Abstract
Objective:
Pregabalin is effective in several neuropathic pain syndromes. This trial evaluated its efficacy, safety, and tolerability for treatment of painful HIV-associated neuropathy.
Methods:
This randomized, double-blind, placebo-controlled, parallel-group trial included a 2-week double-blind dose-adjustment (150–600 mg/day BID) phase, a 12-week double-blind maintenance phase, and an optional 3-month open label extension phase. The primary efficacy measure was the mean Numeric Pain Rating Scale (NPRS) score, an 11-point numeric rating scale. Secondary measures included Patient Global Impression of Change (PGIC) and sleep measurements.
Results:
Baseline mean NPRS score was 6.93 for patients randomized to pregabalin (n = 151) and 6.72 for those to placebo (n = 151). Pregabalin average daily dosage (SD) was 385.7 (160.3) mg/d. At endpoint, pregabalin and placebo showed substantial reductions in mean NPRS score from baseline: −2.88 vs −2.63, p = 0.3941. Pregabalin had greater improvements in NPRS score relative to placebo at weeks 1 (−1.14 vs −0.69, p = 0.0131) and 2 (−1.92 vs −1.43, p = 0.0393), and at weeks 7 (−3.22 vs −2.53 p = 0.0307) and 8 (−3.33 vs −2.53, p = 0.0156). At all other time points, differences between groups were not significant. Sleep measurements and 7-item PGIC did not differ among treatment groups; however, collapsed PGIC scores showed 82.8% of pregabalin and 66.7% of placebo patients rated themselves in 1 of the 3 “improved” categories (p = 0.0077). Somnolence and dizziness were the most common adverse events with pregabalin.
Conclusions:
Pregabalin was well-tolerated, but not superior to placebo in the treatment of painful HIV neuropathy. Factors predicting analgesic response in HIV neuropathy warrant additional research.
Classification of Evidence:
This Class II trial showed that pregabalin is not more effective than placebo in treatment of painful HIV neuropathy.
GLOSSARY
- AE
= adverse events;
- ANCOVA
= analysis of covariance;
- ARF
= activity region finder;
- ARV
= antiretroviral;
- GPS
= Gracely Pain Scale;
- HADS
= Hospital Anxiety and Depression Scale;
- HIV-DSP
= HIV-associated distal sensory polyneuropathy;
- LOCF
= last observation carried forward;
- mBPI-sf
= modified Brief Pain Inventory–short form;
- NPRS
= Numeric Pain Rating Scale;
- NPSI
= Neuropathic Pain Symptom Inventory;
- NRS
= Numeric Rating Scale;
- PGIC
= Patient Global Impression of Change;
- VAS
= visual analog scale.
HIV-associated distal sensory polyneuropathy (HIV-DSP) is the most common neurologic complication of HIV infection and a major cause of morbidity in patients with HIV and AIDS.1–5 Symptoms of HIV-DSP appear more commonly in the advanced stages of HIV infection.4–6 Clinical benefit from numerous agents evaluated to date has been inconsistent.7–10
Anticonvulsants such as gabapentin and pregabalin exert an analgesic effect by binding to the α2-δ subunit of voltage-gated calcium channels on primary afferent nerves and suppressing the release of neurotransmitters from central terminals.11 In one small placebo-controlled trial, gabapentin was effective in reducing pain related to HIV-DSP.12 Pregabalin, which has an improved pharmacokinetic profile, has shown efficacy in relieving neuropathic pain associated with diabetic peripheral neuropathy,13–17 postherpetic neuralgia,18–20 and spinal cord injury,21,22 and pain associated with fibromyalgia.23–25 The current controlled study evaluates the efficacy and safety of pregabalin in painful HIV-DSP.
