Abstract
Purpose
To describe chemotherapy use and adverse events (AEs) for advanced-stage, non–small-cell lung cancer (NSCLC) in community practice, including descriptions according to variation by age.
Methods
We interviewed patients with newly diagnosed, stages IIIB and IV NSCLC in the population-based cohort studied by the Cancer Care Outcomes Research and Surveillance Consortium, and we abstracted the patient medical records. AEs were medical events occurring during chemotherapy. Using logistic regression, we assessed the association between age and chemotherapy; with Poisson regression, we estimated event rate ratios and adjusted the analysis for age, sex, ethnicity, radiation therapy, stage, histology, and presence and grade of 27 comorbidities.
Results
Of 1,371 patients, 58% (95% CI, 55% to 61%) received chemotherapy and 35% (95% CI, 32% to 38%) had AEs. After adjustment, 72% (95% CI, 65% to 79%) of those younger than 55 years and 47% (95% CI, 42% to 52%) of those age 75 years and older received chemotherapy. Platinum-based therapies were less common in the older-age groups. Pretreatment medical event rates were 18.6% for patients younger than 55 years and were only 9.2% for those age 75 years and older (adjusted rate ratio, 0.49; 95% CI, 0.26 to 0.91). In contrast, older adults were more likely to have AEs during chemotherapy. The adjusted rate ratios compared with age younger than 55 years were 1.70 for 65- to 74-year-olds (95% CI, 1.19 to 2.43) and 1.34 for those age 75 years and older (95% CI, 0.90 to 2.00).
Conclusion
Older patients who received chemotherapy had fewer pretherapy events than younger patients and were less likely to receive platinum-based regimens. Nevertheless, older patients had more adverse events during chemotherapy, independent of comorbidity. Potential implicit trade-offs between symptom management and treatment toxicity should be made explicit and additionally studied.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death. Non–small-cell lung cancer (NSCLC) comprises 80% of occurrences, and one half have metastatic disease at the time of diagnosis.1 Clinical trials in advanced NSCLC show that chemotherapy can improve survival and has acceptable adverse event (AE) rates. A meta-analysis of randomized, controlled trials of palliative chemotherapy for NSCLC concluded that cisplatin-based chemotherapy regimens prolong survival compared with best supportive care alone.2 More recent trials have documented that doublet combinations of platinum (ie, cisplatin or carboplatin) with taxanes, vinorelbine, camptothecin analogs, and gemcitabine achieve similar response rates,3,4 and single agents have demonstrated benefits in elderly-specific trials.5,6
However, older adults have been under-represented in lung cancer clinical trials, and retrospective subgroup analysis of results in fit older patients provides limited evidence that these benefits may extend to older persons.7 This is of concern, because NSCLC is primarily a disease of older persons: 47% of patients are at least 70 years old at the time of diagnosis, and 14% are at least 80 years old.8 In marked contrast, only 25% of clinical trial patients were age 65 years or older,9 and the average age is 9 years younger than in the community.10 Although more recent trials have increasingly included people age 70 years and older, the same cannot be said of the group of patients who are 80 years and older.
Thus, the best treatment for older patients with advanced NSCLC is debated.11,12 Perhaps in part because of uncertainty about the balance between toxicity, symptom relief, and changes in quality of life and survival, a pronounced inverse association between age and receipt of recommended therapies has been observed.8,13 Clinical trials have limited utility for understanding care and effects in the community, where physicians may assume that older patients have poor prognoses or are at high risk for toxicity. However, the AE rate in the community and its association with patient age has not been reported. The purpose of this article is to describe the AEs experienced during chemotherapy for advanced-stage NSCLC in a population-based cohort and to describe how this varies by age. We tested whether age was associated with receipt of chemotherapy and, among those who received chemotherapy, whether age was associated with clinically important AEs.
METHODS
Participants were enrolled on the Cancer Care Outcomes Research and Surveillance Consortium (CanCORS).14 The consortium conducted a prospective, population-based cohort study on patients with newly diagnosed lung and colorectal cancer in 2003 to 2005 in multiple regions of the country and at a variety of healthcare delivery systems. Forty-nine percent of eligible patients with lung cancer participated. In CanCORS sites that had affiliated Surveillance, Epidemiology, and End Results (SEER) registries, the CanCORS cohort was similar to SEER in stage (stage IV, 38.1% v 41.1%), age (older than 75 years, 26.9% v 33.1%), and ethnic distribution (African American, 11.8% v 9.8%; Hispanic, 4.8% v 5.3%; Asian, 3.6% v 4.7%).
Data collection included a patient interview at enrollment and at 12 months after diagnosis, medical record abstraction, and surveys of treating physicians and informal caregivers.15 Because of the heavy burden of illness and the significant mortality, surrogate versions of the patient baseline interview were also developed: one for when the patient was alive but too ill to participate, and another if the patient was deceased. Participants (n = 1,371) included in this analysis were all those patients with stage IIIB or IV NSCLC who did not receive primary surgery and for whom medical records were collected.
Age, sex, and ethnicity were obtained from the baseline patient interview. Chemotherapy use data were obtained from the medical record and included the start and end date of each chemotherapy regimen along with the drugs administered. Chemotherapy regimens were categorized as platinum-based or non–platinum-based to explore whether older adults were more likely to receive less-toxic (ie, non–platinum-based) regimens. Radiation therapy (any v none), tumor histology (squamous cell, adenocarcinoma, other), stage (IIIB or IV), and comorbidities also were abstracted from the medical record. Comorbidity at the time of diagnosis was represented as a summary index value (none, mild decompensation, moderate decompensation, severe decompensation) across the 27 comorbidities included in the Adult Comorbidity Evaluation 27 (ACE-27) instrument.16 From the ACE-27, a cardiovascular disease index (none, mild, moderate, severe) was constructed as of the time of diagnosis from three indicators of heart disease (myocardial infarction, angina/coronary artery disease, and congestive heart failure). Similarly, the ACE-27 respiratory indicator was coded at diagnosis as a separate index (none, mild, moderate, severe disease).
