Abstract
Background:
The clinical and MRI presentation differs between earlier- and later-onset pediatric multiple sclerosis (MS), whereas the effect of age on the CSF inflammatory profile is unknown and may contribute to delayed diagnosis.
Objectives:
To compare the CSF cellular and immunoglobulin G (IgG) profiles between earlier- and later-onset pediatric MS.
Methods:
We queried the databases of 6 pediatric MS centers for earlier-onset (onset <11 years) and later-onset (≥11 and <18 years) patients with MS or clinically isolated syndrome who underwent CSF analysis within the first 3 months of presentation (observational study). We compared CSF white blood cell (WBC) differential count, IgG index, and IgG oligoclonal bands between age groups.
Results:
We identified 40 earlier-onset (mean age at onset = 7.2 ± 2.7 years, 60% females) and 67 later-onset pediatric MS patients (15.1 ± 1.7 years, 63% females). Although WBC count tended to be higher in earlier-onset patients (median = 9/mm3 [0–343] vs 6 [0–140], p = 0.15), they had a lower proportion of lymphocytes (70% [0–100] vs 93% [0–100] of WBCs, p = 0.0085; difference = +3% per 1-year increase of age, p = 0.0011) and higher proportion of neutrophils than later-onset patients (0.5% [0–75] vs 0% [0–50] of WBCs, p = 0.16; difference = −1% per 1-year increase of age, p = 0.033). In earlier-onset disease, fewer patients had an elevated IgG index than in the later-onset group (35% vs 68% of patients, p = 0.031).
Conclusion:
Age modifies the CSF profile at pediatric multiple sclerosis (MS) onset, which may mislead the diagnosis. Our findings suggest an activation of the innate rather than the adaptive immune system in the earlier stages of MS or an immature immune response.
GLOSSARY
- ADEM
= acute disseminated encephalomyelitis;
- CI
= confidence interval;
- HR
= hazard ratio;
- IgG
= immunoglobulin G;
- MS
= multiple sclerosis;
- OCB
= oligoclonal bands;
- OR
= odds ratio;
- WBC
= white blood cell.
Pediatric multiple sclerosis (MS) has been increasingly recognized in the past 10 years. Still, its diagnosis remains more challenging than adult MS, especially for very young children.1 Indeed, the clinical and MRI presentations of pediatric MS may differ from the adult disease, especially at disease onset and in earlier-onset pediatric MS patients (i.e., those who develop their first symptoms before age 11 years). When compared with later-onset patients, earlier-onset cohorts include more males, more non-Caucasian subjects, and higher rates of encephalopathy at disease onset along with more frequent optic nerve, brainstem, or cerebellum involvement and decreased spinal cord symptoms.2–5 Earlier-onset patients also have a distinct brain MRI phenotype at initial presentation, including more confluent, ill-defined T2-bright lesions that vanish over time, whereas later-onset patients have an MRI phenotype that is more similar to adults.5
In adults, an elevated immunoglobulin G (IgG) index or the presence of CSF-restricted IgG oligoclonal bands (OCB) are biologic hallmarks of MS and thus are included in the diagnostic criteria.6,7 When present at the time of an initial demyelinating event, these biologic findings increase the likelihood of a second attack.8 Because of conflicting studies, it is unknown whether children with MS have a similar CSF profile as adults. The percentage of pediatric MS patients with OCB (8%–92%), an elevated IgG index (64%–75%), or pleocytosis (33%–73%) varies widely between studies.9–14 These inconsistencies between studies may be related to timing of lumbar puncture with respect to disease onset, the various age ranges studied, and different techniques used for CSF analysis. In addition, there is limited longitudinal data regarding the CSF profile in children and adolescents with MS.
We hypothesized that earlier-onset pediatric MS patients have a distinct CSF profile, and proposed to compare the CSF IgG and cellular profile in earlier- vs later-onset patients at disease presentation. Identifying a distinct earlier-onset CSF profile may help to guide clinicians in the evaluation of young patients with an initial demyelinating event concerning for MS. Furthermore, the demonstration of these differences may advance our understanding of the specific immunologic mechanisms underlying earlier-onset pediatric MS, which may be closer to the biologic origin of the disease.
METHODS
Patients.