METHODS
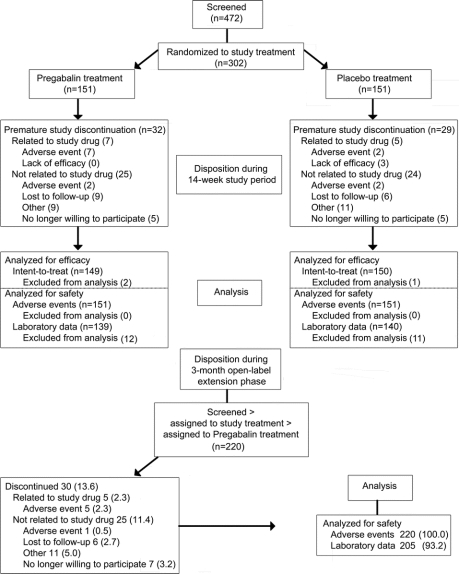
This randomized, placebo-controlled, double-blind, multicenter study evaluated the efficacy, safety, and tolerability of pregabalin for the treatment of pain associated with HIV-DSP (evidence level: Class II). Following trial completion, subjects had the option of enrolling in a 3-month, open-label extension trial (figure 1).
Figure 1 Subject flow through the trial
Eligibility and enrollment.
Eligible subjects had painful HIV-DSP for ≥3 months, confirmed by a neurologist at screening, using established diagnostic criteria,2 and a Karnofsky Performance Score of ≥60 at screening. Patients receiving neurotoxic antiretroviral (ARV) drugs known to cause sensory neuropathy clinically similar to HIV-DSP must have been on stable doses for ≥3 months before screening. Doses of other pain medications had to be stable for ≥1 month before treatment and throughout the study. Subjects receiving serotonin-norepinephrine reuptake inhibitors or antiepileptics were excluded. Nonpharmacologic therapies (e.g., physical therapy, acupuncture) had to be stable for ≥30 days before screening and throughout the study. Patients were excluded for significant pain unrelated to HIV-DSP; a confounding condition associated with neuropathy (e.g., diabetes mellitus, B12 deficiency, alcoholism); or abnormalities in major organ function.
Standard protocol approvals, registrations, and patient consents.
The study was conducted in accordance with the ethics principles of the Declaration of Helsinki, was consistent with Good Clinical Practice guidelines, and was approved by all Institutional Review Boards. Written informed consent was obtained from all participating patients. The trials were registered at the NIH Web site (ClinicalTrials.gov: NCT00232141 and NCT00264875).
Randomization scheme and treatment.
The studies were conducted at 40 centers in the United States and Puerto Rico from October 2005 to November 2007. Subjects who completed at least 4 daily pain diary entries during the last 7 days of a 2-week screening period (no study drug administration) with an average score of ≥4 on the Numeric Pain Rating Scale (NPRS) were randomized in a 1:1 ratio to pregabalin or placebo. Randomization assignments were controlled by a central computerized telerandomization system, such that investigators remained blinded to treatment assignments during the study. Study drug and placebo were identical in appearance in order to preserve blinding. The randomized subjects entered a 2-week dosage-adjustment phase and initially received either pregabalin 150 mg/day or placebo BID taken with or without food (figure 2). On day 3, subjects were contacted to assess pain response and tolerability. Based on these factors, the dose was increased to 300 mg/day (150 mg BID) or maintained at 150 mg/day (75 mg BID). During office visits at the end of weeks 1 and 2, the dose was adjusted up to a maximum of 600 mg/day (300 mg BID) or down to a minimum of 150 mg/day (75 mg BID) or maintained based on individual response and tolerability. After week 2, doses could no longer be adjusted, and subjects entered the 12-week maintenance phase. Subjects remained on the maintenance regimen until the end of week 14; subjects unable to tolerate their maintenance regimen were discontinued.
Figure 2 Study design and dosing schedule
Patients who completed the final visit of the double-blind study could continue into a 3-month, open-label study extension. Open-label pregabalin treatment was initiated at 150 mg/day (75 mg BID); adjustments within the 150 to 600 mg/day dose range were made throughout the study to optimize pain control and tolerability. Subjects were not aware of which treatment they had received during the double-blind trial.
Assessments.
The primary efficacy measure was change from baseline in mean NPRS score at endpoint at 14 weeks (last observation carried forward [LOCF]). Subjects rated pain experienced during the previous 24 hours on an 11-point NPRS ranging from 0 (no pain) to 10 (worst possible pain). A rating of 1 to 3 was considered mild pain; 4 to 6, moderate pain; and 7 to 10, severe pain.26
Secondary efficacy measures included change from baseline to week 14 in Numeric Rating Scale (NRS) Sleep Interference Score, Medical Outcomes Study Sleep Scale, Hospital Anxiety and Depression Scale (HADS), and Patient Global Impression of Change (PGIC). Pain was assessed with the modified Brief Pain Inventory–short form (mBPI-sf), Neuropathic Pain Symptom Inventory (NPSI), and Gracely Pain Scale (GPS). Safety assessments included adverse events (AEs), clinical laboratory tests, vital signs, and physical examination. Neurologic examination including mental status, cranial nerve function, motor function, reflexes, and coordination was performed at screening and last visit.
