Abstract
Purpose
Despite improvements in treatment, approximately 20% of patients with acute lymphoblastic leukemia (ALL) experience relapse and do poorly. The Therapeutic Advances in Childhood Leukemia (TACL) Consortium was assembled to assess novel drugs for children with resistant leukemia. We hypothesize that novel agents and combinations that fail to improve baseline complete remission rates in comparable populations are unlikely to contribute to better outcomes and should be abandoned. We sought to define response rates and disease-free survival (DFS) rates in patients treated at TACL institutions, which could serve as a comparator for future studies.
Patients and Methods
We performed a retrospective cohort review of patients with relapsed and refractory ALL previously treated at TACL institutions between the years of 1995 and 2004. Data regarding initial and relapsed disease characteristics, disease response, and survival were collected and compared with those of published reports.
Results
Complete remission (CR) rates (mean ± SE) were 83% ± 4% for early first marrow relapse, 93% ± 3% for late first marrow relapse, 44% ± 5% for second marrow relapse, and 27% ± 6% for third marrow relapse. Five-year DFS rates in CR2 and CR3 were 27% ± 4% and 15% ± 7% respectively.
Conclusion
We generally confirm a 40% CR rate for second and subsequent relapse, but our remission rate for early first relapse seems better than that reported in the literature (83% v approximately 70%). Our data may allow useful modeling of an expected remission rate for any population of patients who experience relapse.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. The overall survival rate is approximately 80%.1 Successful treatment can be attributed to a number of important strategies, including the use of combination chemotherapy, prophylactic CNS therapy, and risk-based treatment allocation.2 Current stratification algorithms integrate a number of clinical and laboratory features, including age, WBC count at diagnosis, genetic features, and response to induction therapy. The ultimate goal of risk stratification is to maximize response to therapy, but minimize toxicity and adverse effects.
Current relapse rates are approximately 20%, making recurrent ALL a relatively common disease for pediatric oncologists. Reinduction remission rates for patients with first relapse range from 71% to 93%, depending on the timing and site of relapse.3–9 Survival of patients experiencing relapse can be predicted by site of relapse and length of first complete remission (CR1).6,7,10 In general, bone marrow and early relapse (< 36 months from initial diagnosis) have worse prognoses than isolated extramedullary or late relapse (≥ 36 months from initial diagnosis). Although clinical remission can be achieved in most relapses, long-term survival rates range from 40% to 50%.11–14 Reinduction of patients with relapsed ALL commonly includes conventional agents largely identical to those used at initial diagnosis. Hematopoietic stem-cell transplantation (HSCT) is often used as consolidation therapy.
HSCT has been widely used for patients with relapsed ALL.15 However, the benefit of HSCT for patients with late bone marrow relapse or multiple relapses has not been firmly established. HSCT is associated with a 10% to 20% risk of peri-transplantation mortality, depending on donor type, and still has a substantial relapse rate.16 Given the overall poor results with conventional and high-dose therapies for patients with relapse or refractory ALL, new agents and new strategies are urgently needed. The Therapeutic Advances in Childhood Leukemia Consortium (TACL) was established to conduct early-phase studies of new drugs in children with recurrent leukemia.17 A major goal of TACL clinical trials is to provide data to inform larger trials in the Children's Oncology Group. This retrospective chart review establishes baseline remission rates and outcomes for patients with multiply relapsed ALL treated at eight TACL institutions to serve as a benchmark for future TACL trials. With these baseline values, TACL trials will look to assess chemotherapeutic agents and regimens that improve on the current response rates and outcomes of patients with relapsed and refractory ALL and eventually improve initial therapy and decrease the incidence of relapse.
PATIENTS AND METHODS
Study Cohort
The TACL T2005-002 patient cohort comprised all children between the ages of 0 and 21 years originally diagnosed with ALL who were refractory to primary therapy or experienced a relapse at any site and received treatment between 1995 and 2004 at TACL institutions. Participating TACL institutions used a variety of resources to identify all patients who satisfied these criteria, including tumor registries, hospital billing records, and internally maintained patient databases. Patient demographic data and clinical data related to the initial diagnosis and subsequent relapses or treatment failures were abstracted onto case report forms and entered into a central database at the TACL coordinating center at Childrens Hospital Los Angeles. This study was approved by the institutional review board of each participating institution. The analysis cohort for this report comprises ALL patients enrolled onto TACL T2005-002 with relapsed or refractory marrow disease with or without extramedullary involvement.
Study End Points
Patients were considered to have achieved a complete response (CR) if reinduction treatment resulted in an M1 marrow (< 5% blasts by bone marrow aspirate) with no evidence of circulating blasts or extramedullary disease and with recovery of peripheral counts (absolute neutrophil count ≥ 750/μL and platelet count ≥ 75,000/μL). Patients who met this criterion without platelet recovery were designated CRp, but were included as CR for the purpose of statistical analysis. Qualifying marrow and peripheral counts were to have been performed within 1 week. Reinduction treatment not resulting in CR is termed reinduction failure, and surviving patients are termed refractory. Relapse is defined as a pathologically confirmed M3 marrow (≥ 25% leukemic blasts) or the presence of leukemia in any other site (eg, CNS, peripheral blood) in a patient who previously had achieved CR. Relapses and reinduction failures are collectively termed treatment failures in this article. Treatment failures, development of a second malignant neoplasm, or death from any cause are considered events for the purposes of disease-free survival (DFS) analysis. The “time 0” reference for DFS analysis among patients achieving CR is the date of the confirmation of CR or CRp.
