Abstract
Purpose
Hot flashes are a common problem for which effective and safe treatments are needed. The current trial was conducted on the basis of preliminary promising data that pregabalin decreased hot flashes.
Patients and Methods
A double-blind, placebo-controlled, randomized trial design was used to compare pregabalin at target doses of 75 mg twice daily and 150 mg twice daily with a placebo. Hot flash frequencies and scores (frequency times mean severity) were recorded daily during a baseline week and for six treatment weeks. The primary end point for this study was the change-from-baseline hot flash score during treatment week 6 between the 150 mg twice daily target pregabalin treatment and placebo. Nonparametric Wilcoxon rank sum tests, two-sample t tests, and χ2 tests were used to compare the primary and secondary hot flash efficacy end points between pregabalin treatments and placebo.
Results
Hot flash score changes available for 163 patients during the sixth treatment week compared with a baseline week decreased by 50%, 65%, and 71% in the placebo, and target 75 mg twice daily and 150 mg twice daily pregabalin arms, respectively (P = .009 and P = .007, comparing respective pregabalin arms to the placebo arm). While some toxicities were significantly more common in the pregabalin arms, being more evident with the higher dose, pregabalin was generally well tolerated by most patients.
Conclusion
Pregabalin decreases hot flashes and is reasonably well tolerated. A target dose of 75 mg twice daily is recommended. Its effects appear to be roughly comparable to what has been reported with gabapentin and with some newer antidepressants.
INTRODUCTION
Hot flashes are a major clinical problem.1 While they can be treated with hormonal treatment options, there are concerns regarding the safety of hormonal therapy and thus efforts are ongoing to find nonhormonal therapies for hot flashes.
Since the beginning of this millennium, two classes of nonhormonal medications have been demonstrated to effectively ameliorate hot flashes. One group consists of relatively new antidepressants affecting serotonin and/or norepinephrine concentrations. Several of these agents have been studied in placebo-controlled trials and were found to provide a 50% or better reduction in hot flash frequency or score.2 The medications studied in phase III randomized, placebo-controlled trials include venlafaxine,3 desvenlafaxine,4 fluoxetine,5 citalopram,6 sertraline,7–9 and paroxetine.10,11
The second nonhormonal agent is gabapentin, a gamma-aminobutyric acid (GABA) analog that has been used in a variety of neurologic and psychiatric disorders. This agent has also been demonstrated to be effective in controlling hot flashes. The exact mechanism is unclear, but it may reduce noradrenergic hyperactivity.12
Gabapentin was first reported to be a promising new therapy for relief of hot flashes in a case series report.12 This was followed by two pilot trials, again reporting promising results.13,14 Subsequently, a placebo-controlled trial in 59 postmenopausal women reported that gabapentin 900 mg/d decreased the hot flash frequency rate by 45% and the overall hot flash score by 54% after 12 weeks.15 This was significantly better than the placebo, which had decreases of 29% and 31%, respectively. Adverse effects of gabapentin included somnolence and dizziness.
Another randomized, placebo-controlled trial of gabapentin versus placebo in 420 women with breast cancer16 reported that a placebo reduced hot flash scores after 8 weeks of treatment by 15%, gabapentin 100 mg three times per day reduced them by 31%, and gabapentin 300 mg three times per day reduced them by 46% (P = .007), further supporting that gabapentin is an effective nonhormonal agent for the management of hot flashes. More recently, a three-arm study compared gabapentin doses of 2,400 mg/d to estrogen to a placebo in a double-blind clinical trial.17 This trial reported that, at 12 weeks of therapy, there were hot flash score reductions of 71%, 72%, and 54%, respectively (P < .02, for both comparisons to the placebo arm). Another recently published randomized double-blind trial18 demonstrated that gabapentin 300 mg three times daily decreased hot flashes by 51% compared with a placebo reduction of 26% (P < .001).
Pregabalin (Lyrica, Pfizer, New York, NY) is a newer generation compound that appears to work better as an analgesic than does gabapentin.19,20 Given that pregabalin's mechanism of action is similar to that of gabapentin, it was hypothesized that it would effectively alleviate hot flashes. One pilot trial involving eight women who were given pregabalin and then prospectively observed supported this claim.19 In this trial, six of the eight women noted some relief of hot flashes; it was judged to cause excellent relief in four women. The mean hot flash score reduction was 65%, with a median hot flash score reduction of 90%. Pregabalin can be administered twice daily in contrast to gabapentin, which is often given three times per day. This added convenience in administration makes pregabalin a more attractive option. Given the above information, it was decided to formally explore the utility of pregabalin as an agent to alleviate hot flashes. Pursuant to this, the current clinical trial was developed.
