains pSaC. Spectinomycin resistant (Spr), Carbr, Rifampicin resistant (Rifr), and streptomycin sensitive (Sms). | Kim et al., 1996a |
| A136 | C58 heat cured of pTiC58. | Garfinkel et al., 1981 |
| AB150 | UIA5 containing pAtC58 from A136. Spectinomycin resistant (Spr), Rifampicin resistant (Rifr), and Sms. | This work |
| AB152 | UIA5 containing pTiC58. Spr, Rifr, and Sms. | This work |
| AB153 | AB150 containing pTiC58. Spr, Rifr, and Sms. | This work |
| AB154 | AB153 with a pVIK1 12 insertion in attR. Kanamycin resistant (kmr), Spr, Rifr, and Sms. | This work |
| Escherichia coli | ||
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 A1 lac [F' proAB lac!qZ[M15 Tn10 tetracycline resistant; Tetr)] | Stratagene (La Jolla, CA) |
| E. coli S17-1/λ pir | IncP conjugal donor, streptomycin resistant (Smr). | Simon et al., 1983 |
| Plasmids | ||
| pVIK112 | R6K-derived suicide vector, requires R6K replication factor gene pir in host. | Kalogeraki and Winans, 1997 |
| pGN8 | Internal attR fragment (659 bp) cloned into the EcoR I site of pVIK112. | This work |
| pGN7 | Internal attR fragment (659 bp) cloned into pGEM T-Easy. | This work |
| pGEM T-Easy | PCR cloning vector. | Promega (Madison, WI) |
| pSa-C | Carbr, carries the mocC gene. | Kim et al., 1996a |
| pROK2 | Binary vector containing nos-nptII. | Baulcombe et al., 1986 |
| pSW209 | pSM243cd (Stachel and Zambryski, 1986) derivative with a PvirB::lacZ fusion, IncP, Kmr | Steve C. Winans (Cornell University, Ithaca, NY) |
Table II.
Oligonucleotides
List of oligonucleotides used for plasmid-specific PCRS and attR mutagenesis in this study.
| Oligonucleotides | Sequence |
|---|---|
| pAtC58 primers | |
| attDa | 5′CGGGGTACCCCGATGCACAATGGATCAACG3′b |
| 5′CCC AAG CTT GGG TTA GAT AGT TTG TGA GCC3′c | |
| attR (full length)a | 5′CGGGGTACCCCGATTATAAAAACCATTCAT3′b |
| 5′CCCAAGCTTGGGCTAGGCGATTTTGAGAGC3′c | |
| attR (502 bp) | 5′TGACGCAATCCATCCTATCA3′ |
| 5′AGCGGGATAACGCATTCTAA3′ | |
| Primers to amplify attR internal fragment for mutagenesis | 5′ATTCATGGCAGCGATAAAGG3′ |
| 5′AGCGGGATAACGCATTCTAA3′ | |
| attR-lacZ primers to screen insertions in the attR gene | 5′CGGGGTACCCCGATTATAAAAACCATTCAT3′ |
| 5′CCGCCACATATCCTGATCTT3′ | |
| attH1 | 5′CGGTTTCAAGATCGAGTGGT3′ |
| 5′ATAACCTTGTCCCGATGTGC3′ | |
| pTiC58 | |
| virB9 | 5′GCATTTCTCACTCTGGCATGT3′ |
| 5′GATCGTAGGCGGTATTCCAA3′ |
a Primers originally designed for cloning.
b Underlined portion indicates KpnI site.
c Underlined portion indicates HindIII site.
Figure 2.
PCR analysis of isogenic strains. Plasmid-specific primers were used to test for the presence of pTiC58 or pAtC58. The figure here also shows that three AB154 isolates carrying an attR disruption do not show the presence of a full-length attR product but are positive when tested with other att primers and virB9 primers or when the attR full-length forward primer is used in conjunction with the lacZ reverse primer. Reaction volume was 20 μL, and the entire reaction was loaded on a 1% (w/v) Tris-acetate EDTA agarose gel and run at 100 V for 1 h. Refer to text for details of primers and conditions used for PCR.
Virulence of Different Isogenic Strains
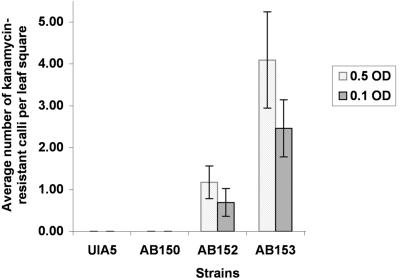
Two methods were used to examine the virulence of the isogenic strains carrying different combinations of plasmids. The first of these is the K. diagremontiana leaf scratch tumorigenesis assay in which varying concentrations of bacteria are delivered to fresh leaf scratches, and the resultant tumors are examined after a period of 3 weeks (for details, see “Materials and Methods”). As expected, UIA5 and AB150 were avirulent because neither of these strains contains a Ti plasmid (Fig. 3). AB153, which has both pAtC58 and pTiC58, induced tumors at all dilutions of the inciting bacterium. However, AB152, which contains pTiC58 but lacks pAtC58, formed smaller tumors than AB153 in response to high concentrations of inoculum and showed little or no tumor formation at the lowest dilution tested (0.01 OD600). Surprisingly, each of the AB154 isolates (attR disruption) was as virulent as the parent strain AB153.
Figure 3.
K. diagremontiana virulence assay. This figure represents a semiquantitative virulence assay. Strains were diluted as described in the methods section and inoculated on K. diagremontiana leaves to assay for relative tumorigenicity of isogenic strains.