Medical record abstractors were trained to record clinically important medical events in an abstraction window from 3 months before diagnosis through 15 months after diagnosis. An event was clinically important if it was acknowledged and reported by a clinician caring for the patient. Abstractors recorded medical events from a drop-down list of 34 distinct terms and associated definitions. All records for a patient, including those from hospitals, oncologist offices, and primary care offices, were reviewed. For example, abstractors were instructed to record fever with neutropenia regardless of whether it resulted in a hospitalization. Only the first occurrence of a particular medical event during the abstraction window was recorded. For this article, we analyzed medical events that occurred before treatment (ie, pretreatment medical events) and those that occurred during treatment. Any medical event that occurred while on chemotherapy was considered an AE. In addition to analyses of all AEs, we analyzed rates of AEs especially likely to be chemotherapy-related: neuropathy, fever with neutropenia, and sepsis. Because an event could be recorded only once, participants with an event in the pretreatment period (ie, between diagnosis and treatment initiation) were excluded from analyses of AEs.
Statistical Analysis
Multivariable logistic regression was used to model the association between age group (< 55, 55 to 64, 65 to 74, and ≥ 75 years) and receipt of chemotherapy for all participants after the analysis was adjusted for demographic and clinical variables. Covariate-adjusted, predicted probabilities of receiving recommended therapy were estimated from these models.13,17 Next, the subset of patients who received chemotherapy was analyzed. Covariate-adjusted probabilities of receiving a platinum-based drug in the first round of chemotherapy and at least one of several types of adverse events were modeled by using logistic regression, whereas rates of the corresponding events (or first instance of the events) were modeled by using Poisson regression. Among those who received chemotherapy, Poisson regression was employed to estimate rate ratios of adverse events experienced during chemotherapy treatment, and analysis was adjusted for differences among age groups and other covariates. In these analyses, our main independent variable was patient age group, and our main dependent variable was the rate of total medical events during chemotherapy, defined as the number of first event occurrences. We classified every person-day of chemotherapy cohort follow-up as before chemotherapy, during chemotherapy (including a 30-day period after the last date chemotherapy was administered), or after chemotherapy. Finally, to examine the possibility of selection bias between age groups among those who received chemotherapy, Poisson regression estimated rate ratios of the mean number of different types of medical events experienced between diagnosis and treatment initiation.
All analyses controlled for interview type (full patient interview, brief patient interview, surrogate interview for live patient, surrogate interview for deceased patient) because of strong differences in receipt of chemotherapy by interview type. Multiple imputation methodology was used to compensate and adjust for missing information in each generalized, linear (regression) model.18
RESULTS
There were 1,646 participants with stage IIIB or IV lung cancer; 1,371 remained after those with prior lung surgery or with missing sex or age data were excluded. Of these remaining patients, medical records indicated receipt of at least one course of chemotherapy for 798 (58%; 95% CI, 55% to 61%; Table 1). Targeted molecular therapy was used by less than 3% of patients because of the time period of study. Almost half of patients received radiation therapy (Table 1); of these, 58% also received chemotherapy (Table 2).
Table 1.
Patients With Stages IIIb or IV NSCLC by Age Group in the CanCORS Study
| Variable | Patients by Age Group (years) |
Total Patients (N = 1,371) |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 55 (n = 174) |
55-64 (n = 333) |
65-74 (n = 451) |