Statistical Methods
The statistical analysis of the dependence of reinduction CR rate on patient characteristics, disease characteristics, and treatment history at the time of reinduction therapy was based on univariate and multivariate logistic regression analysis.18 Analysis of DFS and its dependence on patient and disease characteristics was based on the log-rank test, product-limit (Kaplan-Meier) estimator, and univariate and multivariate Cox regression analysis.19 The analysis of CR rate and DFS used reinduction attempts rather than patients as the primary analytic unit, so that each patient contributed data on one or more reinduction attempts. In the corresponding logistic and Cox regression analyses, accounting for this interpatient correlation gave equivalent results to analyses that ignored this correlation. Results from the latter analytic method are reported. The administration of HSCT as treatment after reinduction was included as a time-dependent covariate in the Cox regression analysis. All P values are two-sided. Estimates of relative risk and relative failure rate are presented with 95% CIs. Statistical computation was performed using STATA software version 9.2 (STATA, College Station, TX) and SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Analysis Cohort
Three hundred thirteen patients with ALL were enrolled onto this study. After further review, 29 were excluded because of atypical diagnoses (12 mixed-lineage B cell/myeloid, two B cell, 15 other or unknown). Of the remaining 284 patients, 227 experienced a combined 485 relapses or (re)induction failures that involved the bone marrow, for which subsequent treatment outcome data were reported for 429 events. Patients experiencing isolated extramedullary relapses only were excluded from our study. Of the 227 patients who experienced marrow relapse, either alone or in combination with another site, a total of 225 patients had at least one treatment for medullary relapse or (re)induction failure. These patients are the subjects of this article. Table 1 lists the number of treatment failures that patients experienced and their post-treatment status. Table 2 lists other clinical characteristics at the patient's initial diagnosis of leukemia.
Table 1.
Patients Who Received Treatment for Medullary or Nonmedullary Relapse/Failure and Their Post-Treatment Status
| Treatment Attempt | No. of Patients Treated | Patients Who Received Treatment for Medullary Relapse/Failure |
Patients Who Received Treatment for Isolated Nonmedullary Relapse/Failure |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Post-Treatment Status* |
No. | Post-Treatment Status* |
|||||||||||
| Medullary† | Nonmedullary‡ | Dead§ | Lost | Alive | Unknown | Medullary† | Nonmedullary‡ | Dead§ | Lost | Alive | ||||
| Second | 284 | 195 | 87 | 12 | 42 | 3 | 50 | 1‖ | 89 | 25 | 22 | 8 | — | 34 |
| Third | 146 | 112 | 49 | 6 | 41 | 1 | 15 | — | 34 | 7 | 9 | 7 | — | 11 |
| Fourth | 71 | 56 | 31 | 2 | 22 | 1 | — | — | 15 | 3 | 2 | 8 | — | 2 |
| Fifth | 39¶ | 34 | 18 | — | 15 | — | 1 | — | 4 | 1 | 2 | 1 | — | — |
| Sixth | 21 | 19 | 7 | 2 | 8 | — | 2 | — | 2 | — | 2 | — | — | — |
| Seventh | 11 | 7 | 4 | — | 3 | — | — | — | 4 | — | 3 | 1 | — | — |
| Eighth | 7 | 4 | 2 | — | 2 | — | — | — | 3 | — | 2 | — | — | 1 |
| Ninth | 4 | 2 | — | — | 1 | — | 1 | — | 2 | — | 1 | 1 | — | — |
| Tenth | 1 | — | — | — | — | — | — | — | 1 | — | — | 1 | — | — |
| Total events | 429 | 154 | ||||||||||||
Patients were included in the “Medullary” and “Nonmedullary” columns only if they had a subsequent relapse/failure for which treatment information was available. If subsequent treatment information was unavailable, that patient was included in “Dead,” “Lost,” or “Alive” on the basis of the status as of the last follow-up date.
Patients who experienced a medullary relapse/reinduction failure and for whom subsequent treatment information was available.
Patients who experienced an isolated, nonmedullary relapse/reinduction failure and for whom subsequent treatment information was available.
Patients who died during/after treatment or who died after a subsequent relapse for which treatment was not given or information was not available.
One patient had a second relapse and was treated, but type of relapse and treatment information were unknown.
One patient (the same patient as ‖) received treatment for the fourth relapse, but whether the fourth relapse was medullary or nonmedullary was unknown. Also, relapse/treatment information for the second and third relapses was missing. This patient had nonmedullary refractory disease after the fifth treatment attempt and died.
Table 2.