The US Network of Pediatric MS Centers of Excellence includes 6 centers across the country that collect common demographic and clinical data (www.nationalmssociety.org/about-multiple-sclerosis/who-gets-ms/pediatric-ms/index.aspx). We identified pediatric patients (aged ≤18 years at onset) meeting criteria for pediatric MS or clinically isolated syndrome according to the current operational definitions,15 who underwent CSF analysis within the first 3 months of the initial clinical presentation. Patients who met criteria for neuromyelitis optica or acute disseminated encephalomyelitis were excluded.15
Because it is close to the current age of puberty (Tanner genital development stage 2 for boys and girls) in the United States,16 we subjectively chose 11 years of age to differentiate earlier-onset (age at onset <11 years) vs later-onset patients (onset from 11 to 18 years). This cutoff has also been used in our previous work.5
CSF analysis.
The CSF analysis was performed for diagnostic purposes. Data were gathered from chart review. We recorded the CSF white blood cell (WBC) count including differential, IgG index, and number of CSF-restricted IgG OCB. The IgG index was considered elevated when compared with the norms of the laboratory where the test was performed. OCB was considered positive when 2 or more bands were present in the CSF but not in the corresponding serum. CSF red blood cell count, protein, and glucose levels were also recorded. Patients with traumatic lumbar puncture (>500 red blood cells/mm3) were excluded.17 The cellular and IgG characteristics from a repeat CSF analysis performed at least 3 months after the first lumbar puncture were recorded, when available.
Data analysis.
Clinical and biologic data were compared between earlier- and later-onset patients using univariate analyses (t test, Wilcoxon rank sum test, or Fisher exact test as appropriate). Multivariate analysis with adjustment for age, gender, and race was performed using multiple linear regression for numerical outcomes and multiple logistic regression for binary outcomes using SAS version 9.1 (SAS Institute, Cary, NC). The influence of CSF characteristics on the time to second event was analyzed using the log-rank test. To determine whether differences were associated with earlier disease onset per se, as opposed to disease duration or age, we fit regression models adjusting for disease duration or age (linear or quadratic adjustments as necessary) and tested for a remaining effect due to early onset. Missing data are indicated in the tables.
Standard protocol approvals, registrations, and patient consents.
The authors received approval from an ethical standard committee on human experimentation for research using human subjects. Written informed consents were obtained from patients, unless the local ethical committee waived the requirement for the investigators to do so.
RESULTS
Patient characteristics.
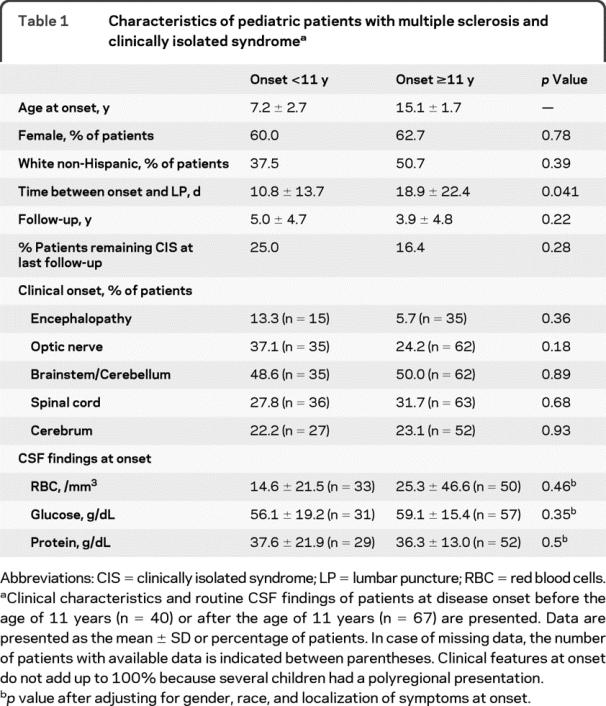
Patient characteristics are presented in table 1. We identified 40 earlier-onset and 67 later-onset pediatric MS patients. There was no significant difference between the 2 groups except that CSF was obtained on average 8 days earlier in earlier- vs later-onset patients. Among those followed up for more than 1 year, 67% of earlier-onset vs 77% of later-onset patients had a second episode during the first year (p = 0.70).