≥ 75 (n = 413) |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Research site | .3331 | ||||||||||
| Eight Northern California counties† | 34 | 19.5 | 62 | 18.6 | 88 | 19.5 | 92 | 22.3 | 276 | 20.1 | |
| State of Alabama | 27 | 15.5 | 38 | 11.4 | 56 | 12.4 | 39 | 9.4 | 160 | 11.7 | |
| Los Angeles County | 34 | 19.5 | 56 | 16.8 | 77 | 17.1 | 75 | 18.2 | 242 | 17.7 | |
| State of Iowa | 43 | 24.7 | 81 | 24.3 | 117 | 25.9 | 113 | 27.4 | 354 | 25.8 | |
| Veterans Health Administration‡ | 12 | 6.9 | 44 | 13.2 | 35 | 7.8 | 35 | 8.5 | 126 | 9.2 | |
| Cancer Research Network§ | 24 | 13.8 | 52 | 15.6 | 78 | 17.3 | 59 | 14.3 | 213 | 15.5 | |
| Female sex | 79 | 45.4 | 117 | 35.1 | 162 | 35.9 | 161 | 39.0 | 519 | 37.9 | .10 |
| Ethnicity | .0014 | ||||||||||
| White | 102 | 58.6 | 246 | 73.9 | 326 | 72.3 | 315 | 76.3 | 989 | 72.1 | |
| African American | 30 | 17.2 | 45 | 13.5 | 48 | 10.6 | 42 | 10.2 | 165 | 12.0 | |
| Hispanic | 11 | 6.3 | 11 | 3.3 | 30 | 6.7 | 20 | 4.8 | 72 | 5.3 | |
| Other | 31 | 17.8 | 31 | 9.3 | 47 | 10.4 | 36 | 8.7 | 145 | 10.6 | |
| Stage IV | 128 | 73.6 | 249 | 74.8 | 342 | 75.8 | 291 | 70.5 | 1,010 | 73.7 | .3220 |
| Histology | .0041 | ||||||||||
| Squamous cell | 20 | 11.5 | 63 | 18.9 | 99 | 22.0 | 86 | 20.8 | 268 | 19.6 | |
| Adenocarcinoma | 74 | 42.5 | 95 | 28.5 | 125 | 27.7 | 117 | 28.3 | 411 | 30.0 | |
| Other‖ | 80 | 46.0 | 175 | 52.6 | 227 | 50.3 | 210 | 50.9 | 692 | 50.5 | |
| Comorbidity at diagnosis | < .0001 | ||||||||||
| Mild | 59 | 33.9 | 125 | 37.5 | 174 | 38.6 | 155 | 37.5 | 513 | 37.4 | |
| Moderate | 32 | 18.4 | 69 | 20.7 | 99 | 22.0 | 98 | 23.7 | 298 | 21.7 | |
| Severe | 20 | 11.5 | 65 | 19.5 | 124 | 27.5 | 115 | 27.9 | 324 | 23.6 | |
| Respiratory disease at diagnosis | < .0001 | ||||||||||
| Mild | 31 | 17.8 | 79 | 23.7 | 138 | 30.6 | 131 | 31.7 | 379 | 27.6 | |
| Moderate | 16 | 9.2 | 17 | 5.1 | 49 | 10.9 | 31 | 7.5 | 113 | 8.2 | |
| Severe | 7 | 4.0 | 31 | 9.3 | 57 | 12.6 | 50 | 12.1 | 145 | 10.6 | |
| Cardiovascular disease at diagnosis | < .0001 | ||||||||||
| Mild | 7 | 4.0 | 33 | 9.9 | 68 | 15.1 | 92 | 22.3 | 200 | 14.6 | |
| Moderate | 6 | 3.5 | 13 | 3.9 | 32 | 7.1 | 36 | 8.7 | 87 | 6.4 | |
| Severe | 4 | 2.3 | 7 | 2.1 | 11 | 2.4 | 15 | 3.6 | 37 | 2.7 | |
| Received chemotherapy | 137 | 78.7 | 213 | 64.0 | 237 | 60.5 | 175 | 42.4 | 798 | 58.2 | < .0001 |
| Chemotherapy type | < .0001 | ||||||||||
| Cisplatin-based | 22 | 16.1 | 32 | 15.0 | 20 | 7.4 | 9 | 5.1 | 83 | 10.4 | |
| Carboplatin-based | 90 | 65.7 | 145 | 62.8 | 182 | 66.7 | 104 | 59.4 | 521 | 65.3 | |
| Nonplatinum | 21 | 15.3 | 33 | 15.5 | 62 | 22.7 | 57 | 32.6 | 173 | 21.7 | |
| Unknown | 4 | 2.9 | 3 | 1.4 | 9 | 3.2 | 5 | 2.9 | 21 | 2.6 | |
| Received radiation therapy | 102 | 58.6 | 178 | 53.5 | 208 | 46.1 | 163 | 39.5 | 651 | 47.5 | |
| Interview type | < .0001 | ||||||||||
| Brief | 13 | 7.5 | 21 | 6.3 | 37 | 8.2 | 39 | 9.4 | 110 | 8.0 | |
| Full | 89 | 51.2 | 131 | 39.3 | 141 | 31.3 | 100 | 24.2 | 461 | 33.6 | |
| Surrogate living | 18 | 10.3 | 29 | 8.7 | 47 | 10.4 | 40 | 9.7 | 134 | 9.8 | |
| Surrogate dead | 54 | 31.0 | 152 | 45.7 | 226 | 50.1 | 234 | 56.7 | 666 | 48.6 | |
Abbreviations: NSCLC, non–small-cell lung cancer; CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
By χ2 test.
Alameda, Contra Costa, Sacramento, San Francisco, San Joaquin, San Mateo, Santa Clara, and Solano counties.
Ten Veterans Health Administration hospitals in the following locations: Baltimore, MD; Biloxi, MS; Chicago, IL; Durham, NC; Minneapolis, MN; Nashville, TN; New York, NY; Portland, OR; Temple, TX; and Tucson, AZ.
Seattle, Washington Group Health Cooperative; Boston, Massachusetts Harvard Pilgrim Health Care; Detroit, Michigan Henry Ford Health System; Hawaii Kaiser Permanente Hawaii; Portland, Oregon, Kaiser Permanente Northwest.
Other histology included non–small-cell carcinoma not otherwise specified (n = 401; International Classification of Diseases for Oncology [ICD-O], revision [ICD-O], code 4086/3), unknown histology (n = 182), large-cell carcinoma not otherwise specified (n = 58; ICD-O 8012/3), carcinoma not otherwise specified (n = 21; ICD-O 8010/3), and other histology codes (n = 30; ICD-O 8000/3, 8001/3, 8013/3, 8020/3, 8022/3, 8031/3, 8032/3, 8034/3, 8343/3, 8560/3, and 8562/3).
Table 2.