One potential problem with the tumorigenesis assays described above is that pTiC58 carries the tzs gene. This gene is expressed in response to vir-inducing conditions and results in the secretion of zeatin, an active cytokinin that could play a role in the tumorous phenotype monitored in the K. diagremontiana leaf scratch assay (Akiyoshi et al., 1985; Krall et al., 2002; Veselov et al., 2003). To eliminate this variable in the measurement of virulence, we electroporated pROK2, a binary vector that carries the plant-expressible nptII gene (Baulcombe et al., 1986), into each of the isogenic strains. These strains were used in assays that estimate the transfer of the T-DNA from pROK2 into tobacco (Nicotiana tabacum) leaf squares by scoring for kanamycin-resistant growth of the plant tissue subsequent to cocultivation (Binns et al., 1995). The results of these assays were similar to those obtained with the K. diagremontiana tumorigenesis assay (Fig. 4). The average number of kanamycin-resistant calli observed for AB152 (pROK2) was much lower than for AB153 (pROK2), whereas the Ti plasmid-less strains UIA5 (pROK2) and AB150 (pROK2) yielded no kanamycin-resistant growths (Fig. 4). Thus, pAtC58 is not required for T-DNA transfer but appears to be necessary for optimal virulence.
Figure 4.
Kanamycin-based plant transformation assay. UIA5, AB150, AB152, and AB153 were tested for the ability to mobilize T-DNA from the binary vector pROK2 that carries the nptII gene, giving rise to kanamycin-resistant calli on tobacco leaf squares. This graph represents the average number of calli per leaf square for each strain at two cell concentrations (OD600). Error bars = se (n = 14).
vir Gene Expression Is Reduced in the Absence of pAtC58
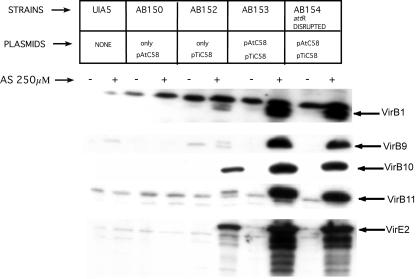
One possible mechanism by which pAtC58 could affect virulence of Ti plasmid-containing strains is through effects on vir gene expression. Therefore, we examined expression levels of several VirB proteins and VirE2 via immunoblot analysis. pTiC58-containing strains AB152 (lacks pAtC58) and AB153 (has pAtC58) expressed the tested VirB proteins and VirE2 under vir-inducing conditions (Fig. 5). However, AB152 expressed very low levels of all the VirB proteins as compared with AB153. Similar results were obtained in analysis of VirE2 expression. This was observed in three independent isolates of AB152 and AB153 (data shown here are for one representative isolate of each). The strain with an attR disruption on pAtC58 and AB154 produced VirB proteins and VirE2 at levels similar to AB153 (Fig. 5). This indicates that a mutation in attR does not affect vir gene regulation. The fact that strains lacking pAtC58 show a remarkable decrease in VirB and VirE2 expression relative to a strain carrying both pTiC58 and pAtC58 suggests that pAtC58 may have a positive effect on vir gene expression.
Figure 5.
Immunoblot analysis of isogenic strains. Immunoblot analysis of Vir protein profiles of various strains using antibodies against VirB1, VirB9, VirB10, VirB11, and VirE2. Protein samples from various strains were run on a 10% (w/v) denaturing polyacrylamide gel, and the ECL Plus western blotting and detection system from Amersham-Pharmacia Biotech was used for analysis.
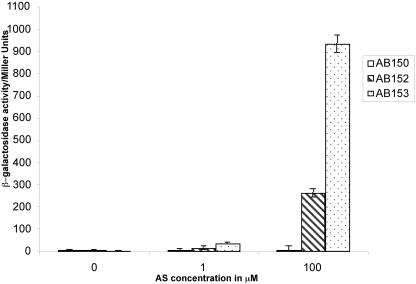
To explore the hypothesis that pAtC58 may affect transcription of the vir genes, plasmid pSW209, which carries a PvirB::lacZ fusion, was electroporated into strains AB150 (pTi–,pAt+), AB152 (pTi+,pAt–), and AB153 (pTi+,pAt+), and the resultant strains were tested for β-galactosidase activity (Fig. 6) after induction at various levels of acetosyringone (AS). As expected, the negative control strain AB150 exhibited no activity. The presence of pAtC58 along with pTiC58 was necessary for maximal lacZ expression: At 1 μm AS, strain AB153 exhibited observable activity, whereas strain AB152 was not significantly above control levels. At an AS concentration of 100 μm, both strains had significant β-galactosidase activity, but strain AB153 showed approximately 4-fold higher level of activity than AB152. These results suggest that pAtC58 is necessary for maximal expression of the pTiC58 vir genes in response to vir-inducing conditions.
Figure 6.
Vir induction assay. A. tumefaciens strains AB150 (Ti–,At+: negative control), AB152 (Ti+,At–), and AB153 (Ti+,At+) carrying pSW209 were grown in induction medium (pH 5.5) containing 0.25% (w/v) l-Ara and different concentrations of acetosyringone (AS) at 25°C. β-galactosidase activity was determined after 20 h, and the results are expressed in Miller units.