Characteristics at Diagnosis of Patients With ALL Who Received at Least One Treatment for Medullary Relapse (n = 225)
| Characteristic | No. of Patients (n = 225) | % |
|---|---|---|
| Age, years | ||
| < 1 (infants) | 8 | 3.6 |
| 1-9 | 151 | 67.1 |
| ≥ 10 | 66 | 29.3 |
| WBC counts/μL | ||
| < 50,000 | 148 | 72.9 |
| ≥ 50,000 | 55 | 27.1 |
| Unknown | 22 | |
| NCI risk criteria at diagnosis | ||
| Non-infants, standard risk | 102 | 45.3 |
| Non-infants, high risk | 99 | 44.0 |
| Non-infants, unknown | 16 | 7.1 |
| Infants | 8 | 3.6 |
| Sex | ||
| Female | 93 | 41.3 |
| Male | 132 | 58.7 |
| Testis positive | ||
| Yes | 4 | 1.9 |
| No | 212 | 98.1 |
| Unknown | 9 | |
| Mediastinal mass | ||
| Yes | 7 | 3.2 |
| No | 209 | 96.8 |
| Unknown | 9 | |
| CNS disease | ||
| Yes | 18 | 8.3 |
| No | 198 | 91.7 |
| Unknown | 9 | |
| Immunophenotype | ||
| Pre B cell | 195 | 86.7 |
| T cell | 30 | 13.3 |
| Karyotype | ||
| Normal | 74 | 43.0 |
| Hypodiploidy | 8 | 4.7 |
| Hyperdiploidy | 16 | 9.3 |
| t(12;21) | 3 | 1.7 |
| t(1;19) | 8 | 4.7 |
| t(4;11) | 2 | 1.2 |
| t(9;22) | 4 | 2.3 |
| Other | 57 | 33.1 |
| Unknown | 53 |
Abbreviations: ALL, acute lymphoblastic leukemia; NCI, National Cancer Institute.
Response to Reinduction Treatment
Table 3, Table 4, Appendix Figure A1 (online only), and Appendix Figure A2 (online only) summarize the relationship between reinduction CR rate, the number of prior treatment attempts, and the outcome of the immediately preceding treatment attempt. The overall CR rate (mean ± SE) after the first reinduction (second therapeutic attempt) was 85% ± 3%, but was less than 50% after the third and subsequent therapeutic attempts (Table 3). The subsequent CR rate was lower when CR was not achieved or was of short duration after the prior treatment attempt (Table 3). Both of these associations were statistically significant at P < .001 (test for trend) in both the univariate analysis and in multivariate analysis that adjusted for other factors (Table 4). The effect of prior CR duration was smaller for the first reinduction than for subsequent ones, but this interaction was not statistically significant (Table 3). Other factors such as National Cancer Institute risk criteria at diagnosis, nonmedullary site of disease, or immunophenotype were not strongly predictive of CR rate, nor were they statistically significant in the multivariate model (Table 4).
Table 3.
CR Status After Treatment of Medullary Relapse or Refractory ALL, Overall and According to Remission Duration and Treatment Attempt
| Prior Remission Duration | Second Treatment Attempt |
Third Treatment Attempt |
Fourth Treatment Attempt |
Fifth Through Ninth Treatment Attempt |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Total | % | No. | Total | % | No. | Total | % | No. | Total | % | |
| Prior CR achieved? | ||||||||||||
| No | 7 | 7 | 100 | 3 | 15 | 20 | 8 | 33 | 24 | 3 | 50 | 6 |
| Yes | ||||||||||||
| < 18 months duration | 40 | 51 | 78 | 25 | 63 | 40 | 5 | 21 | 24 | 4 | 12 | 33 |
| 18 to 36 months duration | 51 | 59 | 86 | 11 | 20 | 55 | 1 | 1 | 100 | 0 | 2 | 0 |
| ≥ 36 months duration | 55 | 59 | 93 | 7 | 8 | 88 | 0 | 0 | — | 0 | 0 | — |
| Duration of prior remission unknown | 10 | 15 | 67 | 2 | 2 | 100 | 1 | 1 | 100 | 1 | 1 | 100 |
| All patients combined | 163 | 191* | 85 | 48 | 108* | 44 | 15 | 56 | 27 | 8 | 65* | 12 |
Abbreviations: CR, complete remission; ALL, acute lymphoblastic leukemia.
Response was unknown for four of the 195 second treatment attempts, four of the 112 third treatment attempts, and one of the 66 fifth through ninth treatment attempts. These treatment attempts are excluded from the table.
Table 4.