Characteristics of Patients With Advanced Stage NSCLC Who Were Receiving Chemotherapy and Adjusted Probability of Receiving Chemotherapy in the CanCORS Study
| Characteristic | Patients |
Adjusted %* | 95% CI | |
|---|---|---|---|---|
| No. | % | |||
| Age, years† | ||||
| < 55 | 137 | 79 | 72 | 65 to 79 |
| 55-64 | 213 | 64 | 62 | 57 to 66 |
| 65-74 | 273 | 61 | 62 | 57 to 66 |
| ≥ 75 | 175 | 42 | 47 | 42 to 52 |
| Sex† | ||||
| Female | 285 | 55 | 53 | 49 to 57 |
| Male | 513 | 60 | 61 | 58 to 64 |
| Radiation therapy | ||||
| No | 414 | 58 | 59 | 55 to 62 |
| Yes | 384 | 59 | 58 | 54 to 61 |
| Research site | ||||
| Eight Northern California counties | 164 | 59 | 61 | 55 to 66 |
| State of Alabama | 89 | 56 | 51 | 44 to 58 |
| Los Angeles County | 147 | 61 | 63 | 57 to 59 |
| State of Iowa | 196 | 55 | 58 | 54 to 63 |
| Veterans Health Administration | 83 | 66 | 55 | 48 to 62 |
| Cancer Research Network | 119 | 56 | 56 | 50 to 62 |
| Ethnicity | ||||
| African American | 98 | 59 | 54 | 47 to 60 |
| Hispanic | 43 | 60 | 63 | 53 to 73 |
| White | 562 | 57 | 58 | 55 to 61 |
| Other | 95 | 66 | 63 | 56 to 71 |
| Stage | ||||
| IIIb | 224 | 62 | 58 | 53 to 63 |
| IV | 574 | 57 | 58 | 55 to 61 |
| Histology‡ | ||||
| Squamous | 139 | 52 | 53 | 47 to 58 |
| Adeno | 271 | 66 | 63 | 58 to 67 |
| Other | 388 | 56 | 58 | 54 to 61 |
| Comorbidity | ||||
| None | 149 | 63 | 54 | 47 to 60 |
| Mild | 320 | 62 | 60 | 56 to 65 |
| Moderate | 166 | 56 | 58 | 53 to 63 |
| Severe | 163 | 50 | 59 | 53 to 64 |
| Respiratory disease‡ | ||||
| None | 451 | 61 | 60 | 57 to 64 |
| Mild | 222 | 59 | 60 | 56 to 65 |
| Moderate | 54 | 48 | 47 | 39 to 56 |
| Severe | 71 | 49 | 51 | 43 to 60 |
| Cardiovascular disease | ||||
| None | 630 | 60 | 59 | 56 to 62 |
| Mild | 107 | 54 | 57 | 51 to 63 |
| Moderate | 43 | 49 | 53 | 44 to 63 |
| Severe | 18 | 49 | 55 | 39 to 71 |
| Interview type† | ||||
| Full | 386 | 84 | 83 | 79 to 86 |
| Brief | 82 | 75 | 76 | 68 to 83 |
| Surrogate living | 89 | 66 | 65 | 58 to 73 |
| Surrogate dead | 241 | 36 | 38 | 34 to 41 |
Abbreviations: NSCLC, non–small-cell lung cancer; CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
Adjusted percentages from a logistic regression model with receipt of chemotherapy as dependent variable and all listed variables as independent variables.
Variable is associated with receipt of chemotherapy after adjustment for all covariates: P < .01.
Variable is associated with receipt of chemotherapy after adjustment for all covariates: P < .05.
Older adults were less likely to receive chemotherapy and to receive radiation therapy, had poorer respiratory function at diagnosis as measured by the ACE-27 respiratory indicator, and had more cardiovascular and other comorbidities at diagnosis (Table 1). Most patients (n = 864) were age 65 years or older, and 30% were age 75 years or older.
Age was strongly and inversely related to receipt of chemotherapy; after adjustment, 72% of patients younger than 55 years received chemotherapy, but only 47% of those age 75 years and older did (Table 2). Interview type, age, sex, histology, and degree of respiratory disease at diagnosis also were statistically significant predictors of the receipt of chemotherapy after analysis was adjusted. There were no significant interactions between age and any of the disease or comorbidity measures, which indicated that the inverse relationship between age and receipt of chemotherapy did not depend on these measures. Men were more likely than women to receive chemotherapy. To additionally explore this, we confirmed this association among the subset of participants for whom marital status was available (marital status was not collected in the brief version of the interview) and found that addition of marital status to the models eliminated the sex differences (data not shown). Those who were unable to complete the full baseline interview were less likely to receive chemotherapy.
We classified the first chemotherapy regimen for each patient as cisplatin based (n = 83; 10.4%), carboplatin based (n = 521; 65.3%), non–platinum based (n = 173; 21.6%), or unknown (n = 21; 2.6%). Most (n = 615; 79.2%) first chemotherapy regimens were multiagent regimens, and, of these, platinum-based regimens (n = 576) accounted for 93.7%. Most (n = 162) single-agent regimens were non-platinum agents. Among those treated with chemotherapy, older adults were less likely to receive a platinum-based first regimen; 84% (adjusted percent) of patients younger than 55 years were receiving a platinum-based treatment, and this decreased to 71% among patients age 75 and older (Table 3). Most of this difference was due to a lower rate of cisplatin use with advancing age (Table 1). After adjustment for all other variables, age, interview type, research site, and comorbidity were statistically significant predictors of the receipt of platinum-based chemotherapy (Table 3).
Table 3.