DISCUSSION
Naturally occurring virulent isolates of A. tumefaciens often carry one or more large “accessory” or “cryptic” plasmids, such as pAtC58, in addition to the Ti plasmid (Schell et al., 1976; Casse et al., 1979; Van Montagu and Schell, 1979; Ogawa et al., 2000; Ogawa and Mii, 2001). Although sequence analysis is beginning to provide information suggesting these plasmids encode a variety of metabolic pathways, their possible role in the virulence of this plant pathogen is poorly understood. One important surprise from the sequence analysis is that the att genes, thought to be required for the attachment of the bacteria to the plant cell and, hence, virulence (Mathysse et al., 2000), are located on pAtC58 (Goodner et al., 2001). However, earlier studies indicate that this plasmid is not essential for virulence in A. tumefaciens (Hooykaas et al., 1977; Rosenberg and Huguet, 1984; Hynes et al., 1985). The studies reported here were carried out to reevaluate the role of pAtC58 in the virulence of A. tumefaciens strain C58. New isogenic strains containing the C58 linear and circular chromosome that additionally carried pAtC58 and/or pTiC58 were constructed and tested for virulence, vir gene induction, and the possible role of the att genes in these processes.
The two different virulence assays—tumor formation on K. diagremontiana leaves and delivery of plant-expressible nptII from the binary vector pROK2 to tobacco leaf squares—demonstrated that strain AB152, carrying only pTiC58, exhibits reduced virulence as compared with AB153, which carries both pTiC58 and pAtC58. These results support earlier reports that pAtC58 does not carry genes essential for virulence (Hooykaas et al., 1977; Rosenberg and Huguet, 1984; Hynes et al., 1985) but indicate that pAtC58 has a quantitatively positive effect on virulence. In both types of assays, transformation with strains carrying only pTiC58 was reduced at the highest tested density of inoculum in comparison with that of strains with both plasmids. At lower levels of inoculum, this difference was even more pronounced, with the strain containing pTiC58 only showing little or no capacity for transformation, whereas strains with both plasmids continued to exhibit virulence.
There are several means by which pAtC58 could be involved in the quantitative increase in virulence that is observed. For example, several genes, located throughout the A. tumefaciens genome, have been shown to affect virulence gene expression (Winans, 1991, 1992; Winans et al., 1994; Zhu et al., 2000). Our results suggest that pAtC58-encoded gene products may be involved in this activity. The levels of VirE2, VirB1, VirB9, VirB10, and VirB11 as estimated by immunoblot analysis were clearly higher in strains that contained pAtC58 (AB153) than those that did not (AB152; Fig. 5). Because VirB2-11 and VirE2 are essential for virulence (Stachel and Nester, 1986; Berger and Christie, 1994), these results are consistent with the reduced virulence phenotype of AB152. AB153 expresses all the tested Vir proteins normally under inducing conditions, thus proving that the vir gene regulatory systems encoded by the Ti plasmid in these strains (VirA and VirG) and other chromosomal genes involved in virulence are intact. Vir induction assays on these strains using the reporter plasmid pSW209 that has a PvirB::lacZ fusion showed that AB153 was highly sensitive to AS, whereas AB152 showed a markedly lower activity under normally inducing conditions. These results demonstrate that the presence of pAtC58 has a positive effect on vir gene expression, although the relationship of this plasmid to the activities of the VirA/VirG regulatory system have not been established. One possibility is that the presence of pAtC58 could have a positive affect on the copy number of pTiC58, thus increasing the abundance of the Vir proteins and, as a result, virulence (Pappas and Winans, 2003). Southern-blot analysis has demonstrated, however, that the copy number of pTiC58 is unaffected by the presence or absence of pAtC58 (data not shown). The possibilities that pAtC58 specifically affects VirA/VirG regulated genes or, more generally, Ti plasmid genes are under investigation.
A second possible role pAtC58 could play in virulence is in the interaction of the inciting bacterium with plant cells at the wound site. Matthysse et al. (1996, 2000) reported that Tn3-HoHo1 insertions into a 29-kb region—the att region—result in strains that are incapable of attaching to plant cells and are avirulent. The fact that strains lacking pAtC58 and, therefore, the att region, are virulent and, by inference, capable of binding plant cells indicates that genetic information on this plasmid is not required for the attachment phenotype. However, the lowered virulence in pAtC58 lacking strains could be due to some quantitative reduction in binding. Alternatively, the att disruptions could lead to the production of some negative factor that interferes with binding and virulence. To test this possibility attR was disrupted in our isogenic strains and, surprisingly, did not effect virulence or vir gene expression. Thus, our results do not correspond to those of Matthysse et al. (2000), indicating that Tn3Hoho1 insertions into attR and several other att genes are avirulent and incapable of attachment. We tested two of these strains in our systems (A205 and B123—insertions in attR and attD, respectively) and found them to be avirulent. However, we have not been able to confirm that they are the expected A. tumefaciens strains. This issue is under further investigation.
A. tumefaciens has a complex genome and it is not surprising that a well-regulated and complex cascade of events that leads to virulence and generation of tumors on susceptible host plants would include all of the genome. The 542.8-kb cryptic plasmid pAtC58 (Allardet-Servent et al., 1993; Jumas-Bilak et al., 1995, 1998) has 547 predicted coding regions, of which 170 are predicted to be genes of novel function (Goodner et al., 2001; Wood et al., 2001). Others are predicted ABC transporters, metabolic pathway genes, transcriptional regulators, virulence gene homologs, and lactamases, among others. Our studies on strain C58 under laboratory conditions indicate this plasmid effects virulence in a quantitative manner, consistent with earlier studies (Ogawa et al., 2000; Ogawa and Mii, 2001). These studies demonstrated that the accessory plasmids pAtCN15c and pAtCN15d are necessary for maximal virulence of A. tumefaciens strain CN15, although the means by which the plasmids are involved in tumorigenicity was not addressed.