Univariate and Multivariate Logistic Regression Analysis of Risk of Reinduction Failure
| Variable | No. | Univariate Analyses |
Multivariate Analyses |
||
|---|---|---|---|---|---|
| OR* | 95% CI* | OR† | 95% CI† | ||
| Treatment attempt | |||||
| Second | 191 | 1.0 | — | 1.0 | — |
| Third | 108 | 7.3 | 4.2 to 12.6 | 4.5 | 2.4 to 8.4 |
| Fourth | 56 | 15.9 | 7.8 to 32.5 | 5.8 | 2.4 to 14.1 |
| Fifth through ninth | 65 | 41.5 | 17.9 to 96.2 | 14.3 | 4.9 to 41.5 |
| P | < .001 | < .001 | |||
| P trend‡ | < .001 | < .001 | |||
| Duration of previous remission | |||||
| CR not achieved | 105 | 4.1 | 2.3 to 7.2 | 2.3 | 1.02 to 5.0 |
| CR achieved, < 18 months duration | 147 | 1.0 | — | 1.0 | — |
| CR achieved, 18 to 36 months duration | 82 | 0.31 | 0.17 to 0.56 | 0.52 | 0.27 to 1.03 |
| CR achieved, ≥ 36 months duration | 67 | 0.082 | 0.031 to 0.21 | 0.20 | 0.07, to 0.55 |
| Missing | 19 | ||||
| P | < .001 | < .001 | |||
| P trend‡ | < .001 | < .001 | |||
| NCI risk criteria at diagnosis | |||||
| Non-infants, standard risk | 176 | 1.0 | — | 1.0 | — |
| Non-infants, high risk | 180 | 1.5 | 0.98 to 2.3 | 1.4 | 0.82 to 2.5 |
| Non-infants, unknown | 53 | 2.0 | 1.1 to 3.7 | 0.74 | 0.32 to 1.7 |
| Infants | 11 | 0.93 | 0.26 to 3.3 | 2.1 | 0.47 to 9.8 |
| P | .011 | .31 | |||
| Extramedullary involvement in relapse | |||||
| No | 331 | 1.0 | — | 1.0 | — |
| Yes | 82 | 0.53 | 0.32 to 0.88 | 0.68 | 0.36 to 1.3 |
| Missing | 7 | ||||
| P | .011 | .23 | |||
| Immunophenotype | |||||
| Pre B cell | 348 | 1.0 | — | 1.0 | — |
| T cell | 72 | 2.0 | 1.2 to 3.3 | 1.2 | 0.60 to 2.4 |
| P | .012 | .61 | |||
Abbreviations: OR, odds ratio of achieving complete remission v < complete remission; CR, complete remission; NCI, National Cancer Institute.
ORs and 95% CIs from univariate logistic models.
ORs and 95% CIs from multivariate logistic models after adjusting for the other variables in the Table.
Test for trend.
HSCT
Appendix Table A1 (online only) presents data on HSCT, both overall and according to reinduction outcome. As shown in Table A1 (online only), HSCT was generally used after achievement of CR, and the median time from remission to HSCT was 2.9 months in second CR. Ninety-three percent of patients underwent HSCT within 6 months of achieving remission. Although most patients underwent HSCT once, seven patients underwent it twice.
Postrecurrence DFS for Patients Achieving CR
Table 5, Figure 1A, and Figure 1B summarize the relationship between postrecurrence DFS and other factors among patients achieving CR. DFS among patients who achieved CR decreased with an increasing number of prior treatment attempts (Fig 1A). Two-year DFS for patients achieving CR after second and third therapeutic attempts was 40% ± 4% and 31% ± 7%, respectively. Five-year DFS for these patients was 27% ± 4% and 15% ± 7%, respectively. DFS increased with increasing duration of the prior remission (Fig 1B). Both effects were significant at P < .001 in univariate analysis (test for trend) and retained significance at P = .024 and P < .001 in the Cox multivariate regression analysis (Table 5). Other factors associated with DFS in multivariate analysis were National Cancer Institute risk criteria at initial diagnosis (P = .004) and immunophenotype (P = .007). The multivariate analysis also demonstrated a survival benefit for children who receive HSCT regardless of the number of relapses experienced, with a hazard ratio of 0.58 (P = .003). Additional extramedullary sites of disease were not significantly associated with DFS in univariate (P = .10) or multivariate analysis (P = .36).
Table 5.