PATIENTS AND METHODS
Patients considered for this clinical trial were adult women with bothersome hot flashes, defined by occurrence at least 28 times per week and sufficient severity to make the patient desire therapeutic intervention. The hot flashes must have been present for at least 1 month before study entry. Patients must have been able to complete questionnaires by themselves or with assistance.
Patients were not allowed on trial if they were receiving antineoplastic chemotherapy, androgens, progestogen agents, or estrogens, with the exception that vaginal estrogen was allowed if it had been used for at least 1 month and it was planned to be continued during the 7-week study period. Patients were not allowed to have used gabapentin or pregabalin in the past. Current or planned use of other agents for hot flashes was not allowed, except stable doses of vitamin E, soy products, and/or antidepressants, with the understanding that the same treatments would be continued throughout the study period. Patients could not have had a concurrent history of renal insufficiency or have childbearing potential.
The protocol was approved per US federal guidelines, and patients needed to provide appropriate informed written consent. Patients were stratified by age (younger than age 50 years v age 50 years or older); the use of tamoxifen, raloxifene, or an aromatase inhibitor (yes v no); the duration of hot flashes (< 9 months v 9 months or more); and the estimated daily frequency of hot flashes (four to nine v > nine). Subsequently, patients were randomly allocated to one of three study arms by the North Central Cancer Treatment Group (NCCTG) randomization office using a dynamic allocation procedure that balanced marginal distributions of the above stratification factors.20 These arms consisted of a placebo treatment versus target pregabalin doses of 75 mg twice daily versus pregabalin doses of 150 mg twice daily.
After random assignment, but before initiation of any study treatment, patients were asked to complete a validated daily hot flash diary for 1 week.21 On this diary, patients were to record on a daily basis the number of mild, moderate, severe, and very severe hot flashes. After this week, patients initiated their study treatment. Patients on the lower pregabalin arm received 50 mg at bedtime for the first week, then 50 mg twice daily for the second week, and then 75 mg twice daily for four more weeks. Patients on the higher pregabalin arm received 50 mg at bedtime for the first week, then 50 mg twice daily for the second week, then 75 mg twice daily for the third week, and then 150 mg twice daily for three more weeks. Patients receiving placebo tablets, which looked identical to the pregabalin tablets, were assigned to take them on the same schedule as the patients receiving pregabalin. While taking the study medications, patients were asked to continue to fill out the daily hot flash diaries.
In addition to the hot flash diaries, patients were asked to complete a symptom experience questionnaire at the end of each study week. This questionnaire asked patients to score, on a scale of 0 to 10 points, the following symptoms: unwanted weight gain, sleepiness, nausea, dizziness, an undesirable increase in appetite, fatigue, mouth dryness, abnormal sweating, constipation, blurred or double vision, trouble sleeping, coordination difficulties, trouble concentrating, swelling of hands and/or feet, vaginal dryness or dyspareunia, less than desirable libido, and trouble achieving an orgasm. In addition, to identify other potential adverse events, specific symptoms were graded by National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE, v3.0) at baseline and then by nurse phone call at the end of weeks 1, 3, 5, and 7. These specific symptoms included limb edema, constipation, dizziness, cognitive disturbance, blurred vision, and diplopia. Patients were also asked to complete the Profile of Mood States (POMS) tool22 and the Hot Flash Related Daily Interference Scale (HFRDIS)23 at the end of the baseline week and at the completion of the last treatment week.
If patients developed symptoms that were felt to be related to pregabalin and were of unacceptable severity, the pregabalin dose could be reduced to previous dose/frequency levels or stopped at the physician's discretion.
On study completion, including completion of all of the questionnaires, patients were unblinded as to treatment arm. Patients choosing to stop pregabalin at this time were counseled to wean themselves from it over 10 to 13 days, depending on the dose they were taking. For example, patients taking 300 mg twice daily were instructed to decrease their dose to 150 mg twice daily for 3 days, then take 150 mg once daily for 3 days, and then take 150 mg every other day for three doses.