Static mechanical allodynia was evoked by applying the plastic base of a von Frey hair in the area of maximum pain for 10 seconds with sufficient pressure to indent the skin. Dynamic mechanical allodynia was evoked by gently stroking the area of maximum pain with a foam brush. Punctate hyperalgesia was evoked by pinprick over the area of maximum pain with a supplied safety pin. Temporal summation was evoked by repeatedly tapping the area of maximum pain with a 300 g (6.65) Von Frey hair at a rate of approximately 2 Hz (2 taps per second) for 60 seconds. Cold allodynia was evoked by applying a standardized cool (15°C ± 2°C) metal rod. Cold hyperalgesia was evoked by applying a cold (4°C) metal rod. Patients were asked to rate the pain produced by the applied stimuli on an 11-point NRS. All assessments were performed once at baseline and at endpoint.
During the open-label extension study, efficacy and safety assessments were done at months 1, 2, and 3. Pain was measured on a 100-mm visual analog scale (VAS), where 0 corresponded to no pain and 100 corresponded to worst possible pain. The VAS was completed at entry and at each monthly visit. Safety assessments included AEs, edema, weight, and vital signs.
Statistical analysis.
The study was designed to provide 90% power to detect a difference of at least 1.1 (a clinically meaningful difference) in the NPRS change from baseline in weekly mean pain score between pregabalin-treated subjects and placebo at an alpha level of 0.05, a tolerance for type II error set at beta = 0.10, and a common SD of 2.2.26
Efficacy and safety measures were assessed for all patients who received study medication based on intention to treat. Comparison of the pregabalin and placebo groups on the change from baseline in mean NPRS scores at endpoint using the LOCF was conducted using an analysis of covariance (ANCOVA) model with treatment and pooled site as fixed effects, and baseline pain score as a covariate.
Secondary analyses included examination of responder rates based on ≥30% and ≥50% reduction in mean weekly pain score using Cochran-Mantel-Haenszel tests. The PGIC was compared between treatment groups using a Cochran-Mantel-Haenszel test. Descriptive statistics were reported for the open-label study.
Evoked pain measures and CD4, CD8, or HIV plasma viral loads were compared within and between treatment groups at baseline, endpoint assessments, and also for change from baseline values.
A post hoc, hypothesis-generating exploratory analysis of evoked pain measures was done to identify patient subgroups with greatly differing treatment effects. Activity region finder (ARF), a data mining technique that uses a recursive partitioning procedure, identified subgroups with a high mean response or a high proportion of respondents.27 Following subgroup identification, pregabalin and placebo groups were compared on the change from baseline in mean NPRS scores at endpoint LOCF, using an ANCOVA model with treatment and pooled site as fixed effects and baseline pain score, the ARF-identified subgroup as a covariate, and an additional term for the interaction of this subgroup with treatment. Least squares estimates of treatment differences from this interaction term were obtained from the ANCOVA model.
RESULTS
Patient characteristics.