Cox Proportional Hazards Model of Disease-Free Survival From Start of Remission for Patients Who Achieved CR
| Variable | No. | Univariate Analyses |
Multivariate Analyses |
||
|---|---|---|---|---|---|
| HR* | 95% CI* | HR† | 95% CI† | ||
| Treatment attempt | |||||
| Second | 162 | 1.0 | — | 1.0 | — |
| Third | 47 | 1.5 | 0.99 to 2.1 | 1.2 | 0.75 to 1.8 |
| Fourth | 15 | 3.4 | 2.0 to 5.9 | 3.4 | 1.7 to 6.7 |
| Fifth through ninth | 8 | 1.7 | 0.73 to 3.8 | 1.4 | 0.53 to 3.8 |
| P | < .001 | .006 | |||
| P trend‡ | < .001 | .024 | |||
| Duration of previous remission | |||||
| CR not achieved | 21 | 0.87 | 0.51 to 1.5 | 0.50 | 0.23 to 1.1 |
| CR achieved, < 18 months duration | 74 | 1.0 | — | 1.0 | — |
| CR achieved, 18 to 36 months duration | 63 | 0.50 | 0.34 to 0.75 | 0.45 | 0.29 to 0.69 |
| CR achieved, ≥ 36 months duration | 62 | 0.34 | 0.22 to 0.51 | 0.32 | 0.20 to 0.50 |
| Missing | 12 | ||||
| P | < .001 | < .001 | |||
| P trend‡ | < .001 | < .001 | |||
| NCI risk criteria at diagnosis | |||||
| Non-infants, standard risk | 109 | 1.0 | — | 1.0 | — |
| Non-infants, high risk | 94 | 1.9 | 1.3 to 2.6 | 1.9 | 1.3 to 2.7 |
| Non-infants, unknown | 22 | 1.7 | 1.01 to 2.8 | 1.2 | 0.65 to 2.4 |
| Infants | 7 | 1.3 | 0.54 to 3.3 | 1.0 | 0.40 to 2.6 |
| P | .002 | .004 | |||
| Extramedullary involvement in relapse | |||||
| No | 170 | 1.0 | — | 1.0 | — |
| Yes | 55 | 0.73 | 0.50 to 1.1 | 0.75 | 0.49 to 1.1 |
| Missing | 7 | ||||
| P | .10 | .36 | |||
| Immunophenotype | |||||
| Pre B cell | 202 | 1.0 | — | 1.0 | — |
| T cell | 30 | 1.8 | 1.2 to 2.8 | 2.1 | 1.3 to 3.5 |
| P | .009 | .007 | |||
| HSCT | |||||
| No | 109 | 1.0 | — | 1.0 | — |
| Yes | 117 | 0.67 | 0.48 to 0.93 | 0.58 | 0.40 to 0.83 |
| Missing | 6 | ||||
| P | .018 | .003 | |||
Abbreviations: CR, complete remission; HR, hazard ratio; NCI, National Cancer Institute; HSCT, hematopoietic stem-cell transplantation.
HRs and 95% CIs from univariate Cox models.
HRs and 95% CIs from Cox models after adjusting for the other variables in the Table.
Test for trend.
Fig 1.
Post recurrence disease-free survival after complete remission (CR) as function of (A) treatment attempt and of (B) duration of first CR (CR 1).
DISCUSSION
Although current chemotherapy regimens successfully cure 80% of children with newly diagnosed ALL, substantial numbers of patients experience relapse and have poor outcomes. Though most patients experiencing relapse achieve remission, a definitive cure continues to be elusive. As reported previously, the most important factors in survival after relapse are the site of and time to relapse.10
Of patients experiencing first relapse, 85% ± 3% achieved CR after their next treatment attempt (Table 3). For early first relapse (< 36 months from diagnosis), the CR rate was 83% ± 4% (n = 110) and for late first relapse (≥ 36 months from diagnosis), the CR rate was 93% ± 3% (n = 59). Breaking down early relapse into very early relapse (< 18 months from diagnosis) and intermediate (18 to 36 months from diagnosis), we found CR rates of 78% ± 6% and 86% ± 5%, respectively. This is generally in keeping with the reported literature in which remission rates for patients in first relapse ranged from 71% to 93%, but it is important to note that some of these data also include isolated extramedullary relapses.5,8,20,21 When further examined, the CR rate for very early and intermediate first marrow relapse also seems better in our series than those reported by Raetz et al.22 In that report, CR2 was 68% ± 6% (n = 69) for overall early relapse events, with 45% ± 11% for very early (< 18 months) relapse (n = 24) and 79% ± 6% for intermediate (18 to 36 months) relapse (n = 45).22
We were also able to assess the efficacy of subsequent therapeutic attempts and observed that there was a significant decrease in those achieving remission, with rates of 44%, 27%, and 12% for third, fourth, and further therapeutic attempts, respectively (Table 3). New agents and combinations might be assessed against this benchmark.
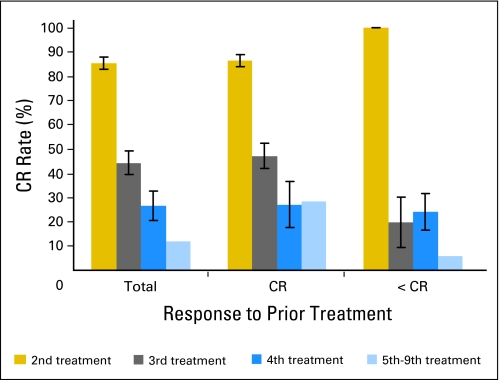
In this analysis, we were also able to observe patients over time through multiple relapses to assess factors contributing to subsequent outcomes. Not surprisingly, patients who did not obtain a CR with the prior therapeutic attempt had a much lower likelihood of obtaining a CR with a subsequent therapeutic attempt (Table 3 and Fig A1 [online only]). (It should be noted in Fig A1 [online only] that patients in < CR who had a high rate of achieving CR with a second therapy includes those patients with primary refractory ALL.) The duration of the prior remission remained a significant factor in predicting subsequent response (Table 3 and Fig A2 [online only]).
We found DFS for patients in CR2 to be 27% ± 4% at 5 years. These results are similar to those found in the literature, though exact comparisons are difficult as a result of different cohorts of patients analyzed with different end points (Appendix Table A2 [online only]). DFS rates ranges from 16% to 39% ± 5% depending on the study, time to end point, and the patient population.5–8,20,23,24 Though slightly different variables were measured, all the results show similar poor outcomes for patients in CR2.