Statistical Methodology
Hot flash scores were computed for each patient by combining both severity (mild, moderate, severe, and very severe) and frequency of hot flashes from daily hot flash diaries, averaging across each study week.21 The primary end point for this study was the change-from-baseline hot flash score during treatment week 6 between the 150 mg twice daily target pregabalin treatment and placebo. Secondary end points included comparison of the 75 mg twice daily target pregabalin treatment change-from-baseline hot flash score versus placebo, comparisons of the change-from-baseline hot flash frequencies between 150 mg twice daily target pregabalin and 75 mg twice daily target pregabalin treatments versus placebo, and comparisons of toxicity profiles, moods (from POMS), and the HFRDIS scores between either treatment arm and placebo.
Depending on the variable of interest, mean (standard deviation), median (range), and frequency (percentage) were used to summarize data in a descriptive manner. Nonparametric Wilcoxon rank sum tests, two-sample t tests and χ2 tests (or Fisher's exact tests) were used to compare the primary and secondary end points between pregabalin treatments and placebo. Because of the exploratory nature of secondary analysis, the P values were not adjusted for multiple comparisons. A P value less than .05% was considered as statistically significant for the primary end point.
RESULTS
Baseline Characteristics
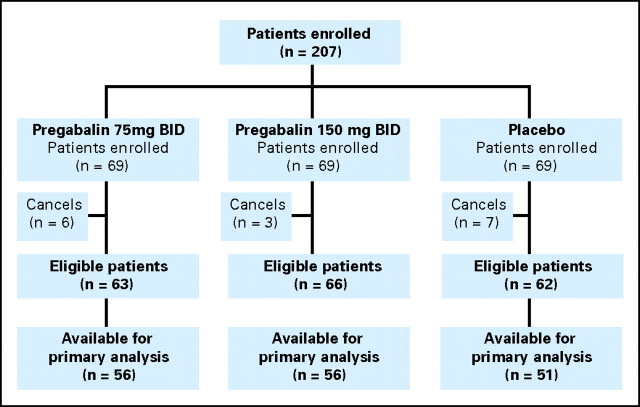
This study opened for accrual on June 20, 2008, and was closed on August 21, 2008, with a final accrual of 207 patients from 19 NCCTG member sites. Baseline patient characteristics, detailed in Table 1, were similar in the three treatment groups. Patient study flow is illustrated in a consort diagram (Fig 1). The reasons that some patients were not evaluable included study dropouts for toxicities and/or other reasons and failures to complete and/or return study diary forms.
Table 1.
On-Study Patient Characteristics
| Characteristic | Placebo (n = 69) |
Pregabalin |
Total (N = 207) |
|||||
|---|---|---|---|---|---|---|---|---|
| 75 mg (n = 69) |
150 mg (n = 69) |
|||||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| 18-49 | 13 | 19 | 15 | 22 | 16 | 23 | 44 | 21 |
| ≥ 50 | 56 | 81 | 54 | 78 | 53 | 77 | 163 | 79 |
| Race | ||||||||
| White | 61 | 88 | 66 | 96 | 66 | 96 | 193 | 93 |
| Black | 6 | 9 | 3 | 4 | 3 | 4 | 12 | 6 |
| American Indian or Alaskan Native | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Not reported | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Breast cancer history | 28 | 41 | 24 | 35 | 30 | 44 | 82 | 40 |
| No. of hot flashes per day | ||||||||
| 4-9 | 40 | 58 | 39 | 56 | 39 | 56 | 118 | 57 |
| 10+ | 29 | 42 | 30 | 44 | 30 | 44 | 89 | 43 |
| Duration of hot flashes (≥ 9 months) | 61 | 88 | 61 | 88 | 61 | 88 | 183 | 88 |
| Concurrent aromatase inhibitor | 17 | 25 | 11 | 16 | 15 | 22 | 43 | 21 |
| Concurrent raloxifene | 2 | 3 | 1 | 1 | 2 | 3 | 5 | 2 |
| Concurrent tamoxifen | 5 | 7 | 11 | 16 | 7 | 10 | 23 | 11 |
NOTE. All χ2 tests between pregabalin and placebo arms were > .05.
Fig 1.
CONSORT diagram. BID, twice per day.