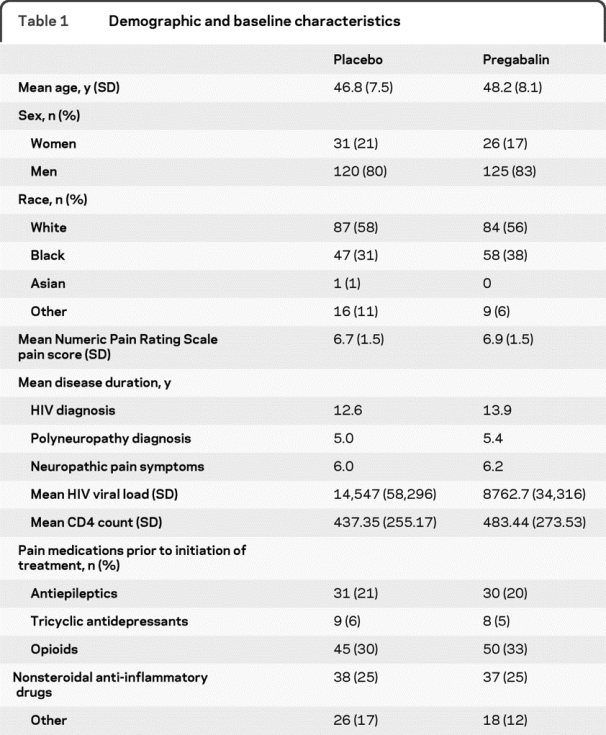
A total of 302 patients (151 pregabalin, 151 placebo; figure 2) were randomized with treatment groups balanced for baseline NPRS score (mean 6.93 pregabalin [SD 1.5], 6.72 placebo [SD 1.5]), duration of neuropathic pain, analgesic treatment, and neurotoxic ARV treatment (table 1). No significant differences between pregabalin and placebo treatment groups were identified at baseline and endpoint assessments with respect to CD4, CD8, or HIV plasma viral load, and likewise, no differences within a treatment group (using change from baseline values) with respect to CD4, CD8, and viral load counts at the endpoint assessments.
Table 1 Demographic and baseline characteristics
Overall, 241 patients (80%) completed the 14-week study; 32 pregabalin subjects (21%) and 29 placebo subjects (19%) terminated early (figure 1). Of 151 patients receiving pregabalin, 17 subjects (11.3%) achieved a maximum dose of 150 mg/day, 53 (35.1%) achieved 300 mg/day, and 91 (53.6%) achieved 600 mg/day. The average daily pregabalin dosage (SD) was 385.7 (160.3) mg/day. A total of 220 subjects elected to enter the open-label extension study, and 169 completed the study. During the open-label study, the average dose was 312.9 mg/day.
Efficacy.
Pain reduction at the 14-week endpoint was similar in placebo and pregabalin groups. NPRS scores at endpoint LOCF relative to baseline for pregabalin showed an average decrease of 2.88 compared with an average decrease of 2.63 for placebo (difference −0.25, p = 0.3914). Weekly pain scores were lower for pregabalin at weeks 1 and 2 (figure 3), with subjects in the pregabalin group experiencing an average decrease of 1.14 in NPRS scores compared with an average decrease of 0.69 for the placebo group at week 1 (p = 0.0131). The mean change from baseline in NPRS scores also differed between the 2 treatment groups at weeks 2 (−1.92 vs −1.43, p = 0.0393), 7 (−3.22 vs −2.53, p = 0.0307), and 8 (−3.33 vs −2.53, p = 0.0156). No difference was observed at weeks 6, 10, and 14 (p = 0.0879, p = 0.3060, and p = 0.1856). Final achieved dosage did not correlate with pain response.
Figure 3 Mean change from baseline in Numeric Pain Rating Scale score
No differences in 30% and 50% responder rates between the pregabalin and placebo groups were observed at any study visit or at endpoint. The endpoint LOCF 50% responder rate for pregabalin was 38.9% and 42.8% for placebo (p = 0.5003). The endpoint LOCF 30% responder rate for pregabalin was 56.3% and 55.9% for placebo (p = 0.9061).
The weekly sleep interference score was lower for pregabalin at time points up to week 6. At week 1, subjects receiving pregabalin had an average decrease of 1.04 in NRS-sleep interference scores compared with an average decrease of 0.68 in the placebo group (p = 0.0469). Differences between the 2 study groups also occurred at weeks 2 (p = 0.0067) and 6 (p = 0.0452), but not at weeks 10 (p = 0.1053) and 14 (p = 0.0711). At study endpoint, the pregabalin and placebo groups did not differ in NRS-sleep interference scores (p = 0.4776).
Pregabalin had a modest effect on the PGIC; 82.8% of subjects receiving pregabalin and 66.7% of subjects receiving placebo considered themselves to be “improved,” 13.3% of subjects receiving pregabalin and 25.4% of subjects receiving placebo experienced “no change,” and 3.9% of subjects receiving pregabalin and 7.9% of subjects receiving placebo felt that they had “worsened” by the end of the study (p = 0.008). However, when patient responses were analyzed by more specific categories (very much improved/much improved/minimally improved/no change/minimally worse/much worse/very much worse), the differences were not significant. The difference in the change in NPSI or HADS scores between pregabalin and placebo groups was not significant.