Few data appear for DFS rates in CR3 and beyond. We found rates of 15% ± 7% at 5 years, whereas Chessels et al8 showed those in CR3 had survival of roughly 20% for HSCT from transplantation and 10% for chemotherapy in patients with relapse of any site. Saarinen-Pihkala et al24 found that patients in CR3 had rates (overall survival, not disease-free) of 36% for those receiving stem-cell transplantation and 15% for those receiving chemotherapy. They also showed that for patients in CR3, 10-year EFS was 28% ± 2% for patients receiving chemotherapy only. Einsiedel et al20 reported that only 12% of patients experiencing a second relapse remained in continuous CR.
We also attempted to assess the utility of HSCT. We examined HSCT as a time-dependent variable and corrected for waiting time bias. We found increased survival (hazard ratio = 0.58; P = .003 in multivariate analysis) for patients undergoing HSCT, regardless of time to relapse or the number of prior relapses. However, we acknowledge that our study is retrospective and selection bias remains. In a small, randomized study, Gaynon et al23 found no advantage for HSCT in early relapse. Eapen et al25 found an advantage for matched sibling donor total-body irradiation–based transplantation for early marrow relapse, but not for late marrow relapse in a large registry study. Malempati et al6 examined the cohort of standard-risk patients experiencing relapse from CCG-1952 and found no advantage for HSCT for patients experiencing early or late relapse.
Historically, more than 90% of candidate new agents that enter the clinic fail to earn licensure. Those that succeed may benefit some cancers, but not others. Validated preclinical models are lacking, and unfortunately, single-agent response rates provide little guidance. One agent may have striking single-agent activity (eg, ifosfamide in rhabdomyosarcoma), yet fail to displace an older agent, namely, cyclophosphamide. Conversely, an agent may have no anticancer activity (eg, leucovorin), yet provide benefit in the proper combination, namely, sequential leucovorin followed by fluorouracil. Agents identified through the Pediatric Preclinical Tumor Panel as showing activity against specific tumors are now entering clinical trials. Hopefully, in the future, we will have a better understanding of the usefulness of the Pediatric Preclinical Tumor Panel for predicting clinical activity.26 Another potential challenge in evaluating the utility of such therapies is how to optimally assess efficacy at an earlier time point other than survival. This would allow a more rapid selection of potentially effective agents. Efficient drug development requires early recognition of winners and losers. A variety of multidrug regimens provide a 40% CR rate in second and subsequent relapse.27 Review of TACL data support this surprisingly uniform benchmark. We hypothesize that candidate agents are best tested in combination, and successful combinations should have CR rates surpassing the 40% benchmark.
Minimal residual disease (MRD), measured either by flow cytometry or polymerase chain reaction, may supplement morphologic response. Recently, Raetz et al22 showed the impact of MRD on outcomes for patients with relapsed ALL. Patients who were MRD negative at the end of the first block of chemotherapy had improved survival compared with those who were MRD positive. MRD positivity was also correlated strongly with the duration of initial remission; those patients experiencing relapse at less than 18 months from initial diagnosis had the highest proportion of MRD positivity. Furthermore, in a follow-up study evaluating the potential benefit of adding a monoclonal CD22 antibody (epratuzumab) to the reinduction platform, a greater proportion of patients experiencing early relapse were MRD negative at the end reinduction compared with historical controls, thus highlighting the possible utility of such measurements in assessing relapse therapy.28 However, MRD remains an unvalidated surrogate at present for patients with relapsed ALL who are treated with novel agents.
The TACL consortium was created to develop novel agents and regimens and bring those deserving forward quickly for testing in larger venues. We propose that agents and regimens that show no improvement over our baseline CR and DFS rates need no further study. Promising agents might be restudied with alternative partners. Response rates depend on the population actually treated. On the basis of our data, we plan to construct a model that will provide us with an expected response rate for any patient population with relapsed or refractory ALL. Future analysis of our data may yield valuable information regarding different chemotherapeutic regimens used and may identify particular regimens that have been more successful than others.
Acknowledgment
We thank all the patients, families, and physicians who contributed clinical information. We also thank all the Therapeutic Advances in Childhood Leukemia staff, data collection managers, and support staff that helped to collect and organize data and prepare this manuscript.
Appendix
Fig A1.
Complete remission (CR) rate of these 19 treatments was 10 of 15 second treatment attempts, two of two third treatment attempts, one of one fourth treatment attempt, and one of one fifth treatment attempt. Bars indicate SE.
Fig A2.
Response to the second to ninth treatment attempts as a function of duration of prior complete remission (CR). Bars indicate SE.
Table A1.