The results described here are consistent with other reports that important interactions occur between processes encoded for by the Ti plasmid and pAtC58. Gene products encoded by pAtC58 can contribute to opine uptake and catabolism (Kim et al., 1996a; Baek et al., 2003), indicating that this plasmid has evolved the capacity to coordinate activities important to the overall success of the virulence strategy utilized by this pathogen. Similarly, the N-acyl homoserine lactone hydrolase encoded by pAtC58 affects the levels of the inducer of the quorum sensing system, oxo-C8-homoserine lactone, that is synthesized by the traI gene product of pTiC58 (Hwang et al., 1994; Carlier et al., 2003) and controls conjugal transfer of the Ti plasmid. Although our studies demonstrate that pAtC58 is clearly important in the expression of the vir genes of pTiC58, the specificity of the interactions necessary for this has not been addressed. For example, is the virulence of other Ti plasmids affected by pAtC58? Is the chromosomal genome involved in this interaction? Future analysis to unravel the role played by accessory plasmids in virulence clearly will be helped by the sequence information currently available and by comparative studies of such genomes in other A. tumefaciens strains. Moreover, characterization of the role played by this plasmid in virulence will provide a model example of how supplementary processes may be involved, generally, in supporting virulence characteristics specified by other parts of the genome.
MATERIALS AND METHODS
Bacterial Strains and Plasmids
Bacterial strains and plasmids used in this study are listed in Table I. All cloning was performed using standard protocols, and all enzymes were used as recommended by manufacturers.
Culture Media, Growth Conditions, and Chemicals
Escherichia coli strains used for cloning were grown in Luria-Bertani media (LB) containing the appropriate antibiotics at 37°C. Agrobacterium tumefaciens strains were grown at 25°C in AB minimal media (Chilton et al., 1974), AB induction media (Winans et al., 1989), or mannitol glutamate luria salts medium (MG/L) (Cangelosi et al., 1991) containing the appropriate concentrations of antibiotics and AS (if used). Antibiotics for E. coli were used at the following concentrations (liquid medium:solid medium): ampicillin (50:125 μg mL–1), kanamycin (40:75 μg mL–1), and streptomycin (200:200 μg mL–1). Antibiotics for A. tumefaciens were used at the following concentrations (liquid medium:solid medium) spectinomycin (50:100 μg mL–1), carbenicillin (30:100 μg mL–1), and kanamycin (10:50 μg mL–1). NOP, AS, isopropyl-beta-d-thiogalactopyranoside, and x-gal were obtained from Sigma (St. Louis). Proteinase K was obtained from Fisher Scientific (Hampton, NH), lysozyme from Boehringer Mannheim/Roche (Indianapolis), restriction enzymes from New England Biolabs (Beverly, MA), Pfu Polymerase from Stratagene, and Taq polymerase from Perkin-Elmer Applied Biosystems (Boston, MA).
AB buffer for NOP and MOP selection was made without ammonium chloride (NH4Cl), and media were made with washed agar to ensure that these remained the sole carbon and nitrogen sources in the medium. NOP stock was made at a concentration of 25 mg mL–1 in 10% (w/v) glacial acetic acid and bitter sterlized using a 0.22 μm filler. For MOP stock, MOP was obtained from Fisher Scientific (Hampton, NH), and a 100 mm stock in distilled water was filter sterilized and stored at –20°C. For AS stock, millimolar stocks were made fresh in dimethyl sulfoxide and added to induction medium as necessary at the appropriate concentration. For A. tumefaciens, x-gal was used at a concentration of 50 μg mL–1 in AB minimal media plates. For E. coli, x-gal was used at 50 μg mL–1 in conjunction with isopropyl-beta-d-thiogalactopyranoside (50 μg mL–1).
Conjugation of pAtC58 Using MOP Selection
UIA5-pSaC was used as a recipient to select for an unmarked pAtC58 from donor strain A136 using MOP selection (Kim et al., 1996a). These strains were grown overnight at 25°C in LB with appropriate antibiotics. Donor and recipient cells were harvested, washed 2× with 0.8% (w/v) NaCl, and resuspended in 1 mL of the same at a final OD600 of 1.0. One hundred microliters of each was mixed and 10 μL spotted on an AB plate and allowed to mate overnight at 25°C. Cells were harvested from the mating plate and resuspended in 100 μL of AB, and 5-μL drops were spotted onto an AB plate containing spectinomycin (100 μg mL–1), carbenicillin (100 μg mL–1), and MOP (4 mm) as the sole carbon source to counterselect against the donor. Colonies were patched again on MOP plates. Single colonies from transconjugants were isolated and screened by PCR (for oligonucleotides, see Table II) for the presence or absence of pAtC58. Positives were subsequently cured of pSaC by passaging in LB without carbenicillin, and colonies that grew on LB plates but not LB with carbenicillin were used for future studies. The absence of pSaC was verified by standard techniques. This strain containing only pAtC58 was named AB150.
DNA Preparation Methods
For colony PCRs, a medium-sized colony was resuspended in 50-μL sterile PCR-grade water. A 10-μL aliquot of 10× PCR buffer was added along with 1 μL of lysozyme (120 μg mL–1) and incubated at 37°C for 15 min. After adding 1 μL of Proteinase K (12 mg mL–1), the sample was incubated at 60°C for 5 min and then at 110°C for 5 min. A pulse spin brought down cell debris, and 2.5 μL of this sample was used for subsequent amplifications (final reaction volume of 20 μL). pTiC58 was purified using the method of Hayman and Farrand (1990) from A. tumefaciens C58. Plasmid isolation from E. coli was done using standard kits (Qiagen USA, Valencia, CA).