Characteristics of Patients With Advanced Stage NSCLC Receiving Chemotherapy Who Received Platinum-Based Regimens and Adjusted Probability of Receiving Platinum-Based Chemotherapy in the CanCORS Study
| Characteristic | Patients |
Adjusted %* | 95% CI (%) | |
|---|---|---|---|---|
| No. | % | |||
| Age, years† | ||||
| < 55 | 115 | 86 | 84 | 77 to 91 |
| 55-64 | 180 | 86 | 85 | 80 to 90 |
| 65-74 | 206 | 78 | 79 | 75 to 84 |
| ≥ 75 | 118 | 69 | 71 | 64 to 78 |
| Sex† | ||||
| Female | 212 | 77 | 75 | 70 to 81 |
| Male | 407 | 81 | 82 | 79 to 85 |
| Radiation therapy | ||||
| No | 304 | 75 | 76 | 71 to 80 |
| Yes | 315 | 85 | 84 | 80 to 88 |
| Research site† | ||||
| Eight Northern California counties | 125 | 78 | 79 | 73 to 85 |
| State of Alabama | 60 | 69 | 65 | 56 to 75 |
| Los Angeles County | 111 | 77 | 79 | 72 to 85 |
| State of Iowa | 146 | 77 | 77 | 71 to 83 |
| Veterans Health Administration | 73 | 90 | 88 | 79 to 96 |
| Cancer Research Network | 104 | 90 | 91 | 85 to 96 |
| Ethnicity | ||||
| African American | 84 | 87 | 85 | 77 to 92 |
| Hispanic | 31 | 76 | 77 | 65 to 89 |
| White | 429 | 78 | 79 | 75 to 82 |
| Other | 75 | 83 | 82 | 74 to 90 |
| Stage | ||||
| IIIb | 185 | 84 | 81 | 75 to 87 |
| IV | 434 | 78 | 79 | 76 to 83 |
| Histology | ||||
| Squamous | 109 | 81 | 80 | 73 to 87 |
| Adeno | 202 | 77 | 77 | 72 to 82 |
| Other | 308 | 81 | 82 | 78 to 86 |
| Comorbidity† | ||||
| None | 117 | 81 | 78 | 71 to 86 |
| Mild | 257 | 83 | 84 | 79 to 88 |
| Moderate | 127 | 78 | 79 | 73 to 85 |
| Severe | 118 | 74 | 74 | 65 to 82 |
| Respiratory disease | ||||
| None | 356 | 81 | 79 | 75 to 83 |
| Mild | 166 | 78 | 79 | 74 to 85 |
| Moderate | 42 | 78 | 79 | 68 to 90 |
| Severe | 55 | 79 | 84 | 75 to 93 |
| Cardiovascular disease | ||||
| None | 495 | 81 | 80 | 77 to 83 |
| Mild | 79 | 75 | 78 | 70 to 85 |
| Moderate | 31 | 74 | 78 | 67 to 90 |
| Severe | 14 | 78 | 84 | 70 to 97 |
| Interview type† | ||||
| Full | 320 | 85 | 84 | 81 to 88 |
| Brief | 68 | 86 | 88 | 81 to 95 |
| Surrogate living | 68 | 79 | 77 | 68 to 87 |
| Surrogate dead | 163 | 69 | 70 | 64 to 76 |
Abbreviations: NSCLC, non–small-cell lung cancer; CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
Adjusted percentages from a logistic regression model with receiving platinum-based chemotherapy as the dependent variable and all listed variables as independent variables.
Variable is associated with receipt of platinum chemotherapy after adjustment for all other covariates: P < .01.
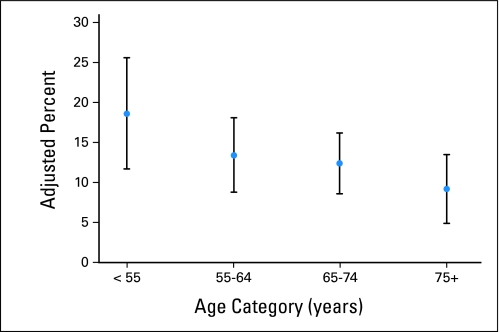
Older adults who received chemotherapy were less likely to have a clinically important acute medical event during the period from diagnosis to the start of chemotherapy than were younger adults, and this rate decreased from 18.6% among those younger than age 55 years to 9.2% among those age 75 years and older (Fig 1). Although the CIs around the adjusted percents (Fig 1) overlap, adjusted rates that incorporated variable person-times were more precise, and the adjusted rate ratio for this difference was statistically significant (adjusted rate ratio, 0.49; 95% CI, 0.26 to 0.91).
Fig 1.
Adjusted percent (and 95% CI) of patients with advanced non–small-cell lung cancer treated with chemotherapy (n = 798) who experienced medical events between diagnosis and treatment initiation by age on the CanCORS (Cancer Care Outcomes Research and Surveillance Consortium) study.
Table 4 displays the frequency of the individual AEs recorded during chemotherapy use. The rates of adverse events among 702 participants who were event free before treatment are listed in Table 5. There were 365 events during 100,769 person-days of chemotherapy among 249 people (35%; 95% CI, 32% to 39%), so some had more than one type of AE during treatment. The highest AE incidence was among 65- to 74-year-olds (adjusted percent, 42.4%; 95% CI, 36.1% to 48.7%) for an adjusted rate ratio of 1.70 (95% CI, 1.19 to 2.43) when compared with those patients age younger than 55 years. Other variables that were related to AE rate after adjustment included interview type, research site, cardiovascular disease at diagnosis, and receipt of radiation (data not shown). There were no age interactions with any covariate, suggesting that AE rate for a given age group did not vary by levels of covariates (eg, cardiovascular and other comorbidity).
Table 4.
Types of Adverse Events Recorded Among Patients Who Received Chemotherapy in the CanCORS Study
| Adverse Event | No. of Events | % With Event* |
|---|---|---|
| Pneumonia | 84 | 10.8 |
| Fever with neutropenia | 45 | 5.8 |
| Neuropathy | 39 | 5.0 |
| Deep vein thrombosis | 35 | 4.5 |
| Pulmonary embolus | 26 | 3.3 |
| Sepsis | 23 | 3.0 |
| Respiratory failure requiring intubation | 16 | 2.1 |
| Pathologic fracture | 14 | 1.8 |
| Stroke | 10 | 1.3 |
| Pneumothorax requiring chest tube | 10 | 1.3 |
| Lower GI bleeding | 9 | 1.2 |
| Superior vena cava syndrome | 9 | 1.2 |
| Cardiac arrest | 8 | 1.0 |
| Myocardial infarction | 8 | 1.0 |
| Syndrome of inappropriate antidiuretic hormone | 8 | 1.0 |
Abbreviation: CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
Adverse events that occurred in fewer than 1% of participants included the following: acute renal failure, seizure, abdominal or pelvic abscess, aspiration, congestive heart failure, hypercalcemia, bowel obstruction or perforation, GI bleeding, spinal cord compression, angina, deep wound infection, indwelling venous catheter clot, bronchopleural fistula, and Cushing's syndrome.
Table 5.