HSCT After Treatment Attempt
| Treatment Attempt and Response | HSCT After Treatment |
Total | Median* Time From Remission to HSCT (months) | ||||
|---|---|---|---|---|---|---|---|
| Yes |
No | Unknown | |||||
| Matched Related Donor | Matched Unrelated Donor | Other | |||||
| Second treatment attempt | |||||||
| CR | 43 | 31 | 15 | 70 | 4 | 163 | 2.9 |
| < CR | 2 | — | — | 25 | 1 | 28 | |
| Unknown | 3 | — | — | 1 | — | 4 | |
| Subtotal | 48 | 31 | 15 | 96 | 5 | 195 | |
| Third treatment attempt | |||||||
| CR | 12 | 10 | 1 | 24 | 1 | 48 | 2.2 |
| < CR | — | 1 | — | 58 | 1 | 60 | |
| Unknown | — | 1 | — | 1 | 2 | 4 | |
| Subtotal | 12 | 12 | 1 | 83 | 4 | 112 | |
| Fourth treatment attempt | |||||||
| CR | — | 3 | — | 12 | — | 15 | 0.5 |
| < CR | 1 | — | 1 | 38 | 1 | 41 | |
| Subtotal | 1 | 3 | 1 | 50 | 1 | 56 | |
| Fifth through ninth treatment attempt | |||||||
| CR | 1 | 2 | — | 5 | — | 8 | 0.6 |
| < CR | — | — | — | 55 | 2 | 57 | |
| Unknown | — | — | — | 1 | — | 1 | |
| Subtotal | 1 | 2 | — | 61 | 2 | 66 | |
| Total | 62 | 48 | 17 | 290 | 12 | 429 | |
NOTE. Duration from remission/disease evaluation date to HSCT: median, 2.5 months (range, 0 to 17 months). A total of 92.8% of the patients who received HSCT received it within 6 months after achieving remission. Seven patients received HSCT twice.
Abbreviations: HSCT, hematopoietic stem-cell transplantation; CR, complete remission.
Median is for patients who achieved CR after a treatment attempt. Patients who did not achieve CR or whose response was unknown were excluded when calculating the median.
Table A2.
Comparison of CR and DFS Rates of Selected Studies
| Study | CR Rate (%) | DFS Rate (%) | Patient Population | No. of Patients With Bone Marrow Involvement |
|---|---|---|---|---|
| TACL | CR2: 85 | CR2: 27 ± 4 at 5 years | TACL institutions: marrow relapse between 1995-2004 | 225 |
| CR3: 44 | CR3: 15 ± 7 at 5 years | |||
| CR4: 27 | CR4: 13 ± 9 at 2 years | |||
| Gaynon et al23 | CR2: 20 ± 7 at 5 years | CCG 1941 (marrow relapse < 12 months from completion of treatment) | 214 | |
| Malempati et al6 | CR2: 49.1 ± 6.1 at 2 years | CCG 1952 (SR patients only) | 217 | |
| CR2: 37.4 ± 4.1 at 3 years | ||||
| Nguyen et al7 | CR2: 39.4 ± 5 at 5 years (combined BM) | Cumulative CCG (1998-2002) | 1,387 | |
| CR2: 24.1 ± 2.1 at 5 years (isolated BM) | ||||
| Raetz et al22 | CR2: 68 (early) | COG AALL01P2: marrow relapse between 2003-2005 | 124 | |
| CR2: 96 (late) | ||||
| Chessels et al8 | CR2: 23 at 6 years (combined BM) | Great Ormond Street 1972-1998 and relapsed before 2001 | 350 | |
| CR2: 15.5 at 6 years (isolated BM) | ||||
| Einsiedel et al20 | CR2: 87 | CR2: 30 ± 4 at 10 years | BFM 1987-1990 | 178 |
| Rivera et al5 | CR2: 71.7 | CR2: 30.7 ± 8.6 at 5 years | St Judes: marrow relapse between 1984-1994 | 106 |
| Saarinen-Pihkala et al24 | CR2: 90 | CR2: 28 ± 2 at 10 years | All Nordic ALL patients 1981-2001 | |
| CR3: 72 | CR3: 12 ± 3 at 20 years | |||
| Reismuller et al21 | CR2: 85 | CR2: 33 ± 9 at 10 years (combined BM) | Relapsed patients BFM-Austria 1981-1999 | 159 |
| CR2: 28 ± 4 at 10 years (isolated BM) |
Abbreviations: CR, compete remission; DFS, disease-free survival; TACL, Therapeutic Advances in Childhood Leukemia; CCG, Children's Cancer Group; BM, bone marrow; SR, standard risk; COG, Children's Oncology Group; BFM, Berlin-Frankfurt-Muenster.
Footnotes
Supported by National Institutes of Health Grant No. NCI K22 CA113557 (M.L.L.), Leukemia & Lymphoma Society Grant No. LLS 2157-08 (M.L.L.), the Frank A. Campini Foundation (M.L.L.), the Therapeutic Advances in Childhood Leukemia Consortium, and the University of Southern California–Childrens Hospital Los Angeles Institute for Pediatric Clinical Research. M.L.L. is a Clinical Scholar of the Leukemia & Lymphoma Society.