The pregabalin and the placebo groups experienced similar average decreases from baseline in GPS score of 2.70 and 2.76. p Values for correlations between the change from baseline at endpoint in the GPS and the change from baseline to endpoint in NPRS scores and mBPI-sf measures (including worst pain, average pain, Pain Severity Index, and Pain Interference Index at endpoint) were <0.0001 for all comparisons.
The mean baseline pain score was 38.61 on the 100-mm VAS at the start of the open-label study. The mean VAS score decreased to 30.75 after 4 weeks of treatment and was maintained at 29.39 at the 3-month endpoint.
The difference in the change from baseline to endpoint for any assessment of the evoked pain measures between the pregabalin and placebo groups was not significant. However, ARF analysis indicated that treatment effects differ greatly in subjects with the greatest sensitivity to pinprick at baseline (baseline punctate hyperalgesia score ≥8, n = 39). For these subjects, the change from baseline in mean NPRS scores at endpoint LOCF showed a 2.14-point greater improvement for pregabalin compared to placebo (p = 0.0111). For subjects with a low-to-moderate sensitivity to pinprick at baseline (a score ≤7 on assessment of punctate hyperalgesia), change from baseline difference was 0.06 points (p = 0.8792). No significant difference in pain score was found between groups when using the same criteria for other evoked pain assessments.
Safety and tolerability.
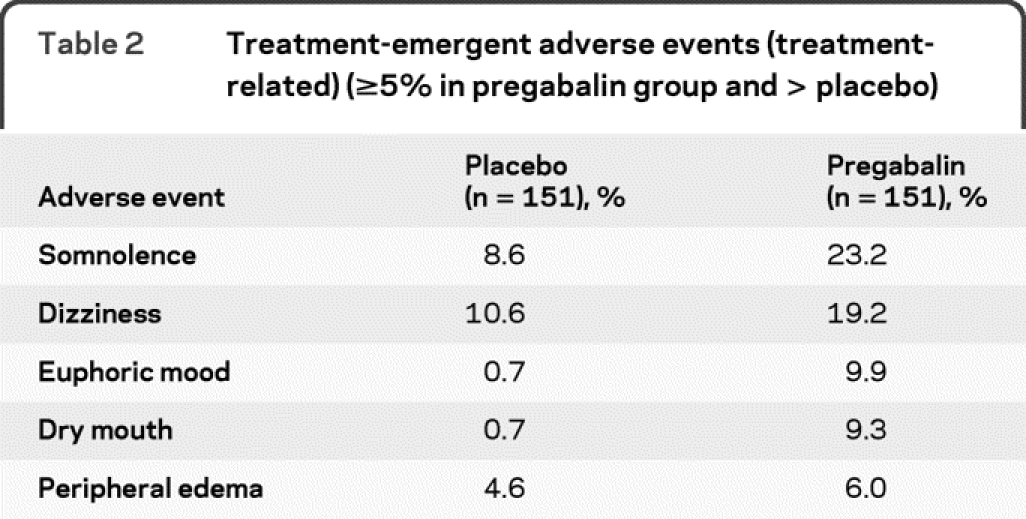
Pregabalin treatment was generally well tolerated. A total of 123 pregabalin-treated subjects (81.5%) and 106 placebo-treated subjects (70.2%) reported AEs. The most commonly reported AEs were generally mild to moderate in intensity and included somnolence, dizziness, euphoric mood, headache, and peripheral edema (table 2).
Table 2 Treatment-emergent adverse events (treatment-related) (≥5% in pregabalin group and > placebo)
Discontinuations due to AEs occurred in 9 subjects (6.0%) treated with pregabalin and 4 (2.6%) of the subjects treated with placebo. The most common reasons for discontinuation due to pregabalin-related AEs were dizziness (4 subjects) and somnolence, confusional state, and disorientation (2 subjects each). The reasons for treatment-related discontinuations in the placebo group were hypoesthesia (1 subject), bladder pain (1 subject), nausea and vomiting (1 subject), and pain (1 subject). No treatment-related serious AEs occurred.