Crude and Adjusted Rates of Adverse Events During Chemotherapy in the CanCORS Study
| Adverse Event by Age, Years | No. of Patients | No. of Person-Days | No. of Events | Patients With Events |
% With Event* |
Rate per 1,000 Person-Days* |
Rate Ratio* |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Adjusted | 95% CI | Adjusted | 95 % CI | Adjusted | 95% CI | ||||
| Any | 702 | 100,769 | 365 | 249 | 35 | ||||||
| < 55 | 120 | 20,066 | 45 | 35 | 29 | 30.6 | 22.1 to 39.2 | 2.676 | 1.619 to 4.424 | 1.0 | |
| 55-64 | 188 | 28,553 | 86 | 55 | 29 | 29.3 | 22.7 to 35.9 | 3.060 | 1.948 to 4.806 | 1.143 | 0.783 to 1.670 |
| 65-74 | 238 | 32,146 | 147 | 100 | 42 | 42.4 | 36.1 to 48.7 | 4.546 | 2.973 to 6.949 | 1.698 | 1.187 to 2.431 |
| ≥ 75 | 156 | 20,004 | 87 | 59 | 38 | 36.0 | 28.7 to 43.3 | 3.589 | 2.337 to 5.511 | 1.341 | 0.898 to 2.004 |
| Neuropathy | 776 | 109,932 | 39 | 39 | 5 | ||||||
| < 55 | 133 | 21,842 | 5 | 5 | 4 | 3.8 | 0.4 to 7.3 | 0.069 | 0.018 to 265 | 1.0 | |
| 55-64 | 209 | 31,785 | 11 | 11 | 5 | 5.2 | 2.3 to 8.1 | 0.110 | 0.035 to 0.347 | 1.594 | 0.519 to 4.899 |
| 65-74 | 264 | 35,249 | 15 | 15 | 6 | 5.7 | 2.9 to 8.6 | 0.132 | 0.042 to 0.413 | 1.921 | 0.648 to 5.696 |
| ≥ 75 | 170 | 21,056 | 8 | 8 | 5 | 4.7 | 1.8 to 7.5 | 0.115 | 0.033 to 0.405 | 1.667 | 0.485 to 5.726 |
| Fever with neutropenia | 776 | 110,019 | 45 | 45 | 6 | ||||||
| < 55 | 133 | 21,842 | 3 | 3 | 2 | 3.0 | 0 to 6.3 | 0.083 | 0.020 to 0.355 | 1.0 | |
| 55-64 | 210 | 31,923 | 10 | 10 | 5 | 4.8 | 1.9 to 7.6 | 0.122 | 0.038 to 0.390 | 1.471 | 0.388 to 5.582 |
| 65-74 | 263 | 35,198 | 19 | 19 | 7 | 6.6 | 3.7 to 9.5 | 0.202 | 0.073 to 0.558 | 2.432 | 0.677 to 8.734 |
| ≥ 75 | 170 | 21,056 | 13 | 13 | 8 | 7.5 | 3.5 to 11.4 | 0.250 | 0.083 to 0.749 | 3.005 | 0.793 to 11.392 |
| Sepsis | 773 | 109,803 | 23 | 23 | 3 | ||||||
| < 55 | 133 | 21,842 | 3 | 3 | 2 | 2.4 | 0 to 5.3 | 0.089 | 0.018 to 0.436 | 1.0 | |
| 55-64 | 209 | 31,893 | 3 | 3 | 1 | 1.5 | 0 to 3.2 | 0.051 | 0.010 to 0.235 | 0.566 | 0.107 to 2.982 |
| 65-74 | 262 | 35,084 | 12 | 12 | 5 | 4.5 | 2.0 to 7.0 | 0.162 | 0.050 to 0.527 | 1.819 | 0.459 to 7.208 |
| ≥ 75 | 169 | 20,984 | 5 | 5 | 3 | 2.7 | 0.3 to 5.0 | 0.106 | 0.027 to 4.15 | 1.181 | 0.242 to 5.770 |
| Neuropathy/febrile neutropenia/sepsis | 772 | 109,665 | 106 | 96 | 12 | ||||||
| < 55 | 133 | 21,842 | 11 | 10 | 8 | 8.5 | 3.2 to 13.7 | 0.284 | 0.111 to 0.726 | 1.0 | |
| 55-64 | 208 | 31,755 | 23 | 22 | 11 | 10.8 | 6.5 to 15.0 | 0.327 | 0.134 to 0.763 | 1.153 | 0.546 to 2.435 |
| 65-74 | 262 | 35,084 | 46 | 41 | 16 | 15.0 | 10.7 to 19.3 | 0.575 | 0.256 to 1.288 | 2.027 | 1.006 to 4.084 |
| ≥ 75 | 169 | 20,984 | 26 | 23 | 14 | 13.2 | 8.2 to 18.2 | 0.523 | 0.233 to 1.174 | 1.843 | 0.852 to 3.989 |
NOTE. Adjusted for interview type, age, sex, PDCR, ethnicity, stage, histology, comorbidity, cardiovascular disease, respiratory disease, radiology, baseline respiratory event, and percent of time on platinum-based chemotherapy. Logistic models were used to calculate adjusted percents, and Poisson models were used to calculate adjusted rates and rate ratios.
Abbreviation: CanCORS, Cancer Care Outcomes Research and Surveillance Consortium.
When AEs that were particularly likely to be chemotherapy-related were analyzed, the age relationship continued to be evident, and the highest rates were observed among those age 65 years and older (Table 5). Rate ratios for the individual chemotherapy-sensitive AEs followed the same age pattern but were individually of too low a frequency to be statistically significant. Other variables positively related to rate of the composite neuropathy/fever with neutropenia/sepsis measure after adjustment included inability to complete the full baseline interview, Los Angeles County and Iowa research sites, and greater percent of chemotherapy time spent on platinum.
DISCUSSION
We have characterized the chemotherapy treatment experience in a population-based study of patients with advanced-stage NSCLC. Overall, 58% of patients on CanCORS who had advanced lung cancer received chemotherapy, and this was the same for both stage IIIB and IV disease. This is a considerably higher frequency than the 41% reported for patients with stage IV disease in the 1996 SEER Patterns of Care (POC) study13 that had a somewhat younger age. This suggests increasing use in more recent periods.