Preparation of A. tumefaciens Competent Cells
Strains were inoculated into 3 mL of MG/L and grown overnight at 25°C to an OD600 of approximately 1.0. A 1-mL aliquot of these cells was spun down and washed six times in sterile distilled water and one time in 10% (w/v) glycerol. The pellet was resuspended in 50 μL of 10% (w/v) glycerol and used for electroporation.
Electroporation of Plasmids
Electroporation of pTiC58 into UIA5 and AB150 and Selection of Transformants
A 1-μl aliquot of a pTiC58 miniprep was used to electroporate strains UIA5 and AB150 at 400 Ω and 2.5 V using a Bio-Rad electroporator (Bio-Rad Laboratories, Hercules, CA; Cangelosi et al., 1991; den Dulk-Ras and Hooykaas, 1995). Cells were recovered in MG/L for 2 h at 25°C.They were pelleted, washed one time in 0.8% (w/v) NaCl to remove traces of MG/L, and dilutions were plated on AB minimal media containing NOP (50 μg mL–1) and Arg (1 mg mL–1) to select for transformants with pTiC58 (von Bodman et al., 1989) because utilization of Arg as a sole carbon source is pTiC58 dependent (von Bodman et al., 1989). Non-Ti plasmid-containing strains used for the electroporation were streaked out on NOP plates before this as negative controls and did not grow on these plates. Transformants from the pTi electroporation were subsequently tested by PCR (for oligonucleotides, see Table II) using virB9 primers to test for the presence of pTiC58 (see “Results” and Fig. 2).
Electroporation of Plasmids into E. coli
Plasmids were introduced into E. coli by electroporation at 200 Ω and 2.5 V using a Bio-Rad electroporator. Cells were recovered in LB medium for 1 h at 37°C and plated on selection media.
Mutagenesis of attR and Selection of attR Knockouts
A 659-bp internal fragment of the attR was amplified using PCR primers as listed in Table II and cloned into pGEM T-Easy (Promega) to yield pGN7. pGN7 was digested with EcoRI to remove the insert, and the EcoRI fragment was cloned into the EcoR I site of pVIK112 (Kalogeraki and Winans, 1997) to yield pGN8. pVIK112 is an R6K-derived suicide vector for strains that lack the R6K replication factor gene pir. The host strain used here was E. coli S17-1/λ pir. Positive clones were verified by PCR and restriction digests and used further. E. coli S17-1/λ pir (pGN8) was mated with AB153 by the drop-mating method described in the pAtC58 conjugation section, and transconjugants were selected on AB minimal media with x-gal, spectinomycin, and kanamycin at appropriate concentrations. Blue colonies were selected and tested by PCR for the absence of a full-length 780-bp attR PCR product and for a positive result with an attR forward primer outside the region of recombination and a lacZ 3′ primer to prove that the insertion had occurred at the attR locus. The insert was designed to truncate the protein at the C terminus by 39 amino acids. These recombinant strains carrying the attR disruption were designated AB154. See Table II for oligonucleotides.
Plant Transformation Assays
Kalanchoë diagremontiana Virulence Assay (Liu et al., 2001)
After strains were grown in MG/L overnight at 25°C with the appropriate antibiotics, the cells were pelleted, resuspended in 0.8% (w/v) NaCl to an OD600 of 1.0 and further diluted as necessary in 0.8 m NaCl. A 2-cm wound made with an 18-guage needle on the youngest expanded leaves of a 5-week-old K. diagremontiana plant with three pairs of leaves was inoculated with 3 μL of these dilutions. Tumor formation was monitored for a period of 25 d and photographed. Assays were done in triplicate and repeated at least thrice.
Tobacco (Nicotinia tabacum) Virulence Assays Using Binary Vector pROK2 (Baulcombe et al., 1986)
Electroporation was used to transform strains with the binary vector pROK2. Assays were done using different dilutions of bacteria as described previously (Binns et al., 1995). pROK2 confers kanamycin resistance to plant tissue. Greenhouse-grown tobacco cv Havana 425 plants were used for all leaf transformation protocols, using procedures previously described (Banta et al., 1994). Kanamycin resistant calli were scored after 21 d.
vir Gene Expression
Immunoblot Analysis for vir Gene Expression
Equal numbers of cells, grown at 25°C in Agrobacterium induction media with or without the phenolic vir gene inducer AS (Sigma) at a final concentration of 250 μm, were collected and resuspended in SDS sample buffer (12% [w/v] Suc, 4% [w/v] SDS, 0.1 m Tris.Cl [pH 6.8], 5 mm EDTA, 0.04% [w/v] bromphenol blue, and 0.1 m dithiothreitol), resolved on a 10% (w/v) SDS-PAGE gel, transferred to a PVDF membrane (Hybond, Amersham-Pharmacia Biotech, Piscataway, NJ), and probed with VirB1, VirB9, VirB10, VirB11, and VirE2 antibodies as described previously (Beaupre et al., 1997; Liu et al., 2001).