Treatment-related AEs were reported by 145 (65.9%) subjects in the open-label study. As during the double-blind study, AEs in the extension phase were generally mild to moderate in intensity. The most commonly reported AEs included somnolence, dizziness, peripheral edema, diarrhea, and fatigue. Discontinuations due to AEs occurred for 2.7% of subjects. No treatment-related serious AEs occurred.
DISCUSSION
Symptomatic therapy for neuropathic pain remains an area in need of more effective therapies. While many neuropathic pain syndromes are clinically similar, the degree to which results derived from one human pain condition may apply to other neuropathic pain conditions caused by different underlying diseases is unclear. In this study we tested pregabalin, a medication demonstrated to be effective for diabetic peripheral neuropathic pain and postherpetic neuralgia, for efficacy in HIV. Control of pain due to peripheral neuropathy either associated with HIV infection or secondary to neurotoxicity of several antiretroviral treatments is a large unmet need. The results of this placebo-controlled, multicenter study show that pregabalin, at doses up to 600 mg/day, is safe and well-tolerated by HIV-infected individuals with DSP. The most common adverse experiences reported were generally of mild or moderate intensity and similar in frequency to those previously reported for pregabalin in studies that enrolled HIV-negative individuals.13–25
Based on the primary efficacy measure, change from baseline in mean NPRS score, the current study revealed no significant difference between placebo and pregabalin treatment arms. Similarly, there were no significant differences in the secondary pain measures, including responder rates based on ≥30% and ≥50% reduction in mean weekly pain score, mBPI-sf, NPSI, and GPS.
Evoked pain was assessed in all patients in this multicenter study. None of the evoked pain measures changed significantly in response to therapy. An exploratory analysis of the relationship between therapeutic response to pregabalin and evoked pain characteristics revealed that the presence of marked sensitivity to pinprick stimuli on punctate hyperalgesia assessment, but not mechanical allodynia, cold hyperalgesia, or cold allodynia, was associated with a significant and clinically meaningful response to pregabalin vs placebo. These data may provide insight into the mechanism of action of pregabalin in this and perhaps other patient populations with neuropathic pain. Punctate hyperalgesia in experimental pain models is a manifestation of central sensitization induced by C-fiber nociceptor activity.28,29 These findings should be examined prospectively in future studies.
Individuals with HIV-associated DSP achieved NPRS treatment effect size similar to those in studies of diabetic peripheral neuropathy13–17 and postherpetic neuralgia.16,18–20 However, the placebo group in the current study had a much higher NPRS change than in the diabetic peripheral neuropathy or postherpetic neuralgia studies. Notably, there were no significant demographic, clinical, or laboratory differences at baseline between the placebo and pregabalin arms in our study (table 1).
Study design might affect placebo responses. We used a flexible-dose design rather than the fixed-dose schema used in most diabetic peripheral neuropathy and postherpetic neuralgia studies. It is unclear whether the difference in study design affected the outcome. We chose a flexible-dose design because this better approximates clinical practice, and there have been reports that this design may potentially decrease the placebo response, at least in trials for treatment of depression.30,31 Furthermore, mild pain at baseline has also been suggested to increase the placebo response.30 However, in this study, the average pain score was in the moderate range.
Dose did not correlate with response; however, this flexible-dose study was not randomized to different doses and not designed to detect dose response. Dose response has been demonstrated in fixed-dose studies of pregabalin.14,15,17,19,20,23,24 In flexible-dose regimens, dose is adjusted for efficacy and tolerability. Direct comparison of dose groups introduces a selection bias (e.g., patients escalated to the highest dose may show less improvement than patients who receive a lower dose, since less responsive patients would receive higher doses).
Notably, several other controlled symptomatic pain studies reported large placebo responses. This was true of a study of amitriptyline in which the substantial placebo response of 0.20 units on the GPS was sufficient to make the slightly greater amitriptyline response insignificant.32 Similarly, large placebo response played a role in determination of efficacy for lamotrigine for HIV-associated neuropathy pain.33 Future studies in HIV-DSP would benefit from study designs that minimize placebo response to allow identification of potentially active therapeutic agents.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Ed Whalen and Dr. Birol Emir.