Presented in part at the American Society of Hematology Annual Meeting, December 8-11, 2007, Atlanta, GA (abstr 854).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Paul S. Gaynon, Genzyme Research Funding: Paul S. Gaynon, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Richard H. Ko, Bruce Bostrom, Richard Sposto, Paul S. Gaynon, Mignon L. Loh
Administrative support: Elena Eckroth
Provision of study materials or patients: Phillip Barnette, Bruce Bostrom, Raymond Hutchinson, Elizabeth Raetz, Nita L. Seibel, Clare J. Twist, Paul S. Gaynon, Mignon L. Loh
Collection and assembly of data: Richard H. Ko, Phillip Barnette, Raymond Hutchinson, Elizabeth Raetz, Nita L. Seibel, Clare J. Twist, Elena Eckroth, Mignon L. Loh
Data analysis and interpretation: Richard H. Ko, Lingyun Ji, Nita L. Seibel, Richard Sposto, Paul S. Gaynon, Mignon L. Loh
Manuscript writing: Richard H. Ko, Lingyun Ji, Richard Sposto, Paul S. Gaynon, Mignon L. Loh
Final approval of manuscript: Richard H. Ko, Phillip Barnette, Bruce Bostrom, Raymond Hutchinson, Elizabeth Raetz, Nita L. Seibel, Clare J. Twist, Elena Eckroth, Richard Sposto, Paul S. Gaynon, Mignon L. Loh
REFERENCES
- 1.Smith MA, Gloeckler-Ries LA, Gurney JG, et al. Bethesda, MD: National Cancer Institute; 2005. Leukemia: SEER Pediatric Monograph. [Google Scholar]
- 2.Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997;90:4243–4251. [PubMed] [Google Scholar]
- 3.Feig SA, Ames MM, Sather HN, et al. Comparison of idarubicin to daunomycin in a randomized multidrug treatment of childhood acute lymphoblastic leukemia at first bone marrow relapse: A report from the Children's Cancer Group. Med Pediatr Oncol. 1996;27:505–514. doi: 10.1002/(SICI)1096-911X(199612)27:6<505::AID-MPO1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Henze G, Fengler R, Hartmann R, et al. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85): A relapse study of the BFM group. Blood. 1991;78:1166–1172. [PubMed] [Google Scholar]
- 5.Rivera GK, Zhou Y, Hancock ML, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368–376. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 6.Malempati S, Gaynon PS, Sather H, et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–5807. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chessells JM, Veys P, Kempski H, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 9.Roy A, Cargill A, Love S, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia: Lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130:67–75. doi: 10.1111/j.1365-2141.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaynon PS, Qu RP, Chappell RJ, et al. Survival after relapse in childhood acute lymphoblastic leukemia: Impact of site and time to first relapse—The Children's Cancer Group Experience. Cancer. 1998;82:1387–1395. doi: 10.1002/(sici)1097-0142(19980401)82:7<1387::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Kolb EA, Steinherz PG. A new multidrug reinduction protocol with topotecan, vinorelbine, thiotepa, dexamethasone, and gemcitabine for relapsed or refractory acute leukemia. Leukemia. 2003;17:1967–1972. doi: 10.1038/sj.leu.2403097. [DOI] [PubMed] [Google Scholar]
- 12.Harris RE, Sather HN, Feig SA. High-dose cytosine arabinoside and L-asparaginase in refractory acute lymphoblastic leukemia: The Children's Cancer Group experience. Med Pediatr Oncol. 1998;30:233–239. doi: 10.1002/(sici)1096-911x(199804)30:4<233::aid-mpo5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein ML, Abshire TC, Pollock BH, et al. Idarubicin and cytosine arabinoside reinduction therapy for children with multiple recurrent or refractory acute lymphoblastic leukemia: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1997;19:68–72. doi: 10.1097/00043426-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Crooks GM, Sato JK. Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1995;17:34–38. doi: 10.1097/00043426-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Butturini A, Rivera GK, Bortin MM, et al. Which treatment for childhood acute lymphoblastic leukaemia in second remission? Lancet. 1987;1:429–432. doi: 10.1016/s0140-6736(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 16.Chessells JM. Relapsed lymphoblastic leukaemia in children: A continuing challenge. Br J Haematol. 1998;102:423–438. doi: 10.1046/j.1365-2141.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 17.Therapeutic Advances in Childhood Leukemia, 2005. https://ipcr-chla.usc.edu/tacl/
- 18.Cox DR, Snell EJ. New York, NY: Chapman and Hall; 1989. Analysis of Binary Data. [Google Scholar]
- 19.Cox DR, Oakes D. New York, NY: Chapman and Hall; 1984. Analysis of Survival Data. [Google Scholar]
- 20.Einsiedel HG, von Stackelberg A, Hartmann R, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: Results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 21.Reismüller B, Attarbaschi A, Peters C, et al. Long-term outcome of initially homogenously treated and relapsed childhood acute lymphoblastic leukaemia in Austria: A population-based report of the Austrian Berlin-Frankfurt-Munster (BFM) Study Group. Br J Haematol. 2009;144:559–570. doi: 10.1111/j.1365-2141.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 22.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse in acute lymphoblastic lymphoma. J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 24.Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: Role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24:5750–5762. doi: 10.1200/JCO.2006.07.1225. [DOI] [PubMed] [Google Scholar]
- 25.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: A collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 27.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. doi: 10.1111/j.1365-2141.2005.05773.x. [DOI] [PubMed] [Google Scholar]
- 28.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: A Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]