vir Gene Induction Assay
pSW209(Table I, Steve Winans, Cornell University) is a reporter plasmid that carries a PvirB::lacZ fusion (PvirB from pTiA6). AB150 (negative control), AB152, and AB153 carrying this plasmid were used for this assay. Strains were assayed for β -galactosidase by the method of Miller (1972) after a 20-h induction in AB induction medium (Winans et al., 1988; pH 5.5) with 0.25% (w/v) l-Ara (Shimoda et al., 1990; Doty et al., 1993) and 0.1 and 100 μm AS concentrations. Assays were done in triplicate and results expressed as Miller units.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
Special thanks to Dr. Stephen K. Farrand (University of Illinois, Urbana Champaign) for his gift of strains UIA5-pSaC, GMI9017, and C58, and, in particular, for his suggestions regarding the use of pSaC and MOP in selection for pAtC58 in the transconjugants. We also wish to thank Steve C. Winans (Cornell University, Ithaca, NY) for pVIK112, Christian Baron (McMaster University, Hamilton, Ontario, Canada) for C58 specific VirB1 and VirE2 antibodies, and Clay Fuqua (Indiana University, Bloomington) for E. coli S17-1/λ pir. We specially acknowledge the invaluable guidance from Dr. Arlene Wise (University of Pennsylvania, Philadelphia, PA) for the vir induction assays. We are grateful to Dr. Colleen McCullen (University of Pennsylvania, Philadelphia, PA) for comments, suggestions, and early readings of the manuscript. Special thanks to Shaunak J. Patel (University of Pennsylvania, Philadelphia, PA), an undergraduate in the lab, for assistance on the project. We wish to thank Dr. Ann G. Matthysse (University of North Carolina at Chapel Hill) for sending us A205 and B123.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030262.
This work was supported by the National Science Foundation (grant nos. MCB98–17149 and NIH RO1 GM47369).
References
- Akiyoshi DE, Regier DA, Gordon MP (1987) Cytokinin production by Agrobacterium and Pseudomonas spp. J Bacteriol 169: 4242–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi DE, Regier DA, Jen G, Gordon MP (1985) Cloning and nucleotide sequence of the tzs gene from Agrobacterium tumefaciens strain T37. Nucleic Acids Res 13: 2773–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allardet-Servent A, Michaux-Charachon S, Jumas-Bilak E, Karayan L, Ramuz M (1993) Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J Bacteriol 175: 7869–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek CH, Farrand SK, Lee KE, Park DK, Lee JK, Kim KS (2003) Convergent evolution of Amadori opine catabolic systems in plasmids of Agrobacterium tumefaciens. J Bacteriol 185: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta LM, Joerger RD, Howitz VR, Campbell AM, Binns AN (1994) Glu-255 outside the predicted ChvE binding site in VirA is crucial for sugar enhancement of acetosyringone perception by Agrobacterium tumefaciens. J Bacteriol 176: 3242–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bash R, Matthysse AG (2002) Attachment to roots and virulence of a chvB mutant of Agrobacterium tumefaciens are temperature sensitive. Mol Plant-Microbe Interact 15: 160–163 [DOI] [PubMed] [Google Scholar]
- Baulcombe DC, Saunders GR, Bevan MW, Mayo MA, Harrison BD (1986) Expression of biologically-active viral satellite RNA from the nuclear genome of transformed plants. Nature 321: 446–449 [Google Scholar]
- Beaupre CE, Bohne J, Dale EM, Binns AN (1997) Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol 179: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger BR, Christie PJ (1994) Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol 176: 3646–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns AN, Beaupre CE, Dale EM (1995) Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol 177: 4890–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi GA, Ankenbauer RG, Nester EW (1990) Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci USA 87: 6708–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi GA, Best EA, Martinetti G, Nester EW (1991) Genetic analysis of Agrobacterium. Methods Enzymol 204: 384–397 [DOI] [PubMed] [Google Scholar]
- Cangelosi GA, Hung L, Puvanesarajah V, Stacey G, Ozga DA, Leigh JA, Nester EW (1987) Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol 169: 2086–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi GA, Martinetti G, Leigh JA, Lee CC, Theines C, Nester EW (1989) Role for Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J Bacteriol 171: 1609–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y, Faure D (2003) The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Appl Environ Microbiol 69: 4989–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casse F, Boucher C, Julliot JS, Michel M, Denarie J (1979) Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J Gen Microbiol 54: 229–240 [Google Scholar]
- Chen L, Chen Y, Wood DW, Nester EW (2002) A new type IV secretion system promotes conjugal transfer in Agrobacterium tumefaciens. J Bacteriol 184: 4838–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA 71: 3672–3676, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie PJ (2001) Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol Microbiol 40: 294–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino NI, Ugalde RA (1989) Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1–2)glucan. J Bacteriol 171: 2842–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dulk-Ras A, Hooykaas PJ (1995) Electroporation of Agrobacterium tumefaciens. Methods Mol Biol 55: 63–72 [DOI] [PubMed] [Google Scholar]
- Dessaux Y, Petit A, Farrand SK, Murphy PJ (1998) Opine and opine-like molecules involved in plant-Rhizobiaceae interactions. In HP Spaink, A Kondorosi, PJJ Hooykaas, eds, The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria. Kluwer Academic Press, Dordrecht, The Netherlands, pp 173–197