Hot Flash Efficacy
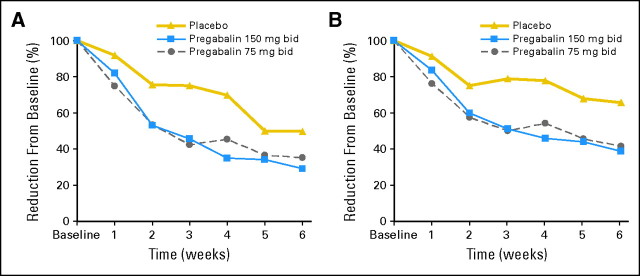
Hot flash score and frequency changes during treatment week 6 were available for 163 (79%) of 207 patients. Hot flash frequency and score decreases from baseline, in terms of actual numerical values and in terms of percent of baseline, are listed in Table 2, with applicable P values comparing each treatment arm against placebo. Data regarding median hot flash score and frequency changes over time are shown in Figure 2. Percent reductions in hot flash scores and frequencies were similarly efficacious in women grouped by whether they had 10 or more hot flashes per day versus four to nine hot flashes per day, a history of breast cancer versus not, and whether they were receiving antiestrogen therapy versus not.
Table 2.
Median Percent and Numerical Changes in Hot Flash Scores and Frequencies During Treatment Week 6, From Baseline
| Measure | Changes From Baseline to Week 6 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo |
Pregabalin |
P |
||||||
| 75 mg Twice a Day |
150 mg Twice a Day |
|||||||
| % | 95% CI | % | 95% CI | % | 95% CI | 75 mg Twice a Day v Placebo | 150 mg Twice a Day v Placebo | |
| Hot flash frequency changes | −36.3 | −51.6 to −20.3 | −58.5 | −74.6 to −48.8 | −61.1 | −72.3 to −48.3 | .007 | .007 |
| Hot flash score changes | −50.1 | −64.5 to −28.3 | −64.9 | −83.6 to −57.8 | −71.0 | −78.2 to −60.6 | .009 | .007 |
| Numerical | Numerical | Numerical | ||||||
|---|---|---|---|---|---|---|---|---|
| Hot flash frequency changes | −2.9 | −3.6 to −1.4 | −4.6 | −5.6 to −3.9 | −4.9 | −6.1 to −4.0 | .003 | .005 |
| Hot flash score changes | −6.1 | −7.9 to −2.9 | −9.7 | −12.1 to −7.3 | −9.6 | −12.9 to −7.6 | .002 | .007 |
Fig 2.
Median changes from baseline for (A) hot flash scores and (B) hot flash frequencies for the three study arms. bid, twice per day.
Information from the HFRDIS revealed that there were improvements at 6 weeks compared with the baseline week for the pregabalin arms compared with the placebo arm. More specifically, the pregabalin 75 mg twice daily target arm patients had improved mood (P = .01) and relations with others (P = .04), while the pregabalin 150 mg twice daily target arm patients recorded improvements in social activities (P = .04), sleep (P = .001), mood (P = .01), enjoyment of life (P = .05), and overall quality of life (P = .02).
With regards to the POMS, there was no significant change in the total POMS scores for either of the individual pregabalin arms versus the placebo arm. For the subscales of the POMS, there was an improvement in the anger-hostility subscale compared with the placebo arm for both the 75 mg twice daily target (P = .006) and the 150 mg twice daily target (P = .002) pregabalin arms.
Toxicity
Toxicity differences between study arms are listed in Table 3, as measured by CTCAE v3.0 and also by the symptom experience diary described in Patients and Methods. For CTCAE-determined toxicity, there was more dizziness with both pregabalin arms and more cognitive difficulties with the higher-dose arm compared with the placebo arm. Toxicity information from the symptom experience diary revealed significantly more toxicity in one or both of the pregabalin arms for undesirable weight gain, sleepiness, dizziness, coordination difficulties, trouble concentrating, and concerns regarding blurred or double vision (Table 3). Changes from baseline for each of these symptoms are illustrated in Figure 3. Of the other symptoms queried on the symptom experience diary (noted in Patients and Methods), there was no difference between the placebo and either of the pregabalin treatments. Table 3 and Figure 3 illustrate that there was more toxicity in the 150 mg twice daily target dose arm than the 75 mg twice daily target dose arm and that some of the symptoms, after peaking, tended to improve with time. Despite the above noted increase in toxicity in the pregabalin arms, the dropout rates for toxicity were statistically similar in all three study arms (6, 3, and 10 patients per arm for the placebo, lower-dose, and higher-dose pregabalin arms, respectively), suggesting that the toxicity was relatively mild.