Table 1 Characteristics of pediatric patients with multiple sclerosis and clinically isolated syndrome
Influence of age on CSF analysis at onset.
CSF findings are presented in tables 1 and 2. Red blood cell count, protein, and glucose levels were similar in both age groups. Whereas the absolute WBC count tended to be higher in earlier- vs later-onset patients, the WBC differential was strikingly different after adjusting for gender, race, and localization of symptoms at onset (table 2). Earlier-onset patients had a lower percentage of lymphocytes, whereas the percentages of monocytes and polynuclear cells, especially neutrophils, were higher than in later-onset patients. Similar results were found using 12 or 13 years as a cutoff between both age categories (not shown). In addition, these differences were found when analyzing changes for each additional year of age at disease onset (table 2).
Table 2 Cellular and IgG profiles in the CSF at pediatric multiple sclerosis presentation
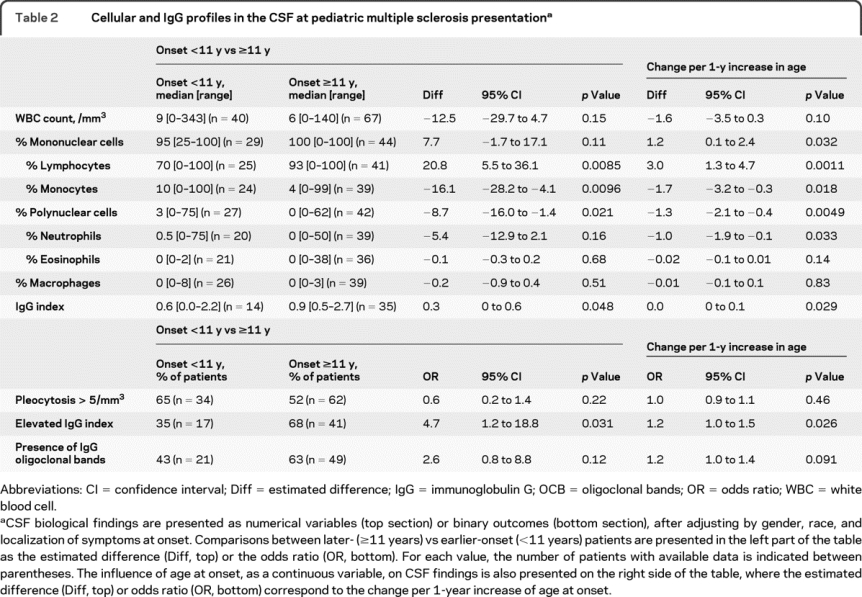
IgG index data were available in 57% of earlier-onset and 50% of later-onset cases (table 2). After adjusting for gender, race, and localization of symptoms at onset, earlier-onset patients were less likely than later-onset patients to have an elevated IgG index (35% vs 68%). However, among only patients with an elevated IgG index, the mean IgG index was not different between both groups (not shown). These differences were also found when analyzing changes for each additional year of age at disease onset (table 2). CSF OCB data were available in 78% of earlier-onset and 67% of later-onset cases (table 2). Earlier-onset patients were less frequently OCB positive. However, among OCB positive patients, there was no difference in the number of CSF bands between the 2 age groups (median number of bands = 3 [2–10] in the earlier-onset vs 5 [1–15] in the later-onset group (p = 0.55). There was no significant association between the presence of an elevated IgG index or positive OCB and WBC count, or the percentage of neutrophils or lymphocytes (data not shown).
Other predictors independently influencing the initial CSF findings after adjusting for age included the clinical involvement of the spinal cord at disease presentation that was associated with increased risk of elevated CSF WBC count (odds ratio [OR] 19.8, 95% confidence interval [CI] 2.35–37.26, p = 0.027) and a non-Caucasian racial background that was associated with a higher risk of elevated IgG index (OR 4.2, 95% CI 1.08–16.1, p = 0.038).
Longitudinal CSF analysis.
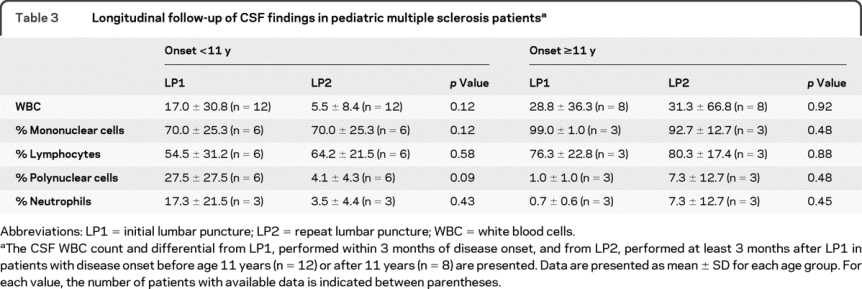
Longitudinal follow-up of CSF findings was available in 12 earlier-onset patients (30%) and 8 later-onset patients (12%) (table 3). The mean ± SD time between the first and the second lumbar puncture was similar in earlier- and later-onset patients (584 ± 629 vs 512 ± 436 days, p = 0.77). The total number of white blood cells and the percentage of polynuclear cells (including neutrophils) tended to decrease over time, whereas the percentage of mononuclear cells (including lymphocytes) tended to increase over time, especially in earlier-onset patients. Among patients who had a repeat lumbar puncture, the proportion of those with either elevated IgG index or positive OCB increased from 12% in the first to 38% in the second analysis. This difference did not reach statistical significance (p = 0.34); however, an association cannot be excluded given the limited number of patients. Repeating the lumbar puncture allowed detection of positive OCB in 2 patients who were initially negative (1 in each age group).
Table 3 Longitudinal follow-up of CSF findings in pediatric multiple sclerosis patients
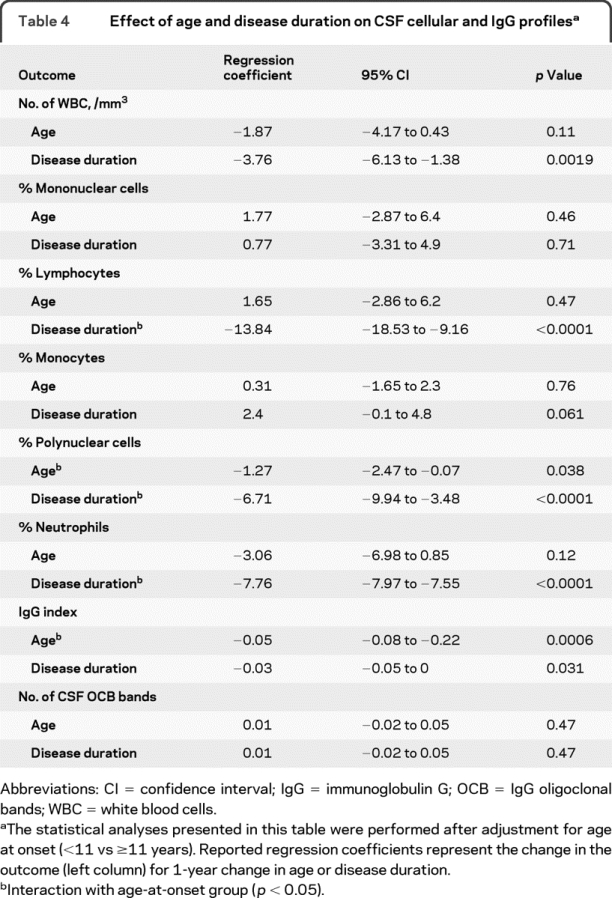
At the time of CSF analysis, when comparing the effect of disease duration (time from clinical onset to the lumbar puncture) vs age in earlier- vs later-onset patients (table 4), we found that the percentage of neutrophils was independently influenced by disease duration and age in earlier-onset patients. The influence of disease duration on the IgG profile was milder than the influence of age, and it was similar in both age groups (table 4).
Table 4 Effect of age and disease duration on CSF cellular and IgG profiles
Influence of initial CSF findings on time to second clinical event.
The results are presented in the figure. Patients who initially had neutrophils in the CSF tended to have a longer time to second event compared with those who did not (median [range] = 210 [25–1,500] vs 125 [34–1604] days, p = 0.12). In contrast, among patients who had positive OCB or an elevated IgG index, the time to second event tended to be shorter compared with those with a negative IgG profile (median [range] = 120 [25–1,500] vs 185 [30–1604] days, p = 0.095). In the Cox models, the hazard ratio (HR) for time to second event was longer in patients with neutrophils in the CSF vs those with none (HR 0.52, 95% CI 0.25–1.05, p = 0.068), and not different in patients with elevated IgG index or positive OCB vs those with negative CSF IgG profile (HR 1.17, 95% CI 0.63–2.2, p = 0.62). The HR for time to second event was also decreased by a higher percentage of neutrophils at onset (HR 0.97 per 1% increase of neutrophils, 95% CI 0.95–1, p = 0.048).
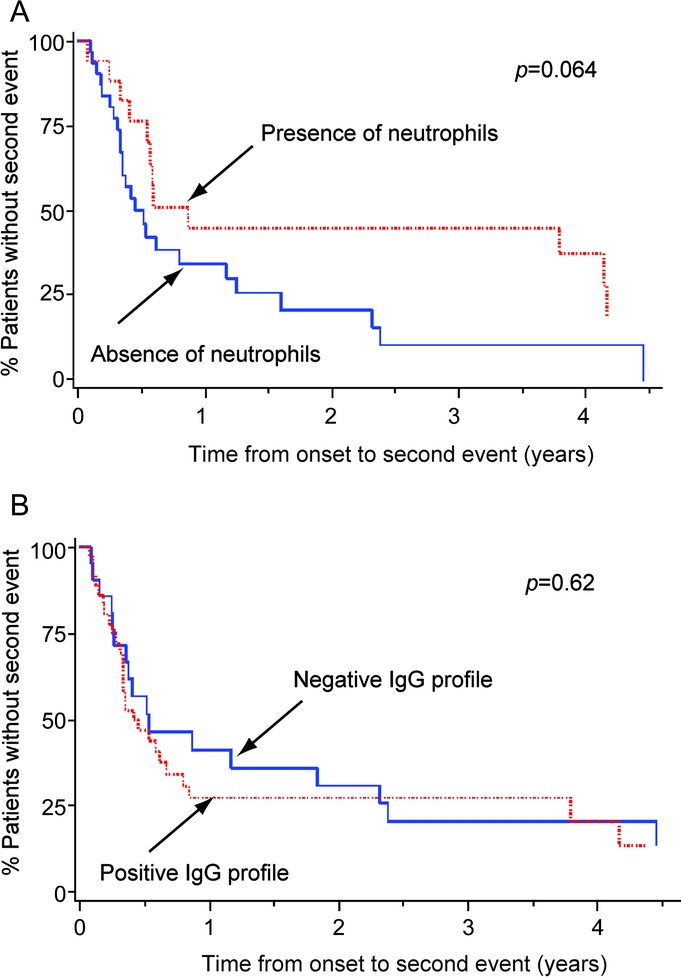
Figure Influence of CSF cellular and IgG profiles at onset on the time to second event
The survival curves represent patients with or without neutrophils (A), and with or without CSF-restricted immunoglobulin G (IgG) oligoclonal bands or an elevated IgG index (B) in the CSF at disease presentation. Log-rank test p values are provided for each analysis.
DISCUSSION
Our data demonstrate that earlier-onset pediatric MS patients have a distinct CSF inflammatory profile at disease presentation compared with later-onset patients. This is in line with their clinical and MRI phenotype specificities at clinical onset.2–5 Our data suggest that a biologic profile considered atypical for adult MS (i.e., neutrophilic pleocytosis, normal IgG index, and negative CSF OCB) should not rule out the disease in children, because our patients had a substantial duration of follow-up (table 1), enabling us to rule out acute disseminated encephalomyelitis (ADEM) and neuromyelitis optica. Besides age at onset, we found an independent influence of disease duration on CSF findings (table 4). Because the initial lumbar puncture was performed 8 days sooner in younger patients, both effects were cumulative. Of note, the CSF was less often sent out for IgG assessment for the initial lumbar puncture in earlier- vs later-onset patients, likely because other diagnoses were considered. This practice of performing IgG index and OCB analysis less frequently in younger patients may also contribute to delaying the diagnosis of pediatric MS, especially when disease strikes before puberty.
We showed that the initial CSF cellular profile tends to become more typical of adult MS as the patients age (less neutrophils, more lymphocytes, potential switch from IgG-negative to IgG-positive profile). This is consistent with previous limited cross-sectional data showing that positive CSF IgG findings in pediatric MS may be more common in patients undergoing lumbar puncture during their second or third attack compared with the first episode.11–13 Thus, repeating the lumbar puncture in younger patients with atypical presentation may help to elucidate the diagnosis.
Although positive CSF findings at onset in children are not associated with a worse disability 8 years later,12 they may have a short-term prognostic role. Indeed, we found that patients with no neutrophils, and likely those with positive IgG profile on their initial lumbar puncture, tended to have a shorter time to second event, suggesting increased disease activity. In adults, the presence of OCB at disease onset was reported to double the risk of having a second attack in a mean follow-up time of 5 months, independently of MRI findings,8 but its role in predicting disability remains controversial.
Our findings in earlier-onset patients (neutrophilic pleocytosis, higher percentage of monocytes, absence of IgG in the CSF) suggests a prominent activation of the innate immune system. In contrast, the profile of later-onset patients (lymphocytic pleocytosis, elevated IgG index in the CSF) rather suggests the activation of the adaptive immune system (antigen specific). In children, we hypothesize that the confluent T2-bright signals seen on the initial brain MRI scans of earlier-onset patients may as well correspond to the activation of innate immunity (e.g., edema or activation of microglial or dendritic cells), whereas more well-defined ovoid lesions rather correspond to the activation adaptive immunity. Both age and disease duration were independently associated with the amount of neutrophils in the CSF of earlier-onset patients. Thus, it is possible that an age-related immaturity of the immune system explains these differences, or that the innate immunity is activated before the adaptive immunity in MS in general. This early activation of the innate immune system may be related to a more recent exposure to an environmental potential triggering factor (e.g., first viral exposure). In adults, the CSF neutrophil content is increased in mildly vs severely disabled patients, suggesting a potential involvement of innate immunity during the earlier stages.18 However, recent adult MS data suggest that it may be involved during later stages as well.19 Whether this is the case in pediatric MS remains to be investigated.
Our study is limited by the lack of a standardized protocol for CSF analysis, especially regarding the assessment of OCB. In addition, we chose the approximate age cutoff of 11 years instead of the actual age of puberty. However, our data suggest that there is an effect of age regardless of the age cutoff, when age is considered as a continuous variable. Finally, whether there is a difference between patients who had lumbar puncture (with assessment for IgG synthesis or not) vs those who did not was not addressed here. Further large-scale prospective analyses should be conducted to analyze potential correlations between CSF and MRI findings. Finally, the predictive value of the CSF findings on long-term disease course and disability in children with MS remains to be investigated.
OCB and IgG index should be analyzed more often in children with a first acute neurologic deficit, because it could be demyelinating in nature. If initially negative, especially in younger patients who often present with atypical clinical and MRI features at MS onset, CSF analysis should be repeated. Pediatric MS operational criteria may be revised to take into account these distinct features in earlier-onset MS. Further studies of various immune cell subtypes in the CSF may confirm whether our findings are explained by predominant activation of the innate immune system at disease onset in younger patients. Finally, biologic differences between earlier-onset pediatric MS and ADEM patients remain to be investigated, and may help in understanding why the first ones relapse and the others do not.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by C. McCulloch and D. Chabas.
DISCLOSURE
Dr. Chabas has received honoraria from Teva Pharmaceutical Industries Ltd., EMD Serono Inc., and Pfizer Inc; has received license fee payments and may accrue revenue on office of technology licensing Stanford Docket 501-085 (issued 5/6/2003): Osteopontin Related Compositions and Methods; and receives research support from the National Multiple Sclerosis Society and the Nancy Davis Foundation. Dr. Ness serves as a consultant to Merck Serono; receives research support from the NIH (NIDDK DK51413 [PI]) and the National Multiple Sclerosis Society; and her spouse receives research support from the NIH (K08 NS43220 [PI]). Dr. Belman and Dr. Yeh report no disclosure. Dr. Kuntz has received honoraria for lectures not funded by industry; and receives research support from the National Multiple Sclerosis Society, the Spinal Muscular Atrophy Foundation, and the Cooperative International Neuromuscular Research Group. Dr. Gorman reports no disclosure. Dr. Strober serves on a speakers' bureau for Athena Diagnostics, Inc. Mr. De Kouchkovsky receives research support from the Consortium of Multiple Sclerosis Centers Foundation. Dr. McCulloch receives royalties from publication of Generalized, Linear, and Mixed Models, 2nd ed. (John Wiley and Sons, 2008), Regression Methods in Biostatistics (Springer, 2004), and Variance Components (John Wiley and Sons, 1992); and receives research support from Amgen and the NIH (NIA U01 AG19069 [Coinvestigator], NIAMS N01-AR2-2258 [Coinvestigator], NINDS P01 NS044155 [Coinvestigator], NIAID R01 AI050587 [Coinvestigator], R01 NS059944 [Coinvestigator], R01 AR046905 [Coinvestigator], NCRR UL1 RR024131 [Coinvestigator], NINDS 2R01 NS34949 [Coinvestigator], NIA/CPMC R01 AG05407 [Coinvestigator], and NIA/CPMC U01 AG018197 [Coinvestigator]). Dr. Chitnis has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; has served/serves as a consultant to Biogen Idec, EMD Serono, Inc., Teva Pharmaceutical Industries Ltd., and Bayer Schering Pharma (Berlex); serves on a speakers' bureau for EMD Serono, Inc.; and receives research support from the NIH (NINDS K08 NS 047669-01 ]PI[), the National Multiple Sclerosis Society, and the Nancy Davis Foundation. Dr. Rodriguez has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; serves on scientific advisory boards for the NIH/NINDS and Fast Forward, LLC; serves on editorial advisory boards for Annals of Neurology, Brain Pathology, the Journal of Neurovirology, and VerusMed; receives royalties from publication of Advances in Multiple Sclerosis (Current Topics in Microbiology and Immunology, vol. 318) (Springer-Verlag, 2008); may accrue revenue on US Patent 5,591,629 (issued January 7, 1997): Monoclonal Antibodies Which Promote Central Nervous System Remyelination and US Patent 5,660,828 (issued August 26, 1997): Method of Treating Autoimmune and/or Viral Induced Diseases that are Mediated by CD8 Phenotype T Cells; and receives research support from Acorda Therapeutics, Inc., Mayo Rehabilitation Research Training Center, the NIH (HD07447 [Coinvestigator]), the National Multiple Sclerosis Society, the Conrad Hilton Foundation, the Donald and Frances Herdrich Foundation, and the Canadian Multiple Sclerosis Society. Dr. Weinstock-Guttman serves on a medical advisory board for the National Multiple Sclerosis Society; has received funding for travel, serves on a speakers' bureau, and/or serves as a consultant to Biogen Idec, Teva Pharmaceutical Industries Ltd., EMD Serono, Inc., Pfizer Inc, Novartis, and sanofi-aventis; serves on the editorial board of aan.com; and receives research support from Biogen Idec, EMD Serono, Inc., Teva Pharmaceutical Industries Ltd., Cyberonics, Inc., and the National Multiple Sclerosis Society. Dr. Krupp has served on scientific advisory boards for Acorda Therapeutics Inc., Genentech, Inc., Pfizer Inc, and sanofi-aventis; has received funding for travel from Acorda Therapeutics Inc., Bayer Schering Pharma, Biogen Idec, EMD Serono, Inc., Genentech, Inc., and Teva Pharmaceutical Industries Ltd.; has received honoraria from Acorda Therapeutics Inc., Bayer Schering Pharma, EMD Serono, Inc., Biogen Idec, Teva Pharmaceutical Industries Ltd., Smith Kline Beecham, the Multiple Sclerosis Association of America, and the France Foundation; has received royalties for publication of Fatigue in Multiple Sclerosis (Demos, 2005); serves as consultant for Leerink Swan, Gerson Lehrman Group, and Guidepoint Global; serves on speakers' bureaus for Biogen Idec, Teva Pharmaceutical Industries Ltd., and EMD Serono, Inc.; receives research support from Teva Pharmaceutical Industries Ltd., Novartis, Genentech, the NIH (HD38107-02 [PI]), the National Multiple Sclerosis Society, and the Montel Williams Foundation; and has received license fee payments for a questionnaire she developed from the following companies: Eli Lilly and Company, MedImmune Vertex Pharmaceuticals Wyeth, ZymoGenetics, EPI-Q, Novartis, Genzyme Corporation, Tibotec Therapeutics, and Genentech, Inc. Dr. Waubant has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; and receives research support from Biogen Idec, Pfizer Inc, sanofi-aventis, the National MS Society, The Immune Tolerance Network, and the Nancy Davis Foundation.
Address correspondence and reprint requests to Dr. Dorothee Chabas, UCSF Regional Pediatric MS Center, 350 Parnassus Ave., Suite 908, San Francisco, CA 94117 dchabas@gmail.com
Study funding: The National Multiple Sclerosis Society sponsored the 6 pediatric MS centers that participated in this study. A UCSF unrestricted fund of private donors supported part of the statistical analysis.
Disclosure: Author disclosures are provided at the end of the article.
Received June 25, 2009. Accepted in final form November 5, 2009.
REFERENCES
- 1.Chabas D, Strober J, Waubant E. Pediatric multiple sclerosis. Curr Neurol Neurosci Rep 2008;8:434–441. [DOI] [PubMed] [Google Scholar]
- 2.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 2000;12:2407–2422. [DOI] [PubMed] [Google Scholar]
- 3.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology 2002;59:1224–1231. [DOI] [PubMed] [Google Scholar]
- 4.Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol 2007;6:773–781. [DOI] [PubMed] [Google Scholar]
- 5.Chabas D, Castillo-Trivino T, Mowry EM, Strober J, Glenn OA, Waubant E. Vanishing MS T2-bright lesions before puberty: a distinct MRI phenotype? Neurology 2008;71:1090–1093. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 7.Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005;62:865–870. [DOI] [PubMed] [Google Scholar]
- 8.Tintore M, Rovira A, Brieva L, et al. Isolated demyelinating syndromes: comparison of CSF oligoclonal bands and different MR imaging criteria to predict conversion to CDMS. Mult Scler 2001;7:359–363. [DOI] [PubMed] [Google Scholar]
- 9.Riikonen R. The role of infection and vaccination in the genesis of optic neuritis and multiple sclerosis in children. Acta Neurol Scand 1989;80:425–431. [DOI] [PubMed] [Google Scholar]
- 10.Hanefeld F, Bauer HJ, Christen HJ, Kruse B, Bruhn H, Frahm J. Multiple sclerosis in childhood: report of 15 cases. Brain Dev 1991;13:410–416. [DOI] [PubMed] [Google Scholar]
- 11.Ruggieri M, Polizzi A, Pavone L, Grimaldi LM. Multiple sclerosis in children under 6 years of age. Neurology 1999;53:478–484. [DOI] [PubMed] [Google Scholar]
- 12.Ghezzi A, Pozzilli C, Liguori M, et al. Prospective study of multiple sclerosis with early onset. Mult Scler 2002;8:115–118. [DOI] [PubMed] [Google Scholar]
- 13.Pohl D, Rostasy K, Reiber H, Hanefeld F. CSF characteristics in early-onset multiple sclerosis. Neurology 2004;63:1966–1967. [DOI] [PubMed] [Google Scholar]
- 14.Atzori M, Battistella PA, Perini P, et al. Clinical and diagnostic aspects of multiple sclerosis and acute monophasic encephalomyelitis in pediatric patients: a single centre prospective study. Mult Scler 2009;15:363–370. [DOI] [PubMed] [Google Scholar]
- 15.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68:S7–S12. [DOI] [PubMed] [Google Scholar]
- 16.Lee PA, Guo SS, Kulin HE. Age of puberty: data from the United States of America. APMIS 2001;109:81–88. [DOI] [PubMed] [Google Scholar]
- 17.Mazor SS, McNulty JE, Roosevelt GE. Interpretation of traumatic lumbar punctures: who can go home? Pediatrics 2003;111:525–528. [PubMed] [Google Scholar]
- 18.Zeman D, Adam P, Kalistova H, Sobek O, Andel J, Andel M. Cerebrospinal fluid cytologic findings in multiple sclerosis: a comparison between patient subgroups Acta Cytol 2001;45:51–59. [DOI] [PubMed] [Google Scholar]
- 19.Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol 2009;65:239–248. [DOI] [PubMed] [Google Scholar]