- Doty SL, Chang M, Nester EW (1993) The chromosomal virulence gene, chvE, of Agrobacterium tumefaciens is regulated by a LysR family member. J Bacteriol 175: 7880–7886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty SL, Yu MC, Lundin JI, Heath JD, Nester EW (1996) Mutational analysis of the input domain of the VirA protein of Agrobacterium tumefaciens. J Bacteriol 178: 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C, Halperin W, Gordon M, Nester E (1985a) Specific attachment of Agrobacterium tumefaciens to bamboo cells in suspension cultures. J Bacteriol 161: 764–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CJ, Staneloni RJ, Rubin RA, Nester EW (1985b) Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol 161: 850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MD, Nester EW (1981) Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27: 43–53 [DOI] [PubMed] [Google Scholar]
- Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67: 16–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodner B, Hinkle G, Gattung S, Miller N, Blanchard M, Qurollo B, Goldman BS, Cao Y, Askenazi M, Halling C et al. (2001) Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294: 2323–2328 [DOI] [PubMed] [Google Scholar]
- Hayman GT, Farrand SK (1990) Agrobacterium plasmids encode structurally and functionally different loci for catabolism of agrocinopine-type opines. Mol Gen Genet 223: 465–473 [DOI] [PubMed] [Google Scholar]
- Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rorsch A (1977) Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent agrobacteria and to explanta. J Gen Microbiol 98: 477–484 [Google Scholar]
- Huang ML, Cangelosi GA, Halperin W, Nester EW (1990) A chromosomal Agrobacterium tumefaciens gene required for effective plant signal transduction. J Bacteriol 172: 1814–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Li PL, Zhang L, Piper KR, Cook DM, Tate ME, Farrand SK (1994) TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA 91: 4639–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes MF, Simon R, Puhler A (1985) The development of plasmid-free strains of Agrobacterium tumefaciens by using incompatibility with a Rhizobium meliloti plasmid to eliminate pAtC58. Plasmid 13: 99–105 [DOI] [PubMed] [Google Scholar]
- Jumas-Bilak E, Maugard C, Michaux-Charachon S, Allardet-Servent A, Perrin A, O'Callaghan D, Ramuz M (1995) Study of the organization of the genomes of Escherichia coli, Brucella melitensis and Agrobacterium tumefaciens by insertion of a unique restriction site. Microbiology 141: 2425–2432 [DOI] [PubMed] [Google Scholar]
- Jumas-Bilak E, Michaux-Charachon S, Bourg G, Ramuz M, Allardet-Servent A (1998) Unconventional genomic organization in the alpha subgroup of the Proteobacteria. J Bacteriol 180: 2749–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C, ed (1998) Agrobacterium-Mediated Horizontal Gene Transfer, Vol 20. Plenum Press, New York [DOI] [PubMed]
- Kalogeraki VS, Winans SC (1997) Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188: 69–75 [DOI] [PubMed] [Google Scholar]
- Kim KS, Chilton WS, Farrand SK (1996a) A Ti plasmid-encoded enzyme required for degradation of mannopine is functionally homologous to the T-region-encoded enzyme required for synthesis of this opine in crown gall tumors. J Bacteriol 178: 3285–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall L, Raschke M, Zenk MH, Baron C (2002) The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP. FEBS Lett 527: 315–318 [DOI] [PubMed] [Google Scholar]
- Lee YW, Jin S, Sim WS, Nester EW (1995) Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA 92: 12245–12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jia Y, Hou Q, Charles TC, Nester EW, Pan SQ (2002) A global pH sensor: Agrobacterium sensor protein ChvG regulates acid-inducible genes on its two chromosomes and Ti plasmid. Proc Natl Acad Sci USA 99: 12369–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PL, Hwang I, Miyagi H, True H, Farrand SK (1999) Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J Bacteriol 181: 5033–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott BB, Lippincott JA (1969) Bacterial attachment to a specific wound site as an essential stage in tumor initiation by Agrobacterium tumefaciens. J Bacteriol 97: 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Jacobs M, Schaff DA, McCullen CA, Binns AN (2001) ChvD, a chromosomally encoded ATP-binding cassette transporter-homologous protein involved in regulation of virulence gene expression in Agrobacterium tumefaciens. J Bacteriol 183: 3310–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Winans SC (1993) The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J Bacteriol 175: 6626–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (1983) Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol 154: 906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (1987) Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol 169: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG (1994) Conditioned medium promotes the attachment of Agrobacterium tumefaciens strain NT1 to carrot cells. Protoplasma 183: 131–136 [Google Scholar]
- Matthysse AG, McMahan S (1998) Root colonization by Agrobacterium tumefaciens is reduced in cel, attB, attD, and attR mutants. Appl Environ Microbiol 64: 2341–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, McMahan S (2001) The effect of the Agrobacterium tumefaciens attR mutation on attachment and root colonization differs between legumes and other dicots. Appl Environ Microbiol 67: 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, White S, Lightfoot R (1995) Genes required for cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 177: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, Yarnall H, Boles SB, McMahan S (2000) A region of the Agrobacterium tumefaciens chromosome containing genes required for virulence and attachment to host cells. Biochim Biophys Acta 1490: 208–212 [DOI] [PubMed] [Google Scholar]
- Matthysse AG, Yarnall HA, Young N (1996) Requirement for genes with homology to ABC transport systems for attachment and virulence of Agrobacterium tumefaciens. J Bacteriol 178: 5302–5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH (1972) Experiments in Molecular Genetics. Cold Spring Harbor Press, Cold Spring Harbor, NY