DISCLOSURE
Dr. Simpson has served on scientific advisory boards for Pfizer Inc., Cephalon, Inc., Eli Lilly and Company, GlaxoSmithKline, Allergan, Inc., Merz Pharmaceuticals LLC, MEDA Pharmaceuticals Ltd., Boehringer Ingelheim, Endo Pharmaceuticals, Biogen Idec, and Alpharma Inc.; serves on the editorial board of AIDS Patient Care; has served on speakers' bureaus for and received speaker honoraria from Eli Lilly and Company and GlaxoSmithKline; has served as a consultant for NeurogesX, Eli Lilly and Company, Regeneron Pharmaceuticals, Inc., GlaxoSmithKline, Allergan, Inc., Merz Pharmaceuticals LLC, and Torrey Pines; and receives research support from NeurogesX, Pfizer Inc., Allergan, Inc., Eli Lilly and Company, and the NIH (NINDS R01-NS-328-05 [Co-I], NINDS R24 MH59724 [Co-I], NIMH 00-AI-0005 [Co-PI], and NINDS R01-NS-41198 [Co-I]). Dr. Schifitto receives research support from the NIH (U01-A1069511 [Co-I], R01-NS036524 [Co-I], P01-MH064570 [Co-PI], and P01-MH64409 [PI]). Dr. Clifford has served on scientific advisory boards for Biogen Idec, Elan Corporation, Roche, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline, Millennium Pharmaceuticals, Inc., Schering-Plough Corp., Bristol-Myers Squibb, Pfizer Inc., and Genzyme Corporation; received a speaker honorarium from GlaxoSmithKline; and receives research support from Pfizer Inc., Schering-Plough Corp., Bavarian Nordic, NeurogesX, GlaxoSmithKline, Tibotec Therapeutics, Boehringer Ingelheim, Gilead Sciences, Inc., Biogen Idec, and the NIH (UO1 NS32228 [PI], UO1 AI69495 [PI], NIMH 22005 CHARTER Project [Washington U Site PI], NIDA RO3 DA022137 [DC investigator], NIMH MH058076 HIV [Site PI], and R21 3857-53187 [PI]) Dr. Murphy is a full-time employee of and holds stock and stock options in Pfizer Inc. Dr. Durso–De Cruz was (until January 2008) a full-time employee of Pfizer Inc. Dr. Glue was (until December 2008) a full-time employee of and holds stock and stock options in Pfizer Inc. Dr. Whalen is a full-time employee of and holds stock and stock options in Pfizer Inc. Dr. Emir is a full-time employee of and holds stock and stock options in Pfizer Inc. Dr. Scott of UBC Scientific Solutions provided editorial and medical writing support, funded by Pfizer Inc. Dr. Freeman serves on scientific advisory boards and as a consultant for Pfizer Inc., Eli Lilly & Company, UCB, Eisai Inc., Solace Pharmaceuticals, GlaxoSmithKline, and Chelsea Therapeutics; receives publishing royalties from UpToDate; serves as an Associate Editor of the Clinical Journal of Pain, Basic and Clinical Editor of Autonomic Neuroscience, and on the editorial board of Clinical Autonomic Research; has served on speakers' bureaus for and received honoraria for speaking and educational activities from Eli Lilly and Company and Pfizer Inc.; and receives research support from Pfizer Inc., the NIH (R01 HL059459 [PI], R01 DK063296 [PI], and R01 NS046710[PI]), and the Langer Family Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. David M. Simpson, Clinical Neurophysiology Laboratories and Neuro-AIDS Program, The Mount Sinai Medical Center, Box 1052, New York, NY 10029 david.simpson@mssm.edu
Supplemental data at www.neurology.org
*Members of the 1066 HIV Neuropathy Study Group are listed in appendix e-1 on the Neurology® Web site at www.neurology.org.
Study funding: Sponsored by Pfizer Inc., which participated in the acquisition of data, statistical analysis, study supervision, and approval of data.
Disclosure: Author disclosures are provided at the end of the article.
Presented in part at the XVII International AIDS Conference, August 7, 2008, Mexico City, Mexico; and the 61st annual meeting of the American Academy of Neurology, April 25–May 2, 2009, Seattle, WA.
Received May 27, 2009. Accepted in final form November 5, 2009.