Older patients were much less likely to receive chemotherapy, and they were more likely to receive nonplatinum chemotherapy regimens when they did receive chemotherapy. Older patients who received chemotherapy had significantly lower baseline (ie, pretreatment) medical event rates, which reflected selection of the fittest elders for treatment. Despite this selection, significantly higher AE rates were seen during chemotherapy among the oldest age groups compared with the youngest age group, and these rates reached 42.4% (95% CI, 36.1% to 48.7%) in patients age 65 to 74 years (RR, 1.70; 95% CI, 1.19 to 2.43) before dropping slightly to 36% (95% CI, 28.7% to 43.3%) in patients age 75 years and older (RR, 1.34; 95% CI, 0.90 to 2.00). These findings are consistent with previous studies that showed that the elderly were much more likely to not receive therapy and that, when treated, the elderly were much more likely to receive less aggressive therapy.13,19–21 None of these age relationships could be explained by a severity-adjusted measure of comorbidity.
AE rates were the highest for the 65- to 74-year-old age group, and the chemotherapy treatment rate for this group was similar to that for 55- to 64-year-old patients. In contrast, treatment rates and pretreatment morbidity levels were much lower for the age group of 75 years or older, which possibly reflected selection of the fittest candidates for treatments. Thus, a likely explanation for the higher AE rate for the 65- to 74-year-old patients is that physicians may think these patients are better able to tolerate treatment and may pursue treatment more aggressively than with the age group of patients age 75 years and older.
Interestingly, we observed a lower proportion of women receiving chemotherapy, and this was not explained by age, as women with lung cancer were younger, on average, than men. Instead, a likely explanation is that married people receive chemotherapy more often, and women are more likely to outlive their husbands. Many studies, especially secondary analyses of administrative and registry data sets, do not have measures of social function, such as marital status. Such factors may be important for understanding apparent disparities in receipt of cancer treatments.
Ethnicity was not associated either with receiving chemotherapy or with adverse events. Analyses of SEER-Medicare data from earlier periods found that elderly black patients were less likely to receive chemotherapy. Absence of such a relationship in our data may reflect progress to narrow previous disparities or may reflect different data sources.
Performance status is an independent predictor of chemotherapy,22–24 and this was not well documented in medical records. However, we were able to control for a surrogate for performance status—interview type—in all analyses. In CanCORS, interview type was a strong predictor of receiving chemotherapy, as those who were unable to complete the full baseline interview were less likely to receive chemotherapy. In particular, only 38% of those who were deceased at the time of the baseline interview received chemotherapy compared with 83% of those who completed the full patient baseline interview.
The two health system research sites (ie, Cancer Research Network and the Veterans Health Administration) had the two highest unadjusted rates of use of platinum-based chemotherapy regimens, and the rates remained somewhat higher after adjustment. This was true for all age groups and, thus, did not reflect greater use in younger patients only (data not shown). Interestingly, these research sites did not have higher AE rates; the highest rates were in Los Angeles County and Iowa. Although it is possible that the health system sites were better able to minimize AEs while delivering platinum-based treatments, an alternative explanation is a geographic variability in abstracting procedures or physician AE documentation practices.
Our results confirm findings on the basis of administrative claims data that document a decline in the use of chemotherapy with increasing patient age after adjustment for other factors.13,20,25,26 Numerous previous authors have questioned whether elderly patients are denied potentially beneficial treatment and participation in clinical trials simply because of their age and out of concern that they are too frail to tolerate treatment.11–13,20,25,26 CanCORS provides important new information, including medical record–based ascertainment of AEs in defined populations with new-onset disease and robust control for 27 comorbid conditions and their severities. Our finding that treated elders have lower pretreatment rates of clinically important medical events suggests that practicing physicians require that elders be even more fit than younger adults before recommending chemotherapy. Our finding of a higher rate of AEs on treatment despite this selection suggests that some differential application of selection criteria by age may be justifiable. Because patients may weigh the quality-of-life impact of disease progression more heavily than that of severe toxicity,27 it is possible that patients would not agree. Regardless, the trade-off between disease-related symptoms and treatment toxicity is a prime example of the need to include patient preferences into medical decision making. To do so in a patient-centered fashion, we need to make these trade-offs explicit and we need to gather the information that will allow these decisions to be made jointly by patient and provider in as evidence-based a way as possible. We believe that our study advances the field in this direction but much more remains to be done.
In conclusion, this study has generated important information about the AEs experienced by a representative community population of elderly patients who received chemotherapy for advanced-stage NSCLC. The purpose of this chemotherapy is to improve quality of survival by reducing disease-related symptoms. Because older patients have at least as many disease symptoms as younger patients, a logical consequence of lower treatment rates is more disease symptoms and worse quality of life for the oldest patients. An unanswered question is whether the increased AEs associated with chemotherapy among the oldest patients who received chemotherapy was offset by improvement in their disease symptoms to provide a net positive effect on quality of life. Future quantifications of quality of life and survival outcomes for the CanCORS cohort will be important contributions to a fuller assessment of this possibility.
Acknowledgment
From the Departments of Epidemiology and Biostatistics, College of Public Health, University of Iowa, Iowa City, IA; Division of Internal Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles; Northern California Cancer Center, Fremont, CA; Departments of Biostatistics and Computational Biology and of Medical Oncology, Dana-Farber Cancer Institute; Department of Biostatistics, Harvard School of Public Health; and Harvard Medical School, Harvard University, Boston, MA; Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, AL; Division of Medical Oncology, Duke University Medical Center; and Veterans Affairs Medical Center, Durham, NC.
Footnotes
See accompanying editorial on page 523
Written on behalf of the Cancer Care Outcomes Research and Surveillance Consortium.
Supported by Grant No. U01 CA093344 from the National Cancer Institute (NCI) to the Statistical Coordinating Center; NCI-supported Primary Data Collection and Research Centers Grants No. U01 CA093332 (Dana-Farber Cancer Institute/Cancer Research Network), U01 CA093324 (Harvard Medical School/Northern California Cancer Center), U01 CA093348 (RAND/University of California, Los Angeles), U01 CA093329 (University of Alabama at Birmingham), U01 CA01013 (University of Iowa), and U01 A093326 (University of North Carolina); a grant from the Department of Veterans Affairs to the Durham Veterans' Affairs Medical Center; and Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics Cooperative Agreement No. 5 U18 HSO16094 (E.A.C., J.F.P., and R.B.W.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth A. Chrischilles, Jane F. Pendergast, Katherine L. Kahn, Robert B. Wallace, Daniela C. Moga, David P. Harrington, Jane C. Weeks, Robert H. Fletcher
Administrative support: Robert B. Wallace, Daniela C. Moga, David P. Harrington, Robert H. Fletcher
Provision of study materials or patients: Elizabeth A. Chrischilles, Katherine L. Kahn, Robert B. Wallace, Jane C. Weeks, Dee W. West, Robert H. Fletcher
Collection and assembly of data: Elizabeth A. Chrischilles, Jane F. Pendergast, Katherine L. Kahn, Robert B. Wallace, Daniela C. Moga, David P. Harrington, Jane C. Weeks, Dee W. West, Robert H. Fletcher
Data analysis and interpretation: Elizabeth A. Chrischilles, Jane F. Pendergast, Katherine L. Kahn, Robert B. Wallace, Daniela C. Moga, David P. Harrington, Catarina I. Kiefe, Jane C. Weeks, Robert H. Fletcher
Manuscript writing: Elizabeth A. Chrischilles, Jane F. Pendergast, David P. Harrington, Catarina I. Kiefe, S. Yousuf Zafar, Robert H. Fletcher
Final approval of manuscript: Elizabeth A. Chrischilles, Jane F. Pendergast, Katherine L. Kahn, Robert B. Wallace, Daniela C. Moga, David P. Harrington, Catarina I. Kiefe, Jane C. Weeks, Dee W. West, S. Yousuf Zafar, Robert H. Fletcher
REFERENCES
- 1.Surveillance Epidemiology and End Results. http://www.seer.cancer.gov/faststats/
- 2.Non–Small-Cell Lung Cancer Collaborative Group. Chemotherapy in non–small-cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Treat J, Belani CP, Edelman MJ, et al. A randomized phase III trial of gemcitabine in combination with carboplatin or paclitaxel versus paclitaxel plus carboplatin in advanced (stage IIIB, IV) non–small-cell lung cancer (NSCLC): Update of the alpha oncology trial (A1-99002L) J Clin Oncol. 2005;23:627s. abstr LBA7025. [Google Scholar]
- 5.Gridelli C. The ELVIS trial: A phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non–small-cell lung cancer: Elderly Lung Cancer Vinorelbine Italian Study. Oncologist. 2001;6(suppl 1):4–7. doi: 10.1634/theoncologist.6-suppl_1-4. [DOI] [PubMed] [Google Scholar]
- 6.Gridelli C, Perrone F, Gallo C, et al. Vinorelbine is well tolerated and active in the treatment of elderly patients with advanced non–small-cell lung cancer: A two-stage phase II study. Eur J Cancer. 1997;33:392–397. doi: 10.1016/s0959-8049(97)89011-9. [DOI] [PubMed] [Google Scholar]
- 7.Hensing TA, Peterman AH, Schell MJ, et al. The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, stage IIIB or IV non–small-cell lung carcinoma treated with carboplatin and paclitaxel. Cancer. 2003;98:779–788. doi: 10.1002/cncr.11548. [DOI] [PubMed] [Google Scholar]
- 8.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: An analysis of the Surveillance, Epidemiology, and End Results Database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 10.Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non–small-cell lung cancer in chemotherapy trials. Intern Med J. 2006;36:216–220. doi: 10.1111/j.1445-5994.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Gridelli C, Shepherd FA. Chemotherapy for elderly patients with non-small cell lung cancer: A review of the evidence. Chest. 2005;128:947–957. doi: 10.1378/chest.128.2.947. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K, Ueoka H, Kiura K, et al. An overview of 48 elderly-specific clinical trials of systemic chemotherapy for advanced non–small-cell lung cancer. Lung Cancer. 2004;46:61–76. doi: 10.1016/j.lungcan.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non–small-cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Ayanian JZ, Chrischilles EA, Wallace RB, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcome: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 16.Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manage. 2003;30:8–14. [Google Scholar]
- 17.Graubard B, Korn E. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 18.Rubin D. New York, NY: John Wiley & Sons; 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 19.Earle CC, Tsai JS, Gelber RD, et al. Effectiveness of chemotherapy for advanced lung cancer in the elderly: Instrumental variable and propensity analysis. J Clin Oncol. 2001;19:1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 20.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non–small-cell lung cancer: Evidence from Surveillance, Epidemiology and End Results–Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Deppermann KM. Influence of age and comorbidities on the chemotherapeutic management of lung cancer. Lung Cancer. 2001;33(suppl 1):S115–S120. doi: 10.1016/s0169-5002(01)00311-7. [DOI] [PubMed] [Google Scholar]
- 23.Janssen-Heijnen MLG, Schipper RM, Razenberg PPA, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: A population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 24.Extermann M, Overcash J, Lyman GH, et al. Comorbid condition and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 25.Hillner BE, McDonald MK, Desch CE, et al. A comparison of patterns of care of non–small-cell lung carcinoma patients in a younger and Medigap commercially insured cohort. Cancer. 1998;83:1930–1937. doi: 10.1002/(sici)1097-0142(19981101)83:9<1930::aid-cncr8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–1246. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 27.Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non–small-cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]