- Neff NT, Binns AN (1985) Agrobacterium tumefaciens interaction with suspension-cultured tomato cells. Plant Physiol 77: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Ishikawa K, Mii M (2000) Highly tumorigenic Agrobacterium tumefaciens strain from crown gall tumors of chrysanthemum. Arch Microbiol 173: 311–315 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Mii M (2001) Ti- and cryptic-plasmid-borne virulence of wild-type Agrobacterium tumefaciens strain CNI5 isolated from chrysanthemum (Dendranthema grandiflora Tzvelev). Arch Microbiol 176: 315–322 [DOI] [PubMed] [Google Scholar]
- Pappas KM, Winans SC (2003) A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol 48: 1059–1073 [DOI] [PubMed] [Google Scholar]
- Peng WT, Lee YW, Nester EW (1998) The phenolic recognition profiles of the Agrobacterium tumefaciens VirA protein are broadened by a high level of the sugar binding protein ChvE. J Bacteriol 180: 5632–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GK, Hommes NG, Kuo J, Castle LA, Morris RO (1988) Inducible expression of cytokinin biosynthesis in Agrobacterium tumefaciens by plant phenolics. Mol Plant-Microbe Interact 1: 235–242 [DOI] [PubMed] [Google Scholar]
- Reuhs BL, Kim JS, Matthysse AG (1997) Attachment of Agrobacterium tumefaciens to carrot cells and Arabidopsis wound sites is correlated with the presence of a cell-associated, acidic polysaccharide. J Bacteriol 179: 5372–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C, Huguet T (1984) The pAtC58 plasmid of Agrobacterium tumefaciens is not essential for tumour induction. Mol Gen Genet 196: 533–536 [Google Scholar]
- Schell J, Van Montagu M, Depicker A, De Waele D, Engler G, Genetello C, Van der Elsacker S, van Larebeke N, Zaenen I (1976) Agrobacterium tumefaciens: What Segment of the Plasmid Is Responsible for the Induction of Crown Gall Tumors? Plenum, New York
- Shimoda N, Toyoda-Yamamoto A, Aoki S, Machida Y (1993) Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J Biol Chem 268: 26552–26558 [PubMed] [Google Scholar]
- Shimoda N, Toyoda-Yamamoto A, Nagamine J, Usami S, Katayama M, Sakagami Y, Machida Y (1990) Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci USA 87: 6684–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Priefer U, Puhler A (1983) A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1: 784–791 [Google Scholar]
- Stachel SE, Nester EW (1986) The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J 5: 1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel SE, Zambryski PC (1986) VirA and VirG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell 46: 325–333 [DOI] [PubMed] [Google Scholar]
- Thomashow MF, Karlinsey JE, Marks JR, Hurlbert RE (1987) Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol 169: 3209–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk SC, Melchers LS, den Dulk-Ras H, Regensburg-Tuink AJ, Hooykaas PJ (1991) Environmental conditions differentially affect vir gene induction in different Agrobacterium strains: role of the VirA sensor protein. Plant Mol Biol 16: 1051–1059 [DOI] [PubMed] [Google Scholar]
- Tzfira T, Citovsky V (2002) Partners-in-infection: host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell Biol 12: 121–129 [DOI] [PubMed] [Google Scholar]
- Uttaro AD, Cangelosi GA, Geremia RA, Nester EW, Ugalde RA (1990) Biochemical characterization of avirulent exoC mutants of Agrobacterium tumefaciens. J Bacteriol 172: 1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J (1974) Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature 252: 169–170 [DOI] [PubMed] [Google Scholar]
- Van Montagu M, Schell J, eds (1979) The Plasmids of Agrobacterium tumefaciens. Elsevier/North Holland Biomedical Press, Amsterdam
- Vaudequin-Dransart V, Petit A, Poncet C, Ponsonnet C, Nesme X, Jones JB, Bouzar H, Chilton WS, Dessaux Y (1995) Novel Ti plasmids in Agrobacterium strains isolated from fig tree and chrysanthemum tumors and their opinelike molecules. Mol Plant-Microbe Interact 8: 311–321 [DOI] [PubMed] [Google Scholar]
- Veselov D, Langhans M, Hartung W, Aloni R, Feussner I, Gotz C, Veselova S, Schlomski S, Dickler C, Bachmann K et al. (2003) Development of Agrobacterium tumefaciens C58-induced plant tumors and impact on host shoots are controlled by a cascade of jasmonic acid, auxin, cytokinin, ethylene and abscisic acid. Planta 216: 512–522 [DOI] [PubMed] [Google Scholar]
- von Bodman SB, McCutchan JE, Farrand SK (1989) Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol 171: 5281–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SC (1991) An Agrobacterium two-component regulatory system for the detection of chemicals released from plant wounds. Mol Microbiol 5: 2345–2350 [DOI] [PubMed] [Google Scholar]
- Winans SC (1992) Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev 56: 12–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SC, Kerstetter RA, Nester EW (1988) Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol 170: 4047–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SC, Kerstetter RA, Ward JE, Nester EW (1989) A protein required for transcriptional regulation of Agrobacterium virulence genes spans the cytoplasmic membrane. J Bacteriol 171: 1616–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SC, Mantis NJ, Chen CY, Chang CH, Han DC (1994) Host recognition by the VirA, VirG two-component regulatory proteins of Agrobacterium tumefaciens. Res Microbiol 145: 461–473 [DOI] [PubMed] [Google Scholar]
- Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, Zhou Y, Chen L, Wood GE, Almeida NF Jr et al. (2001) The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294: 2317–2323 [DOI] [PubMed] [Google Scholar]
- Zhu J, Oger PM, Schrammeijer B, Hooykaas PJ, Farrand SK, Winans SC (2000) The bases of crown gall tumorigenesis. J Bacteriol 182: 3885–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorreguieta A, Geremia RA, Cavaignac S, Cangelosi GA, Nester EW, Ugalde RA (1988) Identification of the product of an Agrobacterium tumefaciens chromosomal virulence gene. Mol Plant-Microbe Interact 1: 121–127 [DOI] [PubMed] [Google Scholar]
- Zupan J, Muth TR, Draper O, Zambryski P (2000) The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J 23: 11–28 [DOI] [PubMed] [Google Scholar]