REFERENCES
- 1.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: The Manhattan HIV Brain Bank. Arch Neurol 2004;61:546–551. [DOI] [PubMed] [Google Scholar]
- 2.Simpson DM, Kitch D, Evans SR, et al, ACTG A5117 Study Group. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology 2006;66:1679–1687. [DOI] [PubMed] [Google Scholar]
- 3.Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995;9:153–161. [PubMed] [Google Scholar]
- 4.Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS 2002;16:2105–2117. [DOI] [PubMed] [Google Scholar]
- 5.Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J Periph Nerv Syst 2001;6:8–13. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Duarte A, Robinson-Papp J, Simpson DM. Diagnosis and management of HIV-associated neuropathy. Neurol Clin 2008;26:821–832. [DOI] [PubMed] [Google Scholar]
- 7.Breitbart W, Rosenfeld BD, Passik SD, McDonald MV, Thaler H, Portenoy RK. The undertreatment of pain in ambulatory AIDS patients. Pain 1996;65:243–249. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart W, Passik S, McDonald MV, et al. Patient-related barriers to pain management in ambulatory AIDS patients. Pain 1998;76:9–16. [DOI] [PubMed] [Google Scholar]
- 9.Frich LM, Borgbjerg FM. Pain and pain treatment in AIDS patients: a longitudinal study. J Pain Symptom Manage 2000;19:339–347. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Estanislao L, Mintz L, Simpson D. Controlling neuropathic pain in HIV. Curr Infect Dis Rep 2004;6:237–242. [DOI] [PubMed] [Google Scholar]
- 11.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 2002;42:229–236. [DOI] [PubMed] [Google Scholar]
- 12.Hahn K, Arendt G, Braun JS, et al, German Neuro-AIDS Working Group. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. J Neurol 2004;251:1260–1266. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628–638. [DOI] [PubMed] [Google Scholar]
- 14.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104–2110. [DOI] [PubMed] [Google Scholar]
- 15.Richter RW, Portenoy R, Sharma U, LaMoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253–260. [DOI] [PubMed] [Google Scholar]
- 16.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254–263. [DOI] [PubMed] [Google Scholar]
- 17.Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 2008;12:203–213. [DOI] [PubMed] [Google Scholar]
- 18.Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003;60:1274–1283. [DOI] [PubMed] [Google Scholar]
- 19.Sabatowski R, Gálvez R, Cherry DA, et al, 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004;109:26–35. [DOI] [PubMed] [Google Scholar]
- 20.van Seventer R, Feister HA, Young JP Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006;22:375–384. [DOI] [PubMed] [Google Scholar]
- 21.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 2006;67:1792–1800. [DOI] [PubMed] [Google Scholar]
- 22.Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 2008;136:150–157. [DOI] [PubMed] [Google Scholar]
- 23.Crofford LJ, Rowbotham MC, Mease PJ, et al, Pregabalin 1008-105 Study Group. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:1264–1273. [DOI] [PubMed] [Google Scholar]
- 24.Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol 2008;35:502–514. [PubMed] [Google Scholar]
- 25.Crofford LJ, Mease PJ, Simpson SL, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain 2008;136:419–431. [DOI] [PubMed] [Google Scholar]
- 26.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 27.Amaratunga D, Cabrera J. Mining data to find subsets of high activity. J Stat Plan Inference 2004;122:23–41. [Google Scholar]
- 28.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 1991;66:190–211. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 1999;122: 2245–2257. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin RH, Katz J, Gitlin MJ. Placebo response in clinical trials of depression and its implications for research on chronic neuropathic pain. Neurology 2005;65:S7–S19. [DOI] [PubMed] [Google Scholar]
- 31.Khan A, Khan SR, Walens G, et al. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28:552–557. [DOI] [PubMed] [Google Scholar]
- 32.Kieburtz K, Simpson D, Yiannoutsos C, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection: AIDS Clinical Trial Group 242 Protocol Team. Neurology 1998;51:1682–1688. [DOI] [PubMed] [Google Scholar]
- 33.Simpson DM, McArthur JC, Olney R, et al, Lamotrigine HIV Neuropathy Study Team. Lamotrigine for HIV-associated painful sensory neuropathies: a placebo-controlled trial. Neurology 2003;60:1508–1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.