Table 3.
Worst Toxicity Data for the Individual Study Arms From the NCI CTCAE, Version 3.0 and the SED
| Measure | Placebo (n = 62) | Pregabalin |
P |
||
|---|---|---|---|---|---|
| 75 mg Twice a Day (n = 63) | 150 mg Twice a Day (n = 66) | 75 mg Twice a Day v Placebo | 150 mg Twice a Day v Placebo | ||
| CTCAE | |||||
| Dizziness, % | .04 | .0006 | |||
| 0-none | 77 | 59 | 47 | ||
| 1-mild | 15 | 32 | 36 | ||
| 2-moderate | 8 | 10 | 12 | ||
| 3-severe | 0 | 0 | 5 | ||
| Cognitive troubles, % | .87 | .01 | |||
| 0-none | 82 | 83 | 62 | ||
| 1-mild | 11 | 16 | 24 | ||
| 2-moderate | 6 | 2 | 14 | ||
| SED | |||||
| Concern about weight gain | 1.0 | 1.0 | 2.0 | .20 | .002 |
| Concern about sleepiness | 1.0 | 1.0 | 2.0 | .97 | .049 |
| Concern about dizziness | 0.0 | 1.0 | 2.0 | .054 | .0001 |
| Concern about coordination | 0.0 | 0.0 | 1.0 | .23 | .008 |
| Concern about concentration | 0.0 | 1.0 | 1.0 | .02 | .006 |
| Concern about blurred/double vision | 0.0 | 0.0 | 1.0 | .64 | .005 |
NOTE. SED, based on a scale of 0 to 10 with 10 being worst toxicity, provides numbers representing the worst median changes from baseline during the treatment period.
Abbreviations: NCI, National Cancer Institute; CTCAE, Common Terminology Criteria for Adverse Events; SED, symptom experience diary.
Fig 3.
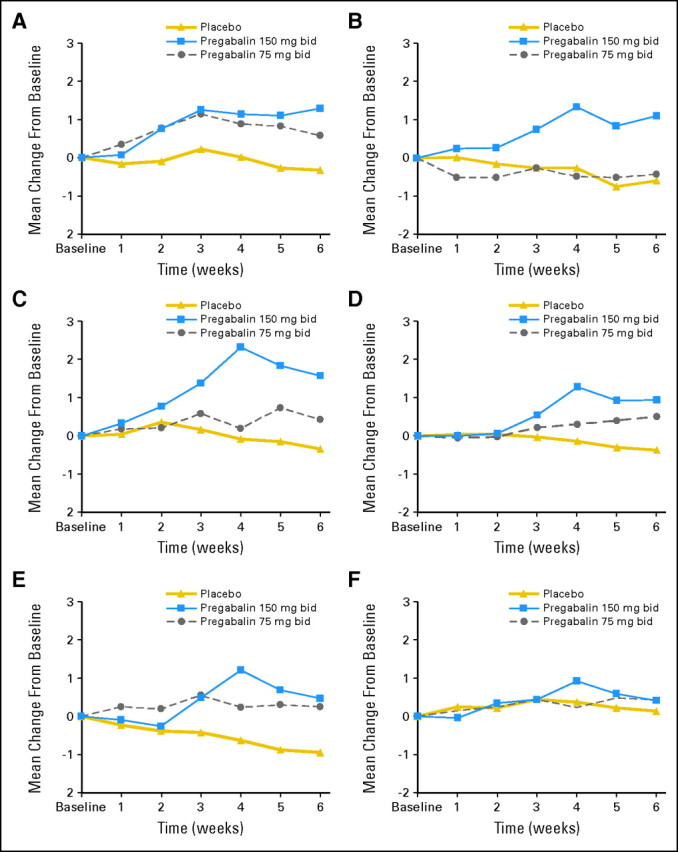
Mean changes over time for each study arm from the symptom experience diary (scale of 0 to 10) for (A) undesirable weight gain, (B) sleepiness, (C) dizziness, (D) coordination difficulties, (E) trouble concentrating, and (F) concerns regarding blurred or double vision. Note that the y-axis is magnified (scale of 0 to 3) to illustrate differences between arms. bid, twice per day.
