Abstract
Although Parkinson disease (PD) is common throughout the world, the evidence for physical therapy interventions that enable long-term improvement in walking is still emerging. This article critiques the major physical therapy approaches related to gait rehabilitation in people with PD: compensatory strategies, motor skill learning, management of secondary sequelae, and education to optimize physical activity and reduce falls. The emphasis of this review is on gait specifically, although balance and falls are of direct importance to gait and are addressed in that context. Although the researchers who have provided the evidence for these approaches grounded their studies on different theoretical paradigms, each approach is argued to have a valid place in the comprehensive management of PD generally and of gait in particular. The optimal mix of interventions for each individual varies according to the stage of disease progression and the patient's preferred form of exercise, capacity for learning, and age.
Parkinson disease (PD) is a common disorder, especially among older adults. Based on an incidence rate of 16 to 19 per 100,000 per year, it is estimated that more than 2 million Americans, and 6 million people worldwide, are currently living with this progressive neurological condition.1 Movement disorders, and in particular gait disorders, are a hallmark of PD.2,3 The slow, short-stepped, shuffling, forward-stooped gait with asymmetrical arm swing is quickly recognizable to clinicians and varies according to the timing of assessment in the PD medication cycle.2–4 In addition to experiencing difficulties with the performance of well-learned movement sequences such as walking, turning, writing, and transfers, some people with PD report falls, cognitive impairment, and autonomic disturbances.5 Together, these problems can can affect quality of life and participation in societal roles.6
Physical therapist management of gait disorders in people with PD has 3 key elements. The first element is teaching the person how to move more easily and to maintain postural stability by using cognitive strategies. This is known as “strategy training” and targets the primary motor control deficit in the basal ganglia, brain stem, and motor cortex. There are 2 forms of strategy training: (1) compensatory strategies to bypass the defective basal ganglia and (2) learning strategies to improve performance through practice. The second element of physical therapy is the management of secondary sequelae affecting the musculoskeletal and cardiorespiratory systems that occur as a result of deconditioning, reduced physical activity, advanced age, and comorbid conditions. The third element is the promotion of physical activities that assist the person in making lifelong changes in exercise and physical activity habits as well as preventing falls. The researchers who have provided the evidence for these approaches have grounded their studies on different theoretical paradigms and studied targeted questions based on those paradigms. Nevertheless, each element has a valid place in the management of PD. In the clinical setting, physical therapists draw from each approach to provide comprehensive, client-centered care.
Measurement of gait-related outcomes includes a variety of perspectives. Included are the assessment of kinematics of gait (eg, stepping rate, stride length), assessment of functional factors (eg, 2- or 6-minute walk distance, ability to climb stairs), and assessment of factors associated with postural control that are closely related to gait (eg, incidence of falls, measures of balance control such as functional reach). These different outcomes have been used by researchers to better understand the effects of a variety of physical intervention approaches related to gait of people in early and middle stages of PD.
Strategy Training
Morris and colleagues2–4,7 provided some of the first evidence that movement strategies can assist people with PD to move, walk, and balance more easily. Their laboratory trials showed that external cues, such as white lines on the floor or a rhythmical beat provided by a metronome or music, enabled elderly people with moderate to severe PD to walk with longer steps and at a more normal stepping rate.4,8,9 These strategies assisted people to walk faster by compensating for the most common movement disorder: hypokinesia. Hypokinesia refers to reduced movement amplitude and speed and is seen as reduced step length and, in some individuals, alterations in the rate and timing of footsteps (cadence). Morris and colleagues3,7 demonstrated that many people with PD who are cognitively intact and do not have marked postural instability can immediately walk with long, fast steps simply by focusing their attention on walking with long steps, even when floor markers are absent.
Through bypassing the defective basal ganglia and instead using the frontal cortex to regulate movement size or timing by consciously thinking about the desired movement, people with PD arguably compensate for the neurotransmitter imbalance in the basal ganglia. Other strategies include visualizing walking with long steps, mentally rehearsing the desired movement pattern before the action is performed, breaking down long or complex motor sequences into parts and focusing on the performance of each individual segment (segmentation), avoiding dual task performance, reading instructions on a cue card, and verbally reciting phrases such as “think big” or “long steps.”2,3,9–13 This model is based on the theory that the ability to move normally is not lost in PD.2,3 Instead, there is an activation problem that can be overcome through targeted physical therapy together with optimal pharmacotherapy.14
Strategy training can be used either to compensate for movement disorders as just described or to assist people to learn methods to move more easily through carefully structured practice. So far, we have discussed how intact regions of the central nervous system have the potential to compensate for the defective basal ganglia via attention strategies or external cues. The randomized controlled trial by Morris et al7 showed that compensatory techniques can be beneficial, yet are sometimes associated with relatively short-term effects. They reported that a 2-week inpatient hospital program of twice-daily therapy of up to 45 minutes per session was effective in reducing disability and improving walking speed and balance, although gains were not fully maintained at a 3-month follow-up. One possible reason for the reversion toward baseline was the relatively small amount of practice. In addition, cognitively driven strategy training can require a lot of mental effort and can be fatiguing for some people. For this reason, therapists usually train people to use the cognitive strategies selectively, when they are needed for key functional tasks, rather than using them continuously throughout the day.2
There is growing evidence that, in the early stages of PD, there remains the capacity to learn new motor skills.15,16 For example, Behrman et al16 reported that the capacity to learn new upper-limb movement sequences was retained in people in the early to middle stages of PD. Subjects with PD and aged-matched comparison subjects repeatedly practiced a series of rapid arm-reaching tasks with different levels of movement complexity over several days.16 Fast performance of sequential targeting tasks improved with practice in both groups and was retained over 48 hours. Similarly, Canning et al15 showed that a multiple-task gait training program, combining walking and cognitive and manual activity practice, resulted in increased multiple-task walking speed in people with mild PD. This improvement in walking performance, achieved within only three 30-minute training sessions, was maintained over a 3-week retention period, suggesting that people with mild PD may have the capacity to learn how to walk under multiple-task conditions.
The message from these lines of evidence is that physical therapists should consider taking different approaches to strategy training according to disease severity. For newly diagnosed individuals and those with mild to moderate disease, it is recommended that therapists provide high-intensity, variable, distributed practice regimens with regular booster sessions over the longer term, with the aim of maximizing motor skill learning. How intensive and sustained the practice needs to be will vary according to the type and severity of movement disorders, the capacity of the person for learning, and whether there are coexisting conditions that limit the ability to practice.
As a guide, physical therapy for people with mild to moderate disease could incorporate practice daily up to 3 times per week, for periods of 6 to 8 weeks, until the motor skill is acquired. Bursts of therapy then could be provided 2 to 3 times per year to promote retention of training. For people who are more severely affected or those with cognitive impairment, very advanced age, or comorbidities that compromise skill acquisition, compensatory strategies are recommended. These strategies typically include repetition and drill practice of a given movement or action sequence, avoidance of multi-tasking, use of external cues and reminders, and segmentation of actions into simple components.2–4,7,17 The incidence of cognitive impairment is high in people whose disease severity is moderate to severe,18 adding weight to the notion that compensatory methods are likely to be more effective than motor skill learning approaches in this group.
Management of Musculoskeletal Sequelae
Schenkman and Butler19 were among the first investigators to propose that physical therapy interventions targeting sequelae such as weakness, loss of range, and reduced aerobic capacity could assist some people with PD to improved balance, gait, and function. This concept recognizes that people with PD can develop sequelae to the disorder that might contribute substantially to their difficulty with activities and participation in societal roles. By using physical therapy interventions to reduce the sequelae, it should be possible to improve function despite the primary central nervous system disorder affecting the basal ganglia. Schenkman and colleagues20–22 have conducted a number of laboratory experiments designed to test whether improved flexibility, muscle strength (force-generating capacity), and cardiovascular condition can improve task performance, including gait, postural control, and overall function. Not all of these studies focused on gait specifically. We contend that the findings are of importance because these factors are intimately related to gait. Studies are under way to measure outcomes of gait more specifically.23
It is well recognized that people with PD may have altered posture, including forward trunk flexion and lack of spinal extension range of motion.22,24 Schenkman and Butler19 proposed that loss of range of motion of axial structures (axial mobility) might contribute to loss of postural control, gait impairment, and decline in overall function. Laboratory experiments confirmed that trunk flexibility was associated with balance (measured by functional reach) and task performance.20–22 Exercises designed to improve axial range of motion were shown to improve functional reach distance.25 Furthermore, a secondary analysis of the data demonstrated nearly significant improvements in 6-minute walk distance for participants in the exercise program.25 Although axial flexibility alone might improve balance and gait to some degree, exercises that target balance, gait, and overall function could maximize the functional benefits from improved axial mobility.
Another line of work has focused on the benefits of strengthening exercises for people with PD. The degree to which people with PD are weak is under debate. Furthermore, it is unclear to what extent weakness is a consequence of deconditioning associated with living with a chronic progressive disease, coupled with aging, and how much of the weakness is related to altered central drive to muscles.26 What is well known is that loss of lower-extremity strength contributes to problems with balance, falls, and functional decline in older people.26,27 Several investigators have begun to explore the benefits of strength training. Hirsch et al28 carried out one of the first laboratory experiments, demonstrating improvements in lower-extremity strength and response to external perturbations. Dibble et al29 showed that a high-intensity eccentric quadriceps muscle strengthening program resulted in increased quadriceps muscle volume, improved 6-minute walk distance, and improved stair descent time. Dibble and colleagues30 also demonstrated the safety of the program. Although it is not clear whether the loss of strength is a sequel to PD, or associated with disuse or aging, it is apparent that lower-extremity strengthening exercise can benefit some people with PD, both in terms of better lower-extremity strength among exercisers compared with controls and in terms of balance and function.26
A third line of inquiry has focused on the cardiovascular system. As with loss of strength, cardiovascular decline can contribute to functional loss in older adults.31 Protas and colleagues32 measured cardiovascular function in people with PD, noting that 8 people in the early to middle stages were able to achieve maximum oxygen consumption comparable to that of 7 older adults without PD. The adults with PD used as much as 20% more oxygen to perform bicycling tasks than did the people without PD, indicating a reduced economy (or efficiency) of movement. These findings have been replicated in nearly 150 people with a walking task, showing that people with PD consume more oxygen than people without PD at every walking speed from 1 to 4 mph.33 Importantly, Schenkman and colleagues34 and other researchers35,36 have demonstrated that aerobic conditioning programs can lead to improvements in maximum oxygen consumption, economy of movement, 6-minute walk distance, and kinematics of gait, as well as overall function.
The message from all of these studies is that some people with mild to moderately severe PD can benefit from interventions that target flexibility, lower-extremity strength, and cardiovascular conditioning. These interventions may improve aspects of balance, gait, and overall functional ability, although further studies are needed to fully define the scope of benefits from each approach. Such exercise is important for people in early stages of PD to prevent sequelae that can interfere with function, to reverse sequelae early in the disease, and to prevent or reverse declines associated with disuse and aging itself. Nevertheless, it is neither realistic nor appropriate to think we can always reverse sequelae in later stages (eg, Hoehn and Yahr stage 3 and onward).
As a guide, individuals should exercise daily to 3 times per week, for periods of 6 to 12 weeks, to improve spinal flexibility, depending on the extent of loss of flexibility.25 They should exercise at least 3 times per week for 4 months to improve cardiovascular fitness.33 In order to achieve muscle strength gains, it is recommended that individuals train 2 or 3 days per week, completing 1 to 3 sets of each exercise using resistance loads equivalent to 8- to 12-repetition maximum, for a minimum of 6 weeks.37
Whichever approach to exercise is used, to sustain benefits, individuals should continue exercising at least a few times per week as part of their daily routine. They should be reassessed by a physical therapist at least annually in the early stages of the disease and more often in later stages of the disease to progress their exercise program.
Promoting Physical Activity and Preventing Falls
Because PD is a chronic progressive disorder, it is probable that sustained exercise is necessary to maintain benefits. Indeed, follow-up data from a number of human exercise interventions have demonstrated a gradual return to baseline abilities after the supervised intervention is finished.7,25,38
Results from several sources (including both animal models and information from humans) support the importance of vigorous exercise for people with PD and raise the question of whether such exercise might play a neuroprotective role. For example, Tillerson et al39 showed that motorized treadmill running twice daily for 10 days enhanced motor performance and brain neurochemistry in 2 different rat models of PD. Likewise, Dobrossy and Dunnett40 reported that rats that received motor training after striatal lesions or striatal grafts showed some recovery in spontaneous movements and skilled motor performance. Data from Fisher and colleagues41 indicate central effects can occur with exercise for people in the early stages of PD. Finally, Thacker and colleagues42 examined the impact of recreational physical activity on future risk of developing PD. Data were examined from 143,325 people who were followed for 8 years. The authors identified a reduced relative risk of developing the disease for those individuals who had reported moderate to vigorous activity at baseline. Although it is not yet clear whether exercise has a neuroprotective effect for people with PD, at a minimum, exercise does assist people to maintain functional ability. Taking all of the results together, we advocate the importance of enabling people with PD to make long-term adaptations to integrate physical activity into their daily lives. Furthermore, we advocate that vigorous exercise begin immediately on diagnosis, if possible, and continue throughout the course of the disease for as long as the individual is able to exercise.
Because weekly intervention with a physical therapist, throughout the entire course of PD, is neither realistic nor desirable, patients need to take responsibility for their physical activity and exercises. Methods have been developed, based on theories of behavior, for improving exercise habits. Strategies include exploration of the patient's beliefs about exercise and barriers to regular exercise and discussing the possibility of looking at things differently to change beliefs and overcome barriers.43–45 Together, the clinician and patient then establish reasonable goals that the patient thinks are attainable; they build on those goals as exercise habits improve. Regular follow-up appointments also are important (eg, monthly, quarterly, annually) to monitor progression and provide support to the patient.
Additionally, to assist patients to achieve a regular exercise regimen, we recommend determining how they prefer to exercise (eg, alone, in a group), where they prefer to exercise (eg, at home, in a community setting), and what type of exercise they prefer (eg, dancing; regular brisk walking, biking). Especially in the earlier stages of PD, any form of activity may be useful in maintaining gains made with specific, targeted, supervised exercise. For example, regular dancing is an attractive strategy for some people. Hackney and colleagues46,47 have shown improvements in balance with both Argentine tango and American ballroom dancing.
Adherence to a regular exercise regimen may be the most difficult challenge for the physical therapist and the patient. The physical therapist needs to shift roles from being the “doer” and “coach” (both of which may be necessary during the supervised interventions) to a role of “consultant” as the patient takes on the responsibility of maintaining activity. When patients embrace the importance of regular exercise, develop the necessary habits, and accept personal responsibility, optimal outcomes are more likely to occur. There is considerable information regarding exercise psychology for older adults.48,49 However, the area of exercise psychology in PD has not been studied extensively, and it cannot be assumed that all of the research in older adults is directly transferable to the PD population. Particularly troubling in this regard is the impact of non-motor signs of PD, such as depression, apathy, and lack of initiative, in many patients.50 Investigations are needed to establish the consequences of these impairments with regard to the patient's ability to adopt and adhere to a regular exercise regimen. We recommend that clinicians be alert to the possibility of such non-motor symptoms with their patients and take appropriate steps when possible to facilitate exercise habits even in the face of apathy. Clearly, this is an important area needing research.
As the disease progresses, falls frequently accompany gait disorders in PD. Thus, minimization of falls is a key goal of physical therapy for patients with locomotor dysfunction.2,3 Between 50% and 70% people with PD experience one or more falls over a 12-month period, which is much higher than the 30% fall rate reported for community-dwelling older people.51,52 Self-reporting is known to markedly underestimate the true fall rate in a range of conditions; thus, there is a need for therapists to look for other indicators of falls such as gait deviations, injuries, increased use of professional services, hospitalization, and reduced participation in societal roles.
Many of the falls in PD occur when people attempt to perform multiple tasks or long or complex movement sequences.2,3,8,9,53 Turning during walking is particularly problematic, as is carrying trays and other objects or walking at the same time as talking. Moreover, evidence is emerging that patients who experience freezing (an episodic inability to initiate or continue stepping) are particularly at risk of falls.14 This group is susceptible to multiple falls, with some individuals falling many times each week despite the best attempts to optimize medication.14 Health promotion activities that educate patients and their families about fall risk factors and how to prevent slips, trips, and falls, therefore, are integral to comprehensive physical therapy management of people with PD. Again, long-term adaptations by the patient are needed to integrate fall prevention strategies into their daily lives. It is important for the clinician to know when, where, and during what tasks the falls occur, as well as the direction.
Although population-based data provide a good guide, the physical therapist still needs to know the specific experience for each individual. Dibble et al54 conducted a systematic review of exercise-based interventions to improve balance in PD and determined that although there is moderate evidence to support the efficacy of exercise in improving postural instability and balance task performance, it remains unclear which specific types and dosages of exercise are optimal for the management of balance disorders in people with PD. However, with exercise interventions as diverse as treadmill training,38 spinal flexibility training,25 Qigong,36 muscle strengthening,55 Tai Chi,56 and tango dancing,46 each proving to be of some benefit to people with PD, it appears that an exercise program tailored to the individual's balance impairment, fall history, lifestyle, and personal interests may be preferable to a “one size fits all” approach.
Evidence Supporting the Efficacy of Physical Therapy for Gait Disorders
A previous systematic review of therapies for PD57 has been published, and Kwakkel et al58 published a subsequent critical review of the literature on physical therapy for PD. The systematic review produced equivocal results, having been performed at a time when few controlled trials of physical therapy for PD had been published. The review by Kwakkel et al58 identified 23 randomized controlled trials investigating the effects of physical therapy on function in people with PD. Only 3 of these studies targeted gait disorders.10,59,60 An additional 6 studies measured gait- and mobility-related outcomes from programs directed toward improving posture and balance.25,55,61–64 These studies were of moderate methodological quality and demonstrated some benefits of physical therapy for gait and mobility. The interventions tested and outcome measures used varied markedly, making between-study comparisons difficult. Interventions included cueing, mental rehearsal, exercises, and cycling. As suggested by Kwakkel et al,58 the quality of physical therapy research in PD has improved in the last decade, yet gaps in the evidence base for specific interventions remain.
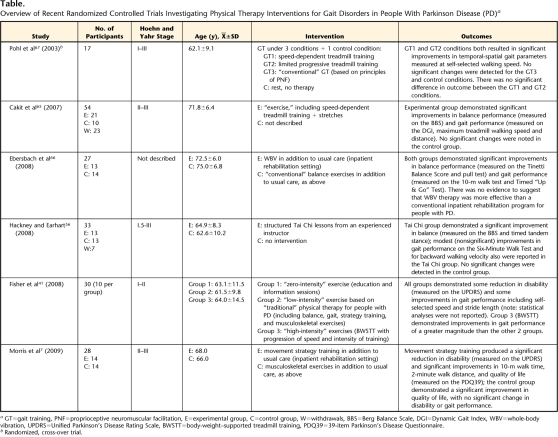
More recently, a number of alternative treatment modalities, such as Tai Chi, treadmill training, and whole-body vibration have been proposed for the management of gait disorders in people with PD.41,56,65–67 These treatment modalities were identified when we conducted a systematic search of the physical therapy literature, which identified an additional 6 randomized controlled trials (Table) published since Kwakkel and colleagues' review. Three of these studies investigated the effects of treadmill training, including high-intensity body-weight–supported training,41 and speed-dependent treadmill training, demonstrating positive results in the small samples tested.65,67 Similarly, whole-body vibration therapy66 and Tai Chi training56 have been reported to produce modest improvements in the gait and balance performance of people with PD. Although all of these studies included a comparison group, none provided control interventions founded on evidence-based “best practice” such as strategy training, cueing, or the management of musculoskeletal sequelae.
Table.
Overview of Recent Randomized Controlled Trials Investigating Physical Therapy Interventions for Gait Disorders in People With Parkinson Disease (PD)a
GT=gait training, PNF=proprioceptive neuromuscular facilitation, E=experimental group, C=control group, W=withdrawals, BBS=Berg Balance Scale, DGI=Dynamic Gait Index, WBV=whole-body vibration, UPDRS=Unified Parkinson's Disease Rating Scale, BWSTT=body-weight–supported treadmill training, PDQ39=39-item Parkinson's Disease Questionnaire.
b Randomized, cross-over trial.
Finally, assistive devices sometimes are prescribed for people with PD to improve gait or reduce falling. A small number of trials have shown up-turned walking sticks to be a useful visual cue for some people (see Morris and colleagues2,3,17 for summaries). In addition, wheeled walking frames sometimes can be of benefit for people with balance impairment and a high risk for falls, provided that the secondary task of maneuvering the frame does not compromise movement. Specific investigations are needed to measure the degree to which assistive devices optimize movement in people with PD, particularly in the more advanced stages of disease progression.
Conclusion
Comprehensive, client-centered physical therapy for people with PD is based on compensatory strategies to bypass the defective basal ganglia, strategies to improve motor learning and performance through practice, management of secondary sequelae affecting the musculoskeletal and cardiorespiratory systems, and fall education, as well as on assisting people to make lifelong changes in physical activity habits. The extent to which strategies, exercises, and health education are used varies according to individual needs and changes over time as the person ages and the disease progresses. Overall, the aim is to enable the person with PD to live well by providing effective physical therapy interventions at optimal times to promote health and well-being and by educating the individual regarding long-term self-management strategies.
Other articles in this issue relate to the promotion of movement in people with PD. For example, Kuo and Donelan68 discuss the principles of dynamic walking; Reisman et al69 distinguish between motor adaptation and motor learning, which is relevant to the interventions reviewed here; and Yogev-Seligmann et al70 and Kizony et al71 explore dual-task methods that are relevant for people with PD who have compromised cognitive performance and are trying to walk.
Footnotes
All authors provided concept/idea/project design, writing, and consultation (including review of manuscript before submission). Dr Morris and Dr Martin provided data collection and analysis and clerical support. Dr Morris provided project management, fund procurement, facilities/equipment, and institutional liaisons.
The authors gratefully acknowledge the support of the Michael J. Fox Foundation, the National Health and Medical Research Council of Australia, The Clinical Centre for Research Excellence in Gait Analysis and Gait Rehabilitation, and the National Institutes of Health (R01 HD043770–04).
References
- 1.Twelves D, Perkins KSM, Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord 2003;18:19–31 [DOI] [PubMed] [Google Scholar]
- 2.Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther 2000;80:578–597 [PubMed] [Google Scholar]
- 3.Morris ME. Locomotor training in people with Parkinson disease. Phys Ther 2006;86:1426–1435 [DOI] [PubMed] [Google Scholar]
- 4.Morris ME, Iansek R. Characteristics of motor disturbance in Parkinson's disease and strategies for movement rehabilitation. Hum Mov Sci 1996;15:649–669 [Google Scholar]
- 5.Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol 2008;64 (suppl 2): S65–S80 [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Verbaan D, van Rooden S, et al. A longitudinal evaluation of health-related quality of life of patients with Parkinson's disease. Value Health 2009;12:392–396 [DOI] [PubMed] [Google Scholar]
- 7.Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson's disease. Mov Disord 2009;24:64–71 [DOI] [PubMed] [Google Scholar]
- 8.Morris ME, Iansek R, Matyas T, Summers JL. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 1994;117:1169–1181 [DOI] [PubMed] [Google Scholar]
- 9.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease: normalization strategies and underlying mechanisms. Brain 1996;119:551–568 [DOI] [PubMed] [Google Scholar]
- 10.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2007;78:134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochester L, Hetherington V, Jones D, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson's disease. Arch Phys Med Rehabil 2005;86:999–1006 [DOI] [PubMed] [Google Scholar]
- 12.Farley B, Koshland G. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Exp Brain Res 2005;167:462–467 [DOI] [PubMed] [Google Scholar]
- 13.Lehman D. Training with verbal instructional cues results in near-term improvement of gait in people with Parkinson disease. J Neurol Phys Ther 2005;29:2–8 [DOI] [PubMed] [Google Scholar]
- 14.Morris ME, Huxham F, Menz HB, et al. Optimizing movement and preventing falls in Parkinson's disease: strategies for patients and caregivers. In: Trail M, Protas EJ, Lai EC. eds. Neurorehabilitation in Parkinson's Disease: An Evidence-Based Treatment Model Thorofare, NJ: Slack Inc; 2008:177–187 [Google Scholar]
- 15.Canning CG, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson's disease: a pilot study. Clin Rehabil 2008;22:226–233 [DOI] [PubMed] [Google Scholar]
- 16.Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson's disease. J Neurol Sci 2000;174:127–136 [DOI] [PubMed] [Google Scholar]
- 17.Morris ME, Iansek R, Galna B. Gait festination and freezing in Parkinson's disease: pathogenesis and rehabilitation. Mov Disord 2008;23(suppl 2): S451–S460 [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol 1997;244:2–8 [DOI] [PubMed] [Google Scholar]
- 19.Schenkman ML, Butler RB. A model for multisystem evaluation treatment of individuals with Parkinson's disease. Phys. Ther 1989;69:932–944 [DOI] [PubMed] [Google Scholar]
- 20.Schenkman ML, Morey M, Kuchibhatla M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson's disease. J Gerontol A Biol Sci Med Sci 2000;55:M441–M445 [DOI] [PubMed] [Google Scholar]
- 21.Schenkman ML, Shipp KM, Chandler J, et al. Relationships between mobility of axial structures and physical performance. Phys Ther 1996;76:276–285 [DOI] [PubMed] [Google Scholar]
- 22.Schenkman ML, Clark K, Xie T, et al. Spinal movement and performance of a standing reach task in participants with and without Parkinson disease. Phys Ther 2001;81:400–1411 [DOI] [PubMed] [Google Scholar]
- 23.Keus SJ, Munneke M, Nijkrake MJ, et al. Physical therapy in Parkinson's disease: evolution and future challenges. Mov Disord 2009;24:1–14 [DOI] [PubMed] [Google Scholar]
- 24.Bloem BR, Beckley DJ, van Dijk JG. Are automatic postural responses in patients with Parkinson's disease abnormal due to their stooped posture? Exp Brain Res 1999;124:481–488 [DOI] [PubMed] [Google Scholar]
- 25.Schenkman ML, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatr Soc 1998;46:1207–1216 [DOI] [PubMed] [Google Scholar]
- 26.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord 2008;23:1–11 [DOI] [PubMed] [Google Scholar]
- 27.Chandler J, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil 1998;79:24–30 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil 2003;84:1109–1117 [DOI] [PubMed] [Google Scholar]
- 29.Dibble LE, Hale TF, Marcus RL, et al. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord 2006;21:1444–1452 [DOI] [PubMed] [Google Scholar]
- 30.Dibble LE, Hale T, Marcus RL, et al. The safety and feasibility of high-force eccentric resistance exercise in persons with Parkinson's disease. Arch Phys Med Rehabil 2006:87:1280–1282 [DOI] [PubMed] [Google Scholar]
- 31.Morey MC, Pieper CF, Cornoni-Huntley J. Physical fitness and functional limitations in community dwelling older adults. Med Sci Sports Exerc 1998;30:715–723 [DOI] [PubMed] [Google Scholar]
- 32.Protas EJ, Stanley RK, Jankovic J, MacNeill B. Cardiovascular and metabolic responses to upper- and lower-extremity exercise in men with idiopathic Parkinson's disease. Phys Ther 1996;76:34–40 [DOI] [PubMed] [Google Scholar]
- 33.Christiansen CL, Schenkman ML, Kohrt WM, et al. Energy expenditure in people with Parkinson's disease during treadmill walking. Presented at: Combined Sections Meeting of the American Physical Therapy Association; February 9–12, 2009; Las Vegas, Nevada [Google Scholar]
- 34.Schenkman ML, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther 2008;88:63–76 [DOI] [PubMed] [Google Scholar]
- 35.Bergen JL, Toole T, Elliott RG, et al. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson's disease patients. NeuroRehabilitation 2002;17:1621–1628 [PubMed] [Google Scholar]
- 36.Burini D, Farabollini B, Iacucci S. A randomized controlled cross-over trial of aerobic training versus qigong in advanced Parkinson's disease. Europa Medicophysica 2006;4:231–238 [PubMed] [Google Scholar]
- 37.Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys Ther 2005;85:1208–1223 [PubMed] [Google Scholar]
- 38.Ellis T, Goede CJ, Feldman R, et al. Efficacy of a physical therapy program in patients with Parkinson's disease: a randomised control trial. Arch Phys Med Rehabil 2005;4:626–632 [DOI] [PubMed] [Google Scholar]
- 39.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neurosciences 2003;119:899–911 [DOI] [PubMed] [Google Scholar]
- 40.Dobrossy MD, Dunnett SB. Motor training effects on recovery of function after striatal lesions and striatal grafts. Exp Neurol 2003;184:274–284 [DOI] [PubMed] [Google Scholar]
- 41.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil 2008;89:1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord 2008;23:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalton CC. The concept of readiness to change. J Adv Nurs 2003;42:108–117 [DOI] [PubMed] [Google Scholar]
- 44.Jette AM, Lachman M, Giorgetti MM, et al. Exercise: it's never too late: the Strong-for-Life Program. Am J Public Health 1999;89:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol 2002;21:452–458 [PubMed] [Google Scholar]
- 46.Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J Neurol Phys Ther 2007;31:173–179 [DOI] [PubMed] [Google Scholar]
- 47.Hackney ME, Earhart G. Effects of dance movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. J Rehabil Med 2009;41:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAuley E, Jerome GJ, Marquez DX, et al. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med 2003;25:1–7 [DOI] [PubMed] [Google Scholar]
- 49.Rhodes RE, Martin AD, Taunton JE, et al. Factors associated with exercise adherence among older adults: an individual perspective. Sports Med 1999;28:397–411 [DOI] [PubMed] [Google Scholar]
- 50.Smith A, Nutt JG, Ransom BR. Parkinson's disease. Lancet 2004;363(9423):1783–1793 [DOI] [PubMed] [Google Scholar]
- 51.Bloem BR, Grimbergen YAM, Cramer M, et al. Prospective assessment of falls in Parkinson's disease. J Neurol 2001;248:950–958 [DOI] [PubMed] [Google Scholar]
- 52.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 2002;72:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris ME, Huxham F, McGinley J, et al. The biomechanics and motor control of gait in Parkinson's disease. Clin Geriatr Med 1996;12:825–845 [DOI] [PubMed] [Google Scholar]
- 54.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematc review across the disability spectrum. J Neurol Phys Ther 2009;33:14–26 [DOI] [PubMed] [Google Scholar]
- 55.Ashburn A, Fazakarley L, Ballinger C, et al. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait Posture 2008;28:456–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deane KHO, Ellis-Hill C, Jones D, et al. Systematic review of paramedical therapies for Parkinson's disease. Mov Disord 2002;17:984–991 [DOI] [PubMed] [Google Scholar]
- 58.Kwakkel G, de Goede CJT, van Wegen EEH. Impact of physical therapy for Parkinson's disease: a critical review of the literature. Parkinsonism Relat Disord 2007;13 (suppl 3): S478–S487 [DOI] [PubMed] [Google Scholar]
- 59.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil 2002;83:1370–1373 [DOI] [PubMed] [Google Scholar]
- 60.Thaut MH, McIntosh GC, Rice RR, et al. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Mov Disord 1996;11:193–200 [DOI] [PubMed] [Google Scholar]
- 61.Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson's disease: a controlled clinical trial. Neurology 1994; 44 (3 pt 1): 376–378 [DOI] [PubMed] [Google Scholar]
- 62.Marchese R, Diverio M, Zucchi F, et al. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord 2000;15:879–883 [DOI] [PubMed] [Google Scholar]
- 63.Müller V, Mohr B, Rosin R, et al. Short-term effects of behavioral treatment on movement initiation and postural control in Parkinson's disease: a controlled clinical study. Mov Disord 1997;12:306–314 [DOI] [PubMed] [Google Scholar]
- 64.Protas EJ, Mitchell K, Williams A, et al. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation 2005;20:183–190 [PubMed] [Google Scholar]
- 65.Cakit BD, Saracoglu M, Genc H, et al. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson's disease. Clin Rehabