Abstract
Proteomics investigations typically yield information regarding static gene expression profiles. The central issues that limit the study of proteome dynamics include how to (i) administer a labeled amino acid in vivo, (ii) measure the isotopic labeling of a protein(s) (which may be low), and (iii) reliably interpret the precursor/product labeling relationships. In this study, we demonstrate the potential of quantifying proteome dynamics by coupling the administration of stable isotopes with mass spectrometric assays. Although the direct administration of a labeled amino acid(s) is typically used to measure protein synthesis, we explain the application of labeled water, comparing 2H2O versus H218O for measuring albumin biosynthesis in vivo. This application emphasizes two distinct advantages of using labeled water over a labeled amino acid(s). First, in long term studies (e.g. days or weeks), it is not practical to continuously administer a labeled amino acid(s); however, in the presence of labeled water, organisms will generate labeled amino acids. Second, to calculate rates of protein synthesis in short term studies (e.g. hours), one must utilize a precursor/product labeling ratio; when using labeled water it is possible to reliably identify and easily measure the precursor labeling (i.e. water). We demonstrate that labeled water permits studies of protein synthesis (e.g. albumin synthesis in mice) during metabolic “steady-state” or “non-steady-state” conditions, i.e. integrating transitions between the fed and fasted state or during an acute perturbation (e.g. following a meal), respectively. We expect that the use of labeled water is applicable to wide scale investigations of proteome dynamics and can therein be used to obtain a functional image of gene expression in vivo.
Proteomics investigations typically yield information regarding static gene expression profiles; i.e. current “state-of-the-art” research programs lack measurements of proteome dynamics (1–3). This deficiency is unfortunate because the ability to measure rates of protein synthesis and breakdown will likely facilitate the identification of biomarkers of disease and yield novel insight regarding underlying homeostatic abnormalities (3, 4). For example, by measuring the concentration of circulating aminotransferase and the synthesis/secretion of albumin, one might be able to determine the degree of liver damage and assess whether hepatic function is compromised, respectively (5). Also, it should be possible to determine the influence of specific factors on the regulation of protein synthesis; e.g. does a therapeutic agent stimulate insulin biosynthesis?
Classic studies of protein biosynthesis have measured the incorporation of a labeled amino acid(s) into a protein(s) of interest and estimated a synthesis rate by using a “precursor/product labeling ratio” (6). Because modern proteomics technologies can rapidly separate and quantify individual proteins from complex mixtures, investigators have started to exploit the use of stable isotope tracers in mass spectrometry-based studies of proteome kinetics. However, the ability to study protein dynamics in vivo presents unique challenges (3, 4, 7–13); e.g. how does one (i) administer an isotope (typically a labeled amino acid) over a prolonged period and (ii) determine the true precursor labeling (because the amino acid will be rapidly turned over and its labeling will be diluted)? We have demonstrated how to quantify protein synthesis using 2H2O in vivo (10, 11); the advantages are that the tracer can be given orally, body water is a homogeneous pool with a relatively slow turnover, and the organism will continuously generate 2H-labeled amino acids (consequently one can study free living subjects, including humans (9, 11, 14)). The assumption of the method is that the equilibration between 2H in body water and a free amino acid(s) is faster than the rate of incorporation of an amino acid(s) into a newly made protein(s); preferably, the labeling of a free amino acid(s) should remain constant regardless of the metabolic status. We have validated that assumption by measuring the time-dependent labeling of alanine in vivo during the administration of 2H2O and by measuring the incorporation of 2H-labeled alanine into plasma albumin and total tissue proteins using gas chromatography-mass spectrometry methods (10, 11, 15). Subsequent reports support our observations (12, 13).
In this study, we demonstrate (as a model example) the application of our 2H2O-based approach for measuring albumin biosynthesis in vivo in mice during long term and short term investigations. Namely, we recently demonstrated how to obtain relatively precise measurements of mass isotopomer profiles of peptides and other relatively large molecules by developing a novel approach for integrating the data (16, 17). Our method allowed us to detect shifts in the isotope distribution profile of albumin-derived peptides from mice given 2H2O (17). In the current report, parallel studies examined the use of H218O because it offers potential advantages over 2H2O, especially during acute studies that involve perturbations such as consumption of a meal. For example, the cleavage of a protein will immediately add a labeled oxygen atom into the carboxyl group of a free amino acid; resonance effects will distribute the label over both carboxyl oxygens. Although repeated cleavage is required to achieve maximal labeling of both oxygens, cleavage of tRNA-bound amino acids will also contribute to the labeling of the carboxyl oxygen (18–21). The synthesis of a new protein(s) then results in the stable incorporation of 18O into the peptide bond; indeed, the oxygen in peptide bonds accounts for a majority of the total oxygen in a protein (18, 19), making it potentially easier to describe precursor/product labeling relationships (6). Finally, during the development of this work pitfalls were identified; thus we discuss strategies to circumvent potential problems.
MATERIALS AND METHODS
Supplies
Unless noted, chemicals and reagents were purchased from Sigma- Aldrich. 2H2O (99%) was purchased from Cambridge Isotopes (Andover, MA), and H218O (95%) was purchased from Isotec (Miamisburg, OH). Gas chromatography and mass spectrometry supplies were purchased from Agilent Technologies (Wilmington, DE). Sequencing grade trypsin (catalog number V5111) was purchased from Promega (Madison, WI).
Biological Methods
Male C57BL/6J mice (∼25 g) were purchased from The Jackson Laboratory (Bar Harbor, ME) and fed standard rodent chow for 5 days before initiating an experiment; mice were housed four or five per cage. The long term and short term experimental protocols were approved by, and conducted in compliance with the policies of, the Case Western Reserve University Institutional Animal Care and Use Committee.
In the long term studies, mice were randomized to receive an intraperitoneal bolus of 2H2O or H218O (14 μl of 2H2O or 12 μl of H218O/g of body weight) saline (9 g of solid NaCl dissolved/1000 ml of labeled water). Following the injection, mice were returned to their cages and allowed to eat and drink ad libitum. In the mice given 2H2O, the drinking water was enriched to twice that of body water (i.e. we expected that the priming bolus would achieve ∼2% 2H labeling; therefore the drinking water was labeled at 4% 2H2O), whereas in mice given H218O, the drinking water was maintained at 3.5% H218O. The rationale for labeling the drinking water more than the expected labeling of body water is based on previous studies (22, 23). For example, the labeling of 2H in body water can be diluted by several sources, including water intake/exchange via respiration and water generated during metabolism (i.e. the reduction of molecular oxygen). We expected that the dilution would be slightly greater for 18O versus 2H because oxygen labeling is diluted via the equilibration/exchange with CO2 in addition to the dilution factors noted above for 2H (14). Food intake, water consumption, and body weights were measured throughout the studies. Mice were killed at various points to obtain blood samples, which were collected in heparinized capillary tubes. Samples were centrifuged, and the plasma was frozen at −20 °C.
For the short term studies, mice were trained for 7 days to eat during a 2-h period of time (9 and 11 a.m.). On the day of the experiment, mice were given an intraperitoneal bolus of labeled water (21 or 25 μl of 2H2O or H218O/g of body weight to achieve ∼3% 2H or ∼3.5% 18O labeling in body water, respectively) immediately before being randomized to either a fasted or a fed group (9 and 11 a.m.). Mice were killed at various intervals after the feeding session (i.e. 45, 90, 180, and 360 min), and blood and liver samples were collected as described above. Note that n = 1 fed and n = 1 fasted mouse were killed at 45, 90, 180, and 360 min to determine the 2H labeling profiles of free amino acids, and n = 5 fed mice were killed at 360 min to quantify the 2H or the 18O labeling of plasma albumin. Finally, a group of control mice (n = 5) was killed to determine the natural isotopic background labeling.
Analytical Methods
2H Labeling of Body Water
The 2H labeling of body water was determined by exchange with acetone (24, 25). Briefly, 10 μl of sample or standard was incubated with 2 μl of 10 n NaOH and 4 μl of a 5% (v/v) solution of acetone in acetonitrile for 24 h. Acetone was extracted by addition of 600 μl of chloroform followed by addition of ∼0.5 g of Na2SO4. Samples were vigorously mixed, and a small aliquot of the chloroform was transferred to a GC1-MS vial.
Acetone was analyzed using an Agilent 5973N-MSD instrument equipped with an Agilent 6890 GC system. A DB-17MS capillary column (30 m × 0.25 mm × 0.25 μm) was used in all analyses. The temperature program was as follows: 60 °C initial, increase by 20 °C/min to 100 °C, increase by 50 °C/min to 220 °C, and hold for 1 min. The sample was injected at a split ratio of 40:1 with a helium flow of 1 ml/min. Acetone eluted at ∼1.5 min. The mass spectrometer was operated in the electron impact mode (70 eV). Selected ion monitoring of m/z 58 and 59 was performed using a dwell time of 10 ms per ion.
18O Labeling of Body Water
The 18O labeling of body water was determined following conversion to trimethylphosphate (TMP) as follows (26). Plasma or standards (5 μl) were added to 12 × 75-mm glass tubes and incubated with ∼3 mg of PCl5 to generate phosphoric acid, and samples were allowed to stand for 20 min. Next, 100 μl of trimethylsilyldiazomethane (Sigma-Aldrich) was added to the sample, which was allowed to stand for 30 min. TMP was extracted by addition of 100 μl of water and 300 μl of chloroform, samples were vigorously mixed, and a small aliquot of the chloroform was transferred to a GC-MS vial.
GC-MS analyses of the TMP derivative were performed using an Agilent 5973N-MSD instrument equipped with an Agilent 6890 GC system. A DB-17MS capillary column (30 m × 0.25 mm × 0.25 μm) was used in all analyses. The temperature program was as follows: 90 °C initial, increase by 30 °C/min to 240 °C, and hold for 1 min. The split ratio was 20:1 with helium flow at 1 ml/min. TMP eluted at ∼2.4 min. The 18O enrichment was determined using electron impact ionization (70 eV) and selected ion monitoring (10-ms dwell time) of m/z 140 and 142.
Gas Chromatography-Mass Spectrometry of Amino Acids
Liver samples (∼0.5 g) were homogenized by addition of 6% perchloroacetic acid (∼2.5 ml). The supernatant was run over an ion exchange column (AG 50W-X8 resin, hydrogen form). The column was first washed with water (∼10 ml), and amino acids were then eluted by washing with 3 m ammonium hydroxide (∼20 ml). To generate the tert-butyldimethylsilyl derivatives the effluent was evaporated to dryness and incubated with 200 μl of tert-butyldimethylsilyl trifluoroacetamide + 10% trimethylchlorosilane (Pierce) + 50 μl of acetonitrile at 100 °C for 90 min (27–29).
GC-MS analyses of the tert-butyldimethylsilyl derivatives were performed using an Agilent 5973N-MSD instrument equipped with an Agilent 6890 GC system (27). A DB-17MS capillary column (30 m × 0.25 mm × 0.25 μm) was used in all analyses. The temperature program was as follows: 70 °C initial, hold for 3 min, increase by 10 °C/min to 290 °C, and hold for 3 min. The split ratio was 10:1 with helium flow at 1 ml/min. The 2H enrichment of individual amino acids was determined using electron impact ionization (70 eV) and selected ion monitoring (10-ms dwell time). To ensure that a sufficient number of data points were collected across a given signal, a selected ion monitoring routine was used; the instrument was programmed to acquire a maximum of four ions per time window (10-ms dwell per ion).
Proteomics-based Assay of Albumin Biosynthesis
Albumin was isolated from plasma as described previously (10, 11). Briefly, 50 μl of plasma was treated with 500 μl of 10% trichloroacetic acid, samples were mixed well and centrifuged, and the supernatant was removed and discarded. Albumin was extracted following addition of 100% ethanol (200 μl). Following evaporation of the ethanol, the residue was dissolved in 25 μl of trypsin solution (i.e. 20 μg of trypsin in 0.5 ml of 25 mm ammonium bicarbonate buffer). Samples were incubated for 18 h at 40 °C, then evaporated to dryness, and redissolved in 0.1% trifluoroacetic acid. Peptide extracts were desalted using a ZipTipC18 (Millipore) and eluted with 2 μl of matrix solution (4 mg of α-cyanohydroxycinnamic acid dissolved in 1 ml of acetonitrile containing 20 μl of trifluoroacetic acid).
MALDI-TOF analyses were done using a prOTOF 2000 instrument (PerkinElmer Life Sciences). The data were acquired by taking 100 laser shots over 42 s; the instrument was programmed to take a six-point circle pattern and record two spectra per location. The acquisition parameters were as follows: laser energy, 75%; laser rate, 100 Hz; declustering voltage, 30 V; cooling flow, 190.0 ml/min; and record a range of 700–5000 Da in the reflectron mode.
We previously reported a novel data processing method that allows us to reliably quantify the isotopic labeling of peptides (16, 17); this method was used to process the spectra. Briefly, the isotope profiles of a given peptide are converted to an ASCII file, and the relative intensities are determined by fitting the data to a series of Gaussian peaks centered at 1-Da intervals using Origin software. Parameters of the fit are constrained to (i) the center of the monoisotopic peak and (ii) a common peak width. The background is allowed to vary during the iterations. The “best fit” areas are used to calculate the abundance of different isotopomer peaks.
Calculations
In mice given either 2H2O or H218O, excess labeling was calculated by normalizing the M1 or M2 isotopomer of a peptide against the M0 isotopomer, i.e. the isotopically substituted species (singly or doubly, respectively) versus the monoisotopic species, and then subtracting the mean background ratio(s) observed in control mice (i.e. mice that did not receive any isotope) (6). In long term studies, the fractional synthesis rate (FSR) was calculated from the exponential increase in protein labeling using the equation
where “t” is the time after exposure to 2H2O and “final labeling” equals the total labeling of a proteolytic peptide (which is influenced by the amino acid composition of the peptide and the equilibration of isotope in the respective amino acids) (6). When 2H2O was used, we modeled the change in the M1/M0 ratio, whereas when H218O was used, we modeled the change in the M2/M0 ratio.
In short term studies (e.g. following consumption of a meal), the FSR was calculated using the equation
where “product labeling8 h” represents the total labeling of a proteolytic peptide 8 h postinjection of the respective tracers (6). As noted earlier, the “precursor labeling” is influenced by the amino acid composition of the peptide and the equilibration of isotope in the respective amino acids. Although one can estimate the precursor labeling from the asymptotic (or steady-state) labeling of albumin that is obtained from a long term study, in cases where 2H2O is used acutely (especially in the fed state), it is difficult to predict the maximum labeling because 2H is incorporated into various carbon-bound positions of amino acids. Therefore, one requires knowledge regarding the equilibration constants between hydrogen in water and peptide-bound amino acids. We estimated the precursor labeling for 2H by integrating the labeling of the free amino acids that make up the peptide LGEYGFQNAILVR. However, when H218O is used, we expect one 18O atom to be stably bound per peptide bond and an additional one or two 18O atoms for certain amino acid side chains (e.g. glutamine or glutamate). We estimated the precursor labeling for 18O by counting the number of peptide bond oxygens and the side chain oxygens, i.e. 12 + 4 = 16.
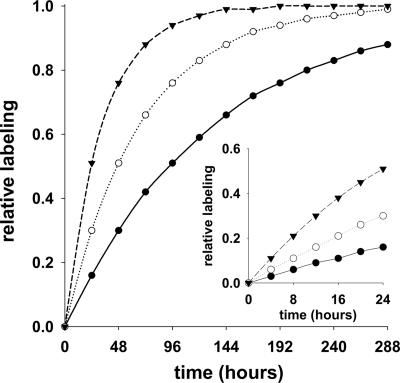
Note that to define a long versus a short term experiment one should consider the half-life of the protein relative to the experimental window (Fig. 1) (30). In the case of albumin, which has a half-life of ∼1.7–2.3 days in a mouse, the fractional synthesis rate (or the rate of change of the proportion of labeled protein) is expected to be ∼0.40–0.30/day (10, 15), i.e. the t½ = ln 2/k where k is the fractional rate constant. Given these values, the maximum proportion of labeled protein (i.e. the steady-state or asymptotic labeling) will be reached after ∼9–12 days of exposure to the tracer. Thus, in our experiments, which rely on Equation 2, “short term” is defined as those conditions in which we expect the change in albumin labeling to be (pseudo)linear, e.g. during the initial 8 h following administration of the tracer.
Fig. 1.
Time-dependent changes in protein labeling. A simulation of labeling profiles for given turnover constants was performed; a range of values was used including 0.0075/h (solid circles), 0.015/h (open circles)/ and 0.03/h (solid triangles). The main panel demonstrates the effect of the turnover constant on the time required to reach maximal (or steady-state) labeling. The inset demonstrates that the change in labeling is (pseudo)linear during the initial 24 h at all of the simulated fractional rate constants.
Statistics
Two-tailed t tests were performed in which we assumed equal variance. Unless noted, data are shown as mean ± S.E.
RESULTS
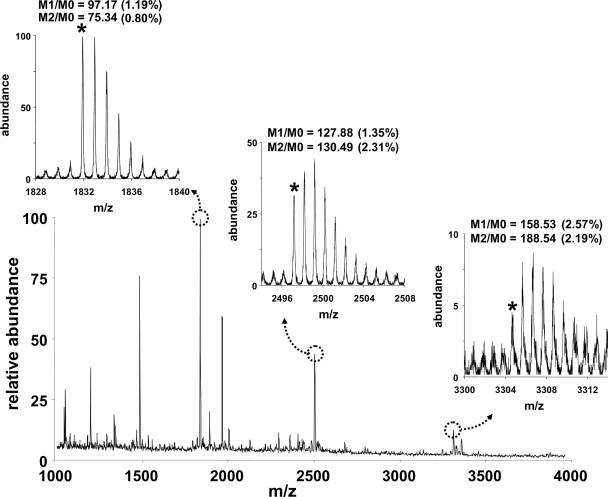
We recently demonstrated how to obtain relatively precise measurements of isotopic profiles of relatively large molecules (e.g. peptides) (16, 17). We considered whether the ionization mode(s) and mass analyzer(s) might affect the reproducibility of the measured isotope ratios. Although MALDI-TOF showed the greatest coefficient of variation, the error was still within an acceptable range for our application; i.e. we could quantify temporal changes in the isotopic distribution profiles of tryptic peptides from albumin using samples that were obtained from mice given 2H2O (17, 32). Fig. 2 demonstrates an example of the MALDI-TOF spectra of plasma albumin following tryptic digestion from a control mouse (which did not receive labeled water). Replicate analyses were performed to determine the precision of natural isotopic abundance distributions using peptides at different signal/noise ratios. Using peptides at m/z 1831.8, 2497.2, and 3304.4, we observed M1/M0 and M2/M0 ratios with coefficients of variation ≤2.6%. Because the peptide at m/z 1479.5 (i.e. LGEYGFQNAILVR (33)) was observed in all samples with precision comparable with that of the other peptides, we used its labeling to calculate rates of albumin synthesis. Although other approaches may offer still better precision of measured isotope ratios (17, 34), the level of reproducibility reported herein is sufficient for general studies of protein dynamics.
Fig. 2.
MALDI-TOF analyses of trypsin-digested albumin. Albumin was purified from plasma and digested using trypsin. MALDI-TOF spectra were acquired in the reflectron mode (positive ions), and replicate analyses were performed to determine the reproducibility of the peptide isotopomer profiles. The insets demonstrate examples of the isotope profiles of different peptides at different signal/noise ratios. The reproducibility of the measured isotope ratios is shown; the mean was determined for four replicate analyses (the coefficient of variation is included in parentheses). The M0 isotopomer is the monoisotopic mass and is marked with an asterisk; M1 and M2 refer to isotopomers with one and two substituted heavy atoms, respectively.
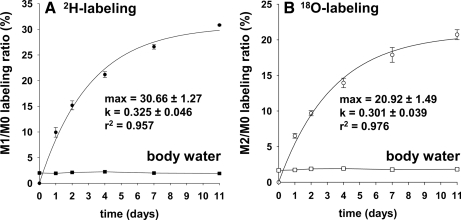
Fig. 3 demonstrates that a steady-state labeling of body water was achieved in long term studies, i.e. 2.03 ± 0.05% 2H and 1.79 ± 0.04% 18O. In our experience one can maintain these conditions for extended periods; e.g. some studies have run for >100 days.2 We typically found that the labeling of body water is ∼50% that of the drinking water because mice produce unlabeled water via endogenous metabolism, etc. The time-dependent labeling of peptide LGEYGFQNAILVR demonstrates comparable fractional rates of albumin synthesis (0.325 ± 0.046 versus 0.301 ± 0.039/day) but a greater absolute labeling in mice given 2H- versus 18O-labeled water (Fig. 3, A versus B, respectively). (Fig. 4 demonstrates the complete time-dependent shifts in the mass isotopomer profiles.) The difference in total peptide labeling was expected and relates in part to the fact that a higher labeling of body water was achieved in mice given 2H2O and in part to the fact that a larger number of 2H atoms are incorporated; e.g. several amino acids incorporated multiple copies of the precursor. For example, Table I demonstrates the time-dependent 2H labeling of intrahepatic free amino acids in fasted versus fed mice. As expected, we observed greater 2H labeling in most of the non-essential amino acids as compared with the essential amino acids, and although we observed a reasonable steady-state 2H labeling in fasted mice, there was a transient perturbation in the fed mice; i.e. the 2H labeling tended to be lower in fed versus fasted mice. This was true of virtually all amino acids and is consistent with an influx of unlabeled amino acids from dietary protein.
Fig. 3.
Time-dependent labeling of body water and incorporation of 2H or 18O into plasma albumin. MALDI-TOF analyses of albumin demonstrated an exponential increase in the labeling of peptide LGEYGFQNAILVR. A demonstrates the change in the M1/M0 ratio in mice given 2H2O, whereas B demonstrates the change in the M2/M0 ratio in mice given H218O for up to 11 days (mean ± S.E., n = 3 mice in each group per day). Note that the background (or endogenous) labeling has been subtracted. Data were best fit to a single exponential. In mice given 2H2O, the asymptotic labeling ratio of M1/M0 was 30.66 ± 1.27%, and the turnover constant was 0.325 ± 0.046 (r2 = 0.957); in mice given H218O; the asymptotic labeling ratio of M2/M0 was 20.92 ± 1.49%; and the turnover constant was 0.301 ± 0.039 (r2 = 0.976).
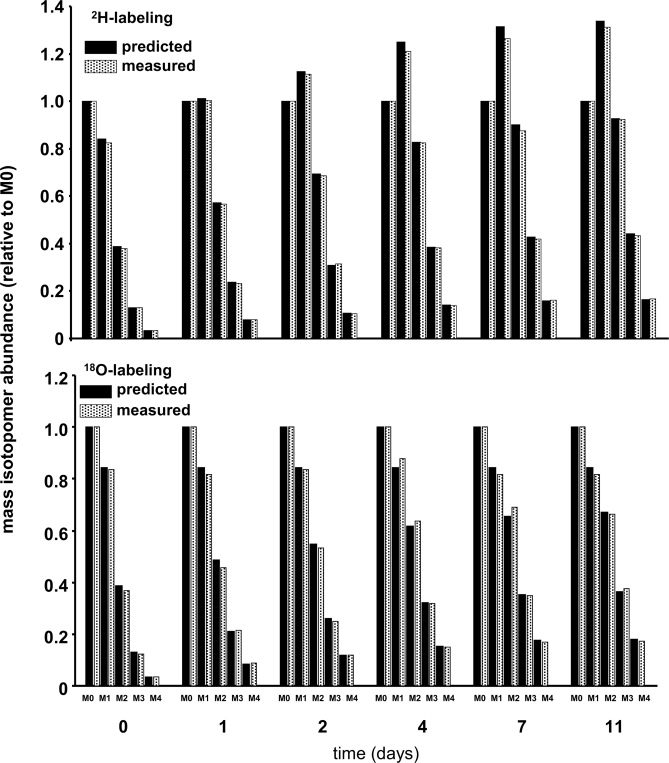
Fig. 4.
Time-dependent labeling of peptide LGEYGFQNAILVR. MALDI-TOF analyses of albumin peptide LGEYGFQNAILVR were performed. Top and bottom panels demonstrate the change in the isotopomer distribution profile in mice given 2H2O and H218O, respectively. The experimentally measured distributions are shown in solid bars. A simulation was run to predict the expected labeling profiles (open bars) assuming a fractional rate constant of 0.30/day. The 2H and 18O labeling in water were 2.03 and 1.79%, respectively. n was set to 25 for 2H and 16 for 18O. Note that the rationale for setting a value for n was based on experimental data in Table I when 2H2O was given (assuming an equilibration constant for arginine similar to that reported by Commerford et al. (37)) and using knowledge of biochemical pathways when H218O was given.
Table I. Time-dependent 2H labeling of individual amino acids.
Mice were given 2H2O and either fasted or fed. Blood and liver samples were collected at various time points (n = 1 mouse per time point) to determine the percentage of 2H labeling of water (in blood) and amino acids (in liver). The 2H labeling of body water was effectively constant over time, 3.18 ± 0.10% in fasted mice and 3.27 ± 0.08% in fed mice (mean ± S.E.). Data are shown as the mean ± S.E. for the analysis of amino acid standards (n = 4 replicate injections). The subscript numbers after an amino acid refer to the m/z signals used to quantify labeling (i.e. the M1/M0 ratio); these correspond to the [M − 57]+ ion cluster of the t-butyldimethylsilyl derivative and therefore contain all carbon-bound hydrogens. Data from individual fasted or fed mice are shown as percent “Excess 2H Labeling” after subtracting the background labeling. Note that “Arg” was included in the standard mixture but could not be detected, and although “Cys” and “Trp” could be detected in the standard mixture, they were not detected (ND) in the biological samples.
| Amino acid background labeling ((M1/M0) × 100) | Excess 2H labeling |
|||||||
|---|---|---|---|---|---|---|---|---|
| Time45 min |
Time90 min |
Time180 min |
Time360 min |
|||||
| Fasted | Fed | Fasted | Fed | Fasted | Fed | Fasted | Fed | |
| % | % | % | % | |||||
| Ala261/260 (22.94 ± 0.05) | 12.21 | 11.21 | 12.73 | 10.77 | 11.43 | 12.62 | 11.16 | 11.87 |
| Gly247/246 (22.59 ± 0.02) | 6.15 | 4.49 | 6.42 | 4.62 | 5.76 | 5.28 | 5.63 | 6.02 |
| Val289/288 (25.22 ± 0.03) | 1.22 | 0.68 | 1.27 | 0.75 | 1.14 | 0.87 | 1.11 | 0.99 |
| Leu303/302 (26.83 ± 0.05) | 1.89 | 0.52 | 1.97 | 1.37 | 1.77 | 1.70 | 1.73 | 1.49 |
| Ile303/302 (26.78 ± 0.02) | 2.05 | 1.58 | 2.14 | 1.64 | 1.92 | 1.32 | 1.88 | 1.55 |
| Pro287/286 (25.13 ± 0.05) | 2.34 | 1.44 | 2.44 | 2.10 | 2.19 | 1.96 | 2.14 | 2.43 |
| Ser391/390 (34.64 ± 0.09) | 2.85 | 1.65 | 2.97 | 1.74 | 2.67 | 1.69 | 2.61 | 2.27 |
| Thr405/404 (25.91 ± 0.07) | 0.54 | 0.18 | 0.57 | 0.20 | 0.51 | 0.37 | 0.50 | 0.38 |
| Met321/320 (25.63 ± 0.03) | 2.21 | 1.28 | 2.31 | 1.51 | 2.07 | 0.98 | 2.02 | 1.89 |
| Asp419/418 (35.89 ± 0.07) | 1.86 | 0.91 | 1.94 | 1.34 | 1.74 | 1.66 | 1.70 | 2.08 |
| Phe337/336 (29.41 ± 0.09) | 0.19 | 0.06 | 0.20 | 0.14 | 0.18 | 0.14 | 0.18 | 0.16 |
| Cys407/406 (35.32 ± 0.08) | ND | ND | ND | ND | ND | ND | ND | ND |
| Glu433/432 (37.74 ± 0.05) | 11.33 | 8.01 | 11.90 | 10.90 | 11.48 | 10.63 | 11.19 | 10.32 |
| Lys432/431 (39.27 ± 0.11) | 0.13 | 0.02 | 0.13 | 0.05 | 0.12 | 0.08 | 0.12 | 0.05 |
| Asn418/417 (36.06 ± 0.09) | 1.35 | 0.41 | 1.40 | 0.97 | 1.26 | 0.94 | 1.23 | 0.87 |
| Gln432/431 (37.82 ± 0.12) | 10.18 | 6.52 | 10.66 | 7.50 | 9.47 | 9.44 | 9.23 | 8.44 |
| Tyr467/466 (41.21 ± 0.09) | 0.54 | 0.27 | 0.57 | 0.29 | 0.51 | 0.38 | 0.50 | 0.43 |
| His441/440 (38.12 ± 0.10) | 4.10 | 2.93 | 4.06 | 2.47 | 3.86 | 3.08 | 3.78 | 2.99 |
| Trp456/455 (41.34 ± 0.09) | ND | ND | ND | ND | ND | ND | ND | ND |
Table II demonstrates the labeling of peptide LGEYGFQNAILVR in control mice and those given either 2H2O or H218O for 8 h. We observed 3.00 ± 0.28% excess 2H in M + 1 and 2.61 ± 0.37% excess 18O in M + 2. In mice given 2H2O, the precursor labeling was determined by fitting the labeling profiles of the respective amino acids (Table I). In mice given H218O, we assumed that the precursor labeling was equal to the 18O labeling of body water times the number of amino acids in the peptide minus one that is (back-)exchanged from the terminal amino acid during tryptic cleavage plus one additional 18O for asparagine and glutamine and two additional 18O atoms for glutamate. Because there is an amplification of the enrichment as labeled water is incorporated into amino acids, the estimated precursor labeling is ∼47.65% 2H1 and ∼52.29% 18O. We calculated rates of albumin synthesis equal to 6.30 ± 0.59 versus 4.96 ± 0.66% newly made albumin over 8 h in mice given 2H2O versus H218O, respectively (p = 0.17).
Table II. Isotopic labeling ratios of LGEYGFQNAILVR.
MALDI-TOF analyses of albumin were performed using samples from control mice (given an intraperitoneal bolus of saline) and mice given 2H2O or H218O, and samples were collected 8 h postinjection. Replicate analyses of each sample (n = 3 analyses per mouse) were performed following tryptic digestion, and data from each mouse were averaged and expressed as the mean ± S.E. per group (n = 5 mice per group). Note that the body water labeling was 3.11 ± 0.08% 2H and 3.29 ± 0.11% 18O. M0 refers to the monoisotopic form of the peptide, i.e. all 12C, 1H, 16O, 14N, etc., whereas M1 and M2 refer to peptide containing one or two substituted heavy isotopes, respectively.
| Experimental group | Isotopic labeling ratios of “LGEYGFQNAILVR” |
|
|---|---|---|
| M1/M0 × 100 | M2/M0 × 100 | |
| Control | 86.79 ± 0.57 | 41.73 ± 0.42 |
| 2H2O | 89.82 ± 0.28 | 44.61 ± 0.30 |
| H218O | 86.36 ± 0.85 | 44.35 ± 0.35 |
DISCUSSION
To use labeled water (e.g. 2H2O) to quantify protein synthesis, one assumes that the labeling of free amino acids is faster than their rate of incorporation into newly made proteins and that solvent-exchangeable sites (e.g. amino-bound hydrogens) will back-exchange with buffer during sample preparation; therefore, changes in the isotopic labeling profile of a peptide reflect the stable incorporation of 2H in carbon-hydrogen bonds. Note that in the presence of 2H2O both non-essential and essential amino acids will become 2H-labeled; thus, the labeling of a protein (or peptide) will reflect the labeling of virtually all amino acids. To predict the maximum labeling of a peptide one must have knowledge of the amino acid composition of that peptide and the equilibration constants for the respective amino acids. For example, it is expected that the total labeling of a peptide (i.e. the product) will be greater than that of body water (i.e. the precursor) because the 2H labeling in body water is copied multiple times in a given peptide; e.g. the incorporation of one alanine will bring approximately four carbon-bound 2H atoms (10–13). The same logic would apply when H218O is used; i.e. protein synthesis acts as a polymerization reaction, incorporating multiple copies of 18O from body water.
Since our initial reports regarding the use of 2H2O in studies of protein synthesis (17, 35), other investigators have also administered 2H2O and measured labeling profiles of proteins and/or peptides (33, 36). The work by Cabral et al. (36) is particularly elegant in that attention was directed toward determining the rise to steady-state labeling of glutathione; the fractional synthesis rate was determined by fitting the labeling curve. They noted that the asymptotic labeling, which reflects the precursor labeling, only reached ∼60% of the maximal (or theoretical) value. Namely, given the structures of the amino acids incorporated into glutathione, they expected that the steady-state labeling could be ∼10 times that of body water because there are 10 carbon-bound hydrogens. Cabral et al. (36) found that ∼6 of the hydrogens are labeled. This incomplete labeling of amino acids is not surprising because all positions may not equilibrate equally with hydrogen (or deuterium) in body water. For example, Commerford et al. (37) demonstrated heterogenous labeling of amino acids in rodents given 3H2O. In another recent report, Xiao et al. (33) measured the 2H labeling of albumin that was isolated from rats maintained on 2H2O for several days. They also concluded that one could use 2H2O to estimate protein biosynthesis by measuring changes in the average mass of a peptide. Although Xiao et al. (33) utilized a different mathematical approach as compared with Cabral et al. (36), their report is particularly interesting in that they observed comparable rates of albumin synthesis using three different tryptic peptides. Xiao et al. (33), like Cabral et al. (36), demonstrated that the apparent precursor labeling (or “n,” i.e. the number of exchangeable hydrogens) is lower than the theoretical maximum. Despite the fact that Xiao et al. (33) provide a clear explanation of the calculations, a major assumption with their approach centers on whether the “n” remains constant when metabolic and/or physiological conditions change, e.g. during feeding (Table I). For example, changes in the average mass of the peptide reflect (i) the labeling of body water, (ii) the number of hydrogens (“n”) that exchange with 2H, and (iii) the fractional synthesis rate. The approach taken by Xiao et al. (33) is valid in cases where “n” is constant (and/or known); otherwise, the average mass of the peptide reflects the combined changes caused by variable precursor labeling and protein synthesis. In total, the studies by Cabral et al. (36) and Xiao et al. (33) and those by our group (17, 32) support the hypothesis that it is possible to measure proteome dynamics in vivo using 2H2O (at least in studies that last several days); however, one must account for the variable 2H labeling of the different amino acids.
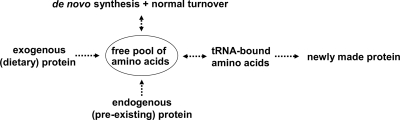
Contrary to amino acid flux in 2H2O, hydrolytic cleavage of peptide (amino acid) bonds and of tRNA-bound amino acids (back to free amino acids) in H218O results in the immediate 18O labeling of carboxyl oxygen (Fig. 5) (18–21). This is especially important in cases where one expects the rapid entry of unlabeled amino acids, e.g. during a meal. If peptides contain amino acids with side chain 18O atoms that are derived from water (e.g. aspartate and glutamate), one should consider the potential for non-homogeneous labeling; e.g. the oxygen in the peptide bond could be labeled to a greater degree than the oxygen in the amino acid side chain. The latter becomes labeled with the complete turnover of the amino acid and/or via de- and reamination reactions; e.g. deamination of glutamine in the presence of H218O will generate [γ-18O]glutamate, which generates [γ-18O]glutamine during reamination (we expect that asparagine and aspartate would undergo a similar series of reactions). Finally, because one expects that a single 18O atom is incorporated per peptide bond and because 18O should label all proteogenic amino acids equally well, assumptions regarding heterogeneity of the precursor “n” and its labeling stability should prove more reliable. In theory, this last point would make H218O a preferred tracer in short term studies of protein synthesis, i.e. in cases where the precursor labeling cannot be determined from the asymptotic labeling of the product (Equation 2 versus Equation 1).
Fig. 5.
Processes that affect amino acid labeling in presence of labeled water. Amino acids are generated via de novo synthesis pathways and/or during the degradation of existing proteins either of dietary origin or that are already present in the organism. In addition, tRNA-bound amino acids are reversibly cleaved back to free amino acids before incorporation into newly made proteins. The hypothesis is that 2H will primarily label amino acids via de novo synthesis and complete turnover, whereas 18O will label the oxygen incorporated in the peptide bonds via all pathways before incorporation into new proteins.
To examine the utility of using labeled water in studies of protein synthesis in vivo, we first initiated a chronic labeling study to measure albumin synthesis. Body water was maintained at steady-state 2H or 18O labeling for 11 days by simply giving a bolus injection of the respective tracer followed by the addition of that tracer to the drinking water. That we observed different asymptotic values for the labeling of peptide LGEYGFQNAILVR (Figs. 3 and 4) is expected because (i) we achieved slightly greater labeling of 2H in body water versus 18O and (ii) a larger number of 2H atoms are incorporated when 2H2O is used versus 18O when H218O is used. However, when the rise to steady state was modeled, we observed comparable fractional rate constants, i.e. ∼0.33 versus 0.30/day in 2H2O versus in H218O, respectively (Fig. 3). Those values are in agreement with the rate constant that we observed when we measured the incorporation of 2H-labeled alanine into plasma albumin over a similar period using gas chromatography-mass spectrometry methods (35). In addition, the values observed in mice are ∼10-fold greater than what we have observed in humans (given 2H2O) (11), which is expected because metabolic rates in mice are ∼10-fold greater than in humans. Taken together, these chronic labeling studies suggest that either type of labeled water can be used to quantify integrative rates of protein synthesis, i.e. in free living subjects over a prolonged period.
The ability to answer a question such as can one measure protein synthesis several hours after administering labeled water? requires precise measurements of the product labeling (to differentiate unlabeled from labeled peptides) and knowledge of the precursor labeling (see Equation 2). Because we (16, 17) and others (33, 36, 38) have demonstrated reasonable precision of the mass spectrometry-based measurements of peptides (Fig. 2), we examined the time-dependent changes in the 2H labeling of amino acids in the fasted versus the fed state (Table I). Several reactions can affect amino acid labeling, including (i) the de novo synthesis of an amino acid, (ii) the digestion of dietary protein, (iii) the degradation of existing endogenous proteins, and (iv) the hydrolysis of tRNA-bound amino acids (Fig. 5). Consistent with previous reports (37), we found that the number of exchangeable hydrogens varies considerably between the amino acids and that, in general, non-essential amino acids achieve a greater degree of labeling (as expected because 2H can be incorporated at multiple sites within a given amino acid during de novo synthesis and normal turnover). Some 2H labeling in essential amino acids is expected because they experience reversible metabolism; e.g. leucine undergoes transamination with α-ketoisocaproate (39). Finally, although the 2H labeling of body water is in rapid equilibrium with the carbon-bound hydrogens of free alanine (10, 13), which is consistent with its role as a central metabolic intermediate (40), this is apparently not true for all amino acids (Table I). The observation of a transient perturbation in the 2H labeling in fed versus fasted mice is consistent with what one expects because the digestion of dietary protein, and subsequent absorption of amino acids, will dilute the labeling of free amino acids. Therefore, the ability to measure protein synthesis via 2H2O during an acute challenge requires measurements of the time-dependent labeling of individual amino acids in the tissue where the protein of interest is being synthesized.
We suspect that our example represents a somewhat extreme scenario regarding changes in 2H labeling in the fed state (Table I). Namely, because mice were trained to consume their normal daily ration of food in a 2-h interval, a relatively large bolus of food entered the system. Also, we specifically focused attention on liver amino acids because we were interested in studying albumin synthesis. Because amino acids are absorbed and enter the systemic circulation through the portal vein, the first pass of diet-derived amino acids will primarily affect the 2H labeling of intrahepatic amino acids. Although knowledge of the equilibration can be obtained from long term studies (i.e. the asymptotic labeling of a peptide yields the precursor labeling), transient changes in amino acid labeling that occur with perturbations (e.g. eating), if unaccounted for, will lead to an underestimation of protein synthesis. By fitting the amino acid labeling profiles, it is possible to quantify albumin (protein) synthesis; i.e. by integrating the labeling curves for each amino acid, we could account for the non-steady-state amino acid labeling (6). Using those data we determined that 6.30 ± 0.59% of the plasma albumin was newly made within the 8 h during the acute perturbation in mice given 2H2O (Table II).
We hypothesized that although 2H labeling may be relatively slow for some amino acids 18O labeling of amino acids should be relatively fast for virtually all amino acids (18–21); thus, H218O might hold advantages over 2H2O. Namely, although the de novo synthesis of an amino acid should result in maximal 2H labeling, the generation of amino acids during protein breakdown (derived from endogenous or exogenous sources) requires extensive turnover of the unlabeled amino acids to achieve maximal 2H labeling; i.e. the release of unlabeled amino acids during protein breakdown will dilute the 2H labeling of newly made proteins if those amino acids do not rapidly turn over in the pool of intracellular amino acids. However, one expects immediate 18O labeling of free amino acids (Fig. 5). We did not attempt to measure the 18O labeling of the individual amino acids because some back-exchange of the label would likely occur during the sample preparation and therein confound the interpretation of the measurements (41, 42). Thus, to explain the protein synthesis data, we relied on knowledge of known pathways and enzymatic reaction mechanisms. Based on the chronic labeling study where we observed extensive incorporation of 18O into albumin (Figs. 3 and 4) and based on the logic outlined in Fig. 5, we expect that H218O should equilibrate more rapidly with amino acids as compared with 2H2O, and in most cases we expect that one 18O atom is incorporated per peptide bond (the exceptions being those amino acids that contain additional side chain oxygen atoms that are derived from water, i.e. asparagine, glutamine, and glutamate in peptide LGEYGFQNAILVR). As with mice given 2H2O, we detected the incorporation of 18O into LGEYGFQNAILVR after 8 h of exposure to H218O in the fed state (Table II); rates of albumin synthesis were estimated by comparing the total labeling in LGEYGFQNAILVR with the theoretical expected labeling (n = 16) and were found to be 4.96 ± 0.66%, effectively the same as in mice given 2H2O.
Calculated rates of protein synthesis are ultimately sensitive to the relative turnover time of the protein of interest and the time course of amino acid labeling. Our observations of comparable rates of protein synthesis in the presence of 2H2O or H218O are consistent with the classic study of Rittenberg and co-workers (18). Although we could quantify the incorporation of 2H and 18O into albumin within several hours, we suspect that H218O offers advantages over 2H2O, especially when considering equilibration time and copy number. To circumvent potential problems regarding slow equilibration of amino acid labeling, we expect that one can either measure the time-dependent labeling of all amino acids (as we did in the mice given 2H2O) or use tandem mass spectrometry. For example, in our previous studies we determined the labeling of specific peptide-bound amino acids by fragmenting peptides (17). Therefore, tandem mass spectrometry can be used to quantify the labeling of peptide-bound amino acids with rapid/known labeling constants, and/or one can quantify the labeling in peptide fragment(s) with minimal side chain oxygen; e.g. because the amino acid sequence AILVR of LGEYGFQNAILVR contains only peptide bond oxygen, the precursor/product labeling ratio can be estimated with a high degree of confidence by simply measuring the 18O labeling of body water. Finally, we expect that tandem mass spectrometry offers additional advantages in that one can better “purify” co-eluting peptides and improve the signal/noise ratio (17).
In summary, we demonstrated the ability to measure protein dynamics in vivo following the administration of either 2H2O or H218O (safe, non-radioactive isotopes), and we expect that it is possible to determine the rate of synthesis of virtually any protein using this approach. Because these tracers are relatively easy to administer and the sensitivity of the proteomics assays allows measurements using small samples, our method should be applicable over a wide range of conditions. Future studies that rely on the use of 2H2O should presumably determine (i) the time-dependent labeling of amino acids in other tissues, (ii) the impact of protein intake on rates of equilibration, and (iii) the impact of health versus disease on amino acid labeling, whereas studies that use H218O should be less influenced by the concerns noted above. Finally, we consider the application of labeled water relative to SILAC methods (4, 43, 44). For example, one advantage of SILAC methods is that they allow investigators to obtain higher levels of product labeling because it is possible to “completely” label an amino acid substrate (using a highly substituted stable isotope of an amino acid, e.g. [13C6]lysine or [13C6]arginine) with the end result being that investigators can achieve a relatively high signal over a relatively low background labeling. However, there are several points worth noting regarding the widespread use of the SILAC method for in vivo studies. First, it is not possible to contrast protein dynamics in fed versus fasted animals; in fact, because the tracer is administered via the diet, all studies must be done in fed animals. Second, it is not possible to readily modify the diet as an experimental variable; i.e. each diet needs to be customized by adding the labeled amino acid. Third, the SILAC methods do not achieve a steady-state labeling in the precursor pool; therefore, the obvious problem that arises is that estimates of protein synthesis will be lower than the true value as noted by Krüger et al. (43).
We conclude with a few general remarks regarding the possible widespread application of our 2H2O or H218O approaches in true proteomics investigations. First, we assume that the method(s) reported herein can easily be implemented at most research centers. For example, one simply needs to capitalize on existing mass spectrometry infrastructure. Second, we recognize that the demands of measuring the isotopic distribution profile of a single peptide conflict with the imperative of identifying the largest number of peptides, making general liquid chromatography-mass spectrometry protocols somewhat unsatisfactory for determining rates of protein synthesis. However, we have found that it is possible to determine the mass isotopomer profile of a peptide with sufficient precision using the “zoom scan mode” (on a Finnigan ion trap) with multiple scans encompassing the chromatographic peak of an entire peptide.2 Therefore, one might anticipate that the conflicting demands on the mass spectrometer acquisition parameters (i.e. full scan to identify peptides that are present and “zoom scan” on a desired peptide to precisely determine its labeling pattern) will limit protein turnover analyses to relatively smaller numbers of peptides/proteins than are present in the proteome. However, by generating a list of candidate peptides, it should be possible to determine water-based rates of protein turnover on multiple proteins for a typical liquid chromatography-mass spectrometry run. Alternatively, in cases where a complex matrix is obtained, the fractionation of protein classes or the isolation of targeted analytes can be used to enhance the application of this method (as demonstrated herein with a quick preparation of albumin). Last, we expect that it is possible to calculate rates of protein breakdown by coupling water-based rates of protein synthesis with existing strategies for quantifying protein abundance (31, 45–48). Namely, changes in protein content (which can be measured by a variety of techniques (31)) reflect the balance between protein synthesis and protein breakdown; as we have shown here, because it is possible to measure protein synthesis, investigators should be able to obtain a functional image of gene expression in vivo.
Acknowledgments
We thank Kathleen Lundberg (Case Western Reserve University Proteomics Center) for skillful assistance with the MALDI-TOF analyses.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grant R01-HL077637 (to M. J. P.). This work was also supported by Juvenile Diabetes Research Foundation Grant 5-2008-310.
2 V. E. Anderson, T. Kasumov, and S. F. Previs, unpublished observations.
1 The abbreviations used are:
- GC
- gas chromatography
- TMP
- trimethylphosphate
- FSR
- fractional synthesis rate
- SILAC
- stable isotope labeling by amino acids in cell culture.
REFERENCES
- 1.Anderson N. L., Polanski M., Pieper R., Gatlin T., Tirumalai R. S., Conrads T. P., Veenstra T. D., Adkins J. N., Pounds J. G., Fagan R., Lobley A. ( 2004) The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol. Cell. Proteomics 3, 311– 326 [DOI] [PubMed] [Google Scholar]
- 2.Beynon R. J., Pratt J. M. ( 2005) Metabolic labeling of proteins for proteomics. Mol. Cell. Proteomics 4, 857– 872 [DOI] [PubMed] [Google Scholar]
- 3.Pratt J. M., Petty J., Riba-Garcia I., Robertson D. H., Gaskell S. J., Oliver S. G., Beynon R. J. ( 2002) Dynamics of protein turnover, a missing dimension in proteomics. Mol. Cell. Proteomics 1, 579– 591 [DOI] [PubMed] [Google Scholar]
- 4.Andersen J. S., Lam Y. W., Leung A. K., Ong S. E., Lyon C. E., Lamond A. I., Mann M. ( 2005) Nucleolar proteome dynamics. Nature 433, 77– 83 [DOI] [PubMed] [Google Scholar]
- 5.Jahoor F., Jackson A. A. ( 1982) Hepatic function in rats with dietary-induced fatty liver, as measured by the uptake of indocyanine green. Br. J. Nutr 47, 391– 397 [DOI] [PubMed] [Google Scholar]
- 6.Wolfe R. R., Chinkes D. L. ( 2004) Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analyses, Wiley-Liss, New York [Google Scholar]
- 7.van Eijk H. M., Deutz N. E. ( 2003) Plasma protein synthesis measurements using a proteomics strategy. J Nutr 133, 2084S– 2089S [DOI] [PubMed] [Google Scholar]
- 8.Whitelegge J. P., Katz J. E., Pihakari K. A., Hale R., Aguilera R., Gómez S. M., Faull K. F., Vavilin D., Vermaas W. ( 2004) Subtle modification of isotope ratio proteomics; an integrated strategy for expression proteomics. Phytochemistry 65, 1507– 1515 [DOI] [PubMed] [Google Scholar]
- 9.Ussing H. H. ( 1941) The rate of protein renewal in mice and rats studied by means of heavy hydrogen. Acta Physiol. Scand 2, 209– 221 [Google Scholar]
- 10.Dufner D. A., Bederman I. R., Brunengraber D. Z., Rachdaoui N., Ismail-Beigi F., Siegfried B. A., Kimball S. R., Previs S. F. ( 2005) Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am. J. Physiol. Endocrinol. Metab 288, E1277– E1283 [DOI] [PubMed] [Google Scholar]
- 11.Previs S. F., Fatica R., Chandramouli V., Alexander J. C., Brunengraber H., Landau B. R. ( 2004) Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am. J. Physiol. Endocrinol. Metab 286, E665– E672 [DOI] [PubMed] [Google Scholar]
- 12.Busch R., Kim Y. K., Neese R. A., Schade-Serin V., Collins M., Awada M., Gardner J. L., Beysen C., Marino M. E., Misell L. M., Hellerstein M. K. ( 2006) Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim. Biophys. Acta 1760, 730– 744 [DOI] [PubMed] [Google Scholar]
- 13.Belloto E., Diraison F., Basset A., Allain G., Abdallah P., Beylot M. ( 2007) Determination of protein replacement rates by deuterated water: validation of underlying assumptions. Am. J. Physiol. Endocrinol. Metab 292, E1340– E1347 [DOI] [PubMed] [Google Scholar]
- 14.Bederman I. R., Dufner D. A., Alexander J. C., Previs S. F. ( 2006) Novel application of the “doubly labeled” water method: measuring CO2 production and the tissue-specific dynamics of lipid and protein in vivo. Am. J. Physiol. Endocrinol. Metab 290, E1048– E1056 [DOI] [PubMed] [Google Scholar]
- 15.Anderson S. R., Gilge D. A., Steiber A. L., Previs S. F. ( 2008) Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted vs. fed mice. Metab. Clin. Exp 57, 347– 354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassano A. G., Wang B., Anderson D. R., Previs S., Harris M. E., Anderson V. E. ( 2007) Inaccuracies in selected ion monitoring determination of isotope ratios obviated by profile acquisition: nucleotide 18O/16O measurements. Anal. Biochem 367, 28– 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Sun G., Anderson D. R., Jia M., Previs S., Anderson V. E. ( 2007) Isotopologue distributions of peptide product ions by tandem mass spectrometry: quantitation of low levels of deuterium incorporation. Anal. Biochem 367, 40– 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borek E., Ponticorvo L., Rittenberg D. ( 1958) Protein turnover in micro-organisms. Proc. Natl. Acad. Sci. U.S.A 44, 369– 374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernlohr R. W. ( 1972) Oxygen probes of protein turnover, amino acid transport, and protein synthesis in Bacillus licheniformis. J. Biol. Chem 247, 4893– 4899 [PubMed] [Google Scholar]
- 20.Fuller J. C., Jr., Nissen S. L., Huiatt T. W. ( 1993) Use of 18O-labelled leucine and phenylalanine to measure protein turnover in muscle cell cultures and possible futile cycling during aminoacylation. Biochem. J 294, 427– 433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernlohr R. W., Webster G. C. ( 1958) Transfer of oxygen-18 during amino acid activation. Arch. Biochem. Biophys 73, 276– 278 [DOI] [PubMed] [Google Scholar]
- 22.Brunengraber D. Z., McCabe B. J., Kasumov T., Alexander J. C., Chandramouli V., Previs S. F. ( 2003) Influence of diet on the modeling of adipose tissue triglycerides during growth. Am. J. Physiol. Endocrinol. Metab 285, E917– E925 [DOI] [PubMed] [Google Scholar]
- 23.McCabe B. J., Previs S. F. ( 2004) Using isotope tracers to study metabolism: application in mouse models. Metab. Eng 6, 25– 35 [DOI] [PubMed] [Google Scholar]
- 24.McCabe B. J., Bederman I. R., Croniger C., Millward C., Norment C., Previs S. F. ( 2006) Reproducibility of gas chromatography-mass spectrometry measurements of 2H labeling of water: application for measuring body composition in mice. Anal. Biochem 350, 171– 176 [DOI] [PubMed] [Google Scholar]
- 25.Yang D., Diraison F., Beylot M., Brunengraber D. Z., Samols M. A., Anderson V. E., Brunengraber H. ( 1998) Assay of low deuterium enrichment of water by isotopic exchange with [U-13C3]acetone and gas chromatography-mass spectrometry. Anal. Biochem 258, 315– 321 [DOI] [PubMed] [Google Scholar]
- 26.Brunengraber D. Z., McCabe B. J., Katanik J., Previs S. F. ( 2002) Gas chromatography-mass spectrometry assay of the (18)O-enrichment of water as trimethyl phosphate. Anal. Biochem 306, 278– 282 [DOI] [PubMed] [Google Scholar]
- 27.Patterson B. W., Carraro F., Wolfe R. R. ( 1993) Measurement of 15N enrichment in multiple amino acids and urea in a single analysis by gas chromatography/mass spectrometry. Biol. Mass. Spectrom 22, 518– 523 [DOI] [PubMed] [Google Scholar]
- 28.Gehrke C. W., Leimer K. ( 1970) Effect of solvents on derivatization using bis(trimethylsilyl)trifluoracetamide. J. Chromatogr 53, 201– 208 [DOI] [PubMed] [Google Scholar]
- 29.Gehrke C. W., Leimer K. ( 1971) Trimethylsilylation of amino acids: derivatization and chromatography. J. Chromatogr 57, 219– 238 [DOI] [PubMed] [Google Scholar]
- 30.Toffolo G., Foster D. M., Cobelli C. ( 1993) Estimation of protein fractional synthetic rate from tracer data. Am. J. Physiol. Endocrinol. Metab 264, E128– E135 [DOI] [PubMed] [Google Scholar]
- 31.Sechi S. ( 2007) Quantitative Proteomics by Mass Spectrometry, Humana Press, Totowa, NJ [Google Scholar]
- 32.Sun G., Wang B., Previs S. F., Anderson V. E. ( 2004) Simultaneous determination of multiple protein synthesis rates by in vivo deuterium labeling. J. Am. Soc. Mass Spectrom 15, 92S [Google Scholar]
- 33.Xiao G. G., Garg M., Lim S., Wong D., Go V. L., Lee W. N. ( 2008) Determination of protein synthesis in vivo using labeling from deuterated water and analysis of MALDI-TOF spectrum. J. Appl. Physiol 104, 828– 836 [DOI] [PubMed] [Google Scholar]
- 34.Fahey A. J., Messenger S. ( 2001) Isotopic ratio measurements by time-of-flight secondary ion mass spectrometry. Int. J. Mass Spectrom 208, 227– 242 [Google Scholar]
- 35.Previs S. F., Gilge D. A., Rachdaoui N. ( 2007) Protein and amino acid kinetics, in Clinical Diabetes Research: Methods and Techniques ( Roden M. ed) pp. 169– 187, J. Wiley and Sons, West Sussex, UK [Google Scholar]
- 36.Cabral C. B., Bullock K. H., Bischoff D. J., Tompkins R. G., Yu Y. M., Kelleher J. K. ( 2008) Estimating glutathione synthesis with deuterated water: a model for peptide biosynthesis. Anal. Biochem 379, 40– 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Commerford S. L., Carsten A. L., Cronkite E. P. ( 1983) The distribution of tritium among the amino acids of proteins obtained from mice exposed to tritiated water. Radiat. Res 94, 151– 155 [PubMed] [Google Scholar]
- 38.MacCoss M. J., Wu C. C., Matthews D. E., Yates J. R., 3rd ( 2005) Measurement of the isotope enrichment of stable isotope-labeled proteins using high-resolution mass spectra of peptides. Anal. Chem 77, 7646– 7653 [DOI] [PubMed] [Google Scholar]
- 39.Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. ( 1982) Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism 31, 1105– 1112 [DOI] [PubMed] [Google Scholar]
- 40.Yang R. D., Matthews D. E., Bier D. M., Lo C., Young V. R. ( 1984) Alanine kinetics in humans: influence of different isotopic tracers. Am. J. Physiol. Endocrinol. Metab 247, E634– E638 [DOI] [PubMed] [Google Scholar]
- 41.Murphy R. C., Clay K. L. ( 1990) Preparation of labeled molecules by exchange with oxygen-18 water. Methods Enzymol 193, 338– 348 [DOI] [PubMed] [Google Scholar]
- 42.Murphy R. C., Clay K. L. ( 1979) Synthesis and back exchange of 18O labeled amino acids for use as internal standards with mass spectrometry. Biomed. Mass Spectrom 6, 309– 314 [DOI] [PubMed] [Google Scholar]
- 43.Krüger M., Moser M., Ussar S., Thievessen I., Luber C. A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. ( 2008) SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 134, 353– 364 [DOI] [PubMed] [Google Scholar]
- 44.Doherty M. K., Hammond D. E., Clague M. J., Gaskell S. J., Beynon R. J. ( 2009) Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. J. Proteome Res 8, 104– 112 [DOI] [PubMed] [Google Scholar]
- 45.Previs M. J., VanBuren P., Begin K. J., Vigoreaux J. O., LeWinter M. M., Matthews D. E. ( 2008) Quantification of protein phosphorylation by liquid chromatography-mass spectrometry. Anal. Chem 80, 5864– 5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong S. E., Foster L. J., Mann M. ( 2003) Mass spectrometric-based approaches in quantitative proteomics. Methods 29, 124– 130 [DOI] [PubMed] [Google Scholar]
- 47.Gygi S. P., Rist B., Gerber S. A., Turecek F., Gelb M. H., Aebersold R. ( 1999) Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol 17, 994– 999 [DOI] [PubMed] [Google Scholar]
- 48.MacCoss M. J., Matthews D. E. ( 2005) Quantitative MS for proteomics: teaching a new dog old tricks. Anal. Chem 77, 294A– 302A [DOI] [PubMed] [Google Scholar]