At study completion, patients were asked by study nurses, while both parties were still blinded, whether they were satisfied with their hot flash control and whether they thought the benefit of the treatment was worth the toxicities that they attributed to it. Table 4 illustrates that the patients in the pregabalin treatment arms were much more satisfied than patients in the placebo arm.
Table 4.
Patient Impressions of the Treatment They Received at the End of the Study, While Still Blinded to the Study Arm
| Impression | Placebo (n = 60) |
Pregabalin |
P |
|||||
|---|---|---|---|---|---|---|---|---|
| 75 mg Twice a Day (n = 61) |
150 mg Twice a Day (n = 64) |
|||||||
| No. | % | No. | % | No. | % | 75 mg Twice a Day v Placebo | 150 mg Twice a Day v Placebo | |
| Satisfied with hot flash control | 19 | 33 | 43 | 69 | 42 | 74 | < .0001 | .0001 |
| Impression of the study agent | ||||||||
| Treatment beneficial with limited or no toxicities | 19 | 34 | 35 | 56 | 36 | 62 | ||
| Symptom improvement not worth the toxicities | 3 | 5 | 18 | 29 | 12 | 21 | < .0001 | < .0001 |
| No treatment benefit | 34 | 61 | 10 | 16 | 10 | 17 | ||
DISCUSSION
The results from this study support the prestudy primary hypothesis that pregabalin at a target dose of 150 mg twice daily would decrease hot flashes significantly more than would a placebo. The findings also support that the lower 75 mg twice daily target dose of pregabalin decreases hot flashes to a degree similar to that of the higher 150 mg twice daily target dose. Cross-study comparisons, crude as they may be, suggest that the hot flash reduction observed with pregabalin is comparable to that seen with gabapentin15–18 and with newer antidepressant agents such as venlafaxine, desvenlafaxine, paroxetine, and citalopram.2–11 Each of these agents appears to decrease hot flashes by approximately 20% to 30% more than does a placebo.2
The higher pregabalin arm appeared to have significantly more toxicity than did the placebo arm. Toxicity was less apparent in the lower-dose pregabalin treatment arm. This, however, did not lead to a significantly higher rate of patient dropout because of toxicity in the pregabalin arms compared with the placebo arm.
Data from this trial lend support for the methodology used to conduct it. Keeping in mind that both of the pregabalin arms received identical doses of pregabalin for the first three treatment weeks as the doses were being titrated upward, Figure 2 illustrates that the data points for each of the two pregabalin treatment arms are remarkably similar for these three treatment weeks. This supports that the numbers of patients involved in each study arm of this trial and the hot flash daily diary and questionnaire that were used to measure hot flashes represent valid means of conducting clinical trials that examine treatments to alleviate hot flashes. While some studies24–27 have suggested that hot flash trials should include physiologic measures of sweating as a means of measuring hot flashes, hot flash daily diaries are an appropriate means of measuring hot flashes in patients participating in clinical trials.28–30
The current trial required 28 hot flashes per week for study entry, as opposed to previous Mayo Clinic/NCCTG hot flash trials, which required 14 hot flashes per week.3,5,31–36 This requirement was made because of concerns previously raised by some parties that 14 hot flashes per week might be too few to study and because only approximately 10% of patients in previous Mayo Clinic/NCCTG hot flash trials had fewer than 28 hot flashes per week so that accrual would not be substantially affected. Of note, this concern about requiring a minimum of 14 hot flashes per week is not substantiated by work that has addressed this issue.21 Unbeknownst to the authors of this study when this study was developed, new data had demonstrated that placebo effects are more pronounced in studies where the number of baseline hot flashes is higher,37 which fits with the higher placebo effect that was seen in this trial (approximately 50%) compared with similar previous trials.3,5,31–36
Although this trial did not include men with hot flashes, similarities between hot flash treatment efficacies in women and men for other agents,32,38 especially data that demonstrate that gabapentin does decrease hot flashes in men to a degree similar to that in women,38 suggest that pregabalin might also be helpful for treating male hot flashes related to androgen deprivation therapy.
Thus, in total, pregabalin at a target dose of 75 to 150 mg twice daily appears to be a clinically useful means of treating hot flashes in women. The lower dose appears to be as beneficial as the higher dose, with less toxicity.
Appendix
The following institutions and members participated in the study: Siouxland Hematology-Oncology Associates, Sioux City, IA (Donald Wender, MD); Sioux Community Cancer Consortium, Sioux Falls, SD (Loren K. Tschetter, MD); Toledo Community Hospital Oncology Program, Toledo, OH (Paul L. Schaefer, MD); Medcenter One Health Systems, Bismarck, ND (Edward J. Wos, DO); Meritcare Hospital Community Clinical Oncology Program (CCOP), Fargo, ND (Preston Steen, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Robin Zon, MD); Missouri Valley Cancer Consortium, Omaha, NE (Gamini S. Soori, MD); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN (Patrick J. Flynn, MD); Virginia Mason Medical Center CCOP, Seattle WA (Andrew D. Jacobs, MD); St. Vincent Regional Cancer Center CCOP, Green Bay, WI (Anthony J. Jaslowski, MD); Geisinger Clinic & Medical Center CCOP, Danville, PA (Albert M. Bernath, Jr., MD); Columbus CCOP, Columbus, OH (J. Philip Kuebler, MD, PhD); Mayo Clinic Scottsdale, AZ (Tom R. Fitch, MD).
Footnotes
See accompanying article on page 634
Supported in part by Public Health Service Grants No. CA-25224, CA-37404, CA-63848, CA-35195, CA-37417, CA-35448, CA-35267, CA-63849, CA-35113, CA-35103, CA-35415, and CA-35431.
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00702949.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Charles L. Loprinzi
Financial support: Charles L. Loprinzi
Administrative support: David L. Graham, Nancy K. Erwin, Shaker R. Dakhil, Donald J. Jurgens
Provision of study materials or patients: Charles L. Loprinzi, Ernie P. Baclueva, Kathleen A. Flynn, Kendrith M. Rowland Jr, David L. Graham, Nancy K. Erwin, Shaker R. Dakhil, Donald J. Jurgens
Collection and assembly of data: Charles L. Loprinzi, Rui Qin, Kelli N. Burger
Data analysis and interpretation: Charles L. Loprinzi, Rui Qin, Kelli N. Burger
Manuscript writing: Charles L. Loprinzi, Rui Qin, Kelli N. Burger
Final approval of manuscript: Charles L. Loprinzi, Rui Qin, Ernie P. Baclueva, Kathleen A. Flynn, Kendrith M. Rowland Jr, David L. Graham, Nancy K. Erwin, Shaker R. Dakhil, Donald J. Jurgens, Kelli N. Burger
REFERENCES
- 1.McKinlay SM, Jefferys M. The menopausal syndrome. Br J Prev Soc Med. 1974;28:108–115. doi: 10.1136/jech.28.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 4.Speroff L, Gass M, Constantine G, et al. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: A randomized controlled trial. Obstet Gynecol. 2008;111:77–87. doi: 10.1097/01.AOG.0000297371.89129.b3. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 6.Barton DL, LaVasseur B, Sloan JA, et al. A phase III trial evaluating three doses of citalopram for hot flashes: NCCTG trial N05C9. J Clin Oncol. 2008;26(suppl):511s. doi: 10.1200/JCO.2009.26.6379. abstr 9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon PR, Kerwin JP, Boesen KG, et al. Sertraline to treat hot flashes: A randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13:568–575. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 8.Grady D, Cohen B, Tice J, et al. Ineffectiveness of sertraline for treatment of menopausal hot flushes: A randomized controlled trial. Obstet Gynecol. 2007;109:823–830. doi: 10.1097/01.AOG.0000258278.73505.fa. [DOI] [PubMed] [Google Scholar]
- 9.Kimmick GG, Lovato J, McQuellon R, et al. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12:114–122. doi: 10.1111/j.1075-122X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 10.Stearns V, Beebe KL, Iyengar M, et al. Paroxetine controlled release in the treatment of menopausal hot flashes: A randomized controlled trial. JAMA. 2003;289:2827–2834. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 11.Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: Results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919–6930. doi: 10.1200/JCO.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 12.Guttuso TJ., Jr Gabapentin's effects on hot flashes and hypothermia. Neurology. 2000;54:2161–2163. doi: 10.1212/wnl.54.11.2161. [DOI] [PubMed] [Google Scholar]
- 13.Loprinzi C, Barton DL, Sloan JA, et al. Pilot evaluation of gabapentin for treating hot flashes. Mayo Clin Proc. 2002;77:1159–1163. doi: 10.4065/77.11.1159. [DOI] [PubMed] [Google Scholar]
- 14.Thummala AR, Griggs J, Rosenblatt J, et al. Pilot study using gabapentin for tamoxifen-induced hot flashes in women with breast cancer. Proc Am Soc Clin Oncol. 2002;21(suppl):362a. doi: 10.1023/B:BREA.0000010676.54597.22. abstr 1445. [DOI] [PubMed] [Google Scholar]
- 15.Guttuso T, Jr, Kurlan R, McDermott MP, et al. Gabapentin's effects on hot flashes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2003;101:337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 16.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy SY, Warner H, Guttuso T, Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: A randomized controlled trial. Obstet Gynecol. 2006;108:41–48. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- 18.Butt DA, Lock M, Lewis JE, et al. Gabapentin for the treatment of menopausal hot flashes: A randomized controlled trial. Menopause. 2008;15:310–318. doi: 10.1097/gme.0b013e3180dca175. [DOI] [PubMed] [Google Scholar]
- 19.Presant CA, Kelly C. Palliation of vasomotor instability (hot flashes) using pregabalin. Community Oncol. 2007;4:83–84. [Google Scholar]
- 20.Therneau TM. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials. 1993;14:98–108. doi: 10.1016/0197-2456(93)90013-4. [DOI] [PubMed] [Google Scholar]
- 21.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 22.Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol Assess. 1995;7:80–83. [Google Scholar]
- 23.Carpenter JS. The hot flash related daily interference scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26:1311–1317. [PubMed] [Google Scholar]
- 25.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–1326. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 26.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 27.Otte JL, Flockhart D, Hayes D, et al. Comparison of subjective and objective hot flash measures over time among breast cancer survivors initiating aromatase inhibitor therapy. Menopause. 2009;16:653–659. doi: 10.1097/gme.0b013e3181a5d0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loprinzi CL, Barton DL, Sloan JA. Whose opinion counts? J Clin Oncol. 2006;24:5183–5185. doi: 10.1200/JCO.2005.04.4388. [DOI] [PubMed] [Google Scholar]
- 29.Loprinzi CL, Barton DL. On hot flash mechanism, measurement, and treatment. Menopause. 2009;16:621–623. doi: 10.1097/gme.0b013e3181a85107. [DOI] [PubMed] [Google Scholar]
- 30.Loprinzi CL, Barton DL. Gadgets for measuring hot flashes: Have they become the gold standard? J Support Oncol. 2009;27:136–137. [PubMed] [Google Scholar]
- 31.Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155–158. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 33.Loprinzi CL, Levitt R, Sloan JA, et al. Medroxyprogesterone acetate (MPA) versus venlafaxine for hot flashes: A North Central Cancer Treatment Group Trial. J Clin Oncol. 2005;23(suppl):732s. abstr 8014. [Google Scholar]
- 34.Barton DL, Loprinzi CL, Quella SK, et al. Prospective evaluation of vitamin E for hot flashes in breast cancer survivors. J Clin Oncol. 1998;16:495–500. doi: 10.1200/JCO.1998.16.2.495. [DOI] [PubMed] [Google Scholar]
- 35.Pockaj BA, Gallagher JG, Loprinzi CL, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol. 2006;24:2836–2841. doi: 10.1200/JCO.2005.05.4296. [DOI] [PubMed] [Google Scholar]
- 36.Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409–1414. doi: 10.1200/JCO.2005.04.7324. [DOI] [PubMed] [Google Scholar]
- 37.Jones JM, Loprinzi CL, Qin R, et al. Hot flash placebo responses: Related to baseline hot flash frequency? J Clin Oncol. 2009;27(suppl):515s. abstr 9628. [Google Scholar]
- 38.Loprinzi CL, Dueck AC, Khoyratty BS, et al. A phase III randomized, double-blind, placebo-controlled trial of gabapentin in the management of hot flashes in men (N00CB) Ann Oncol. 2009;20:542–549. doi: 10.1093/annonc/mdn644. [DOI] [PMC free article] [PubMed] [Google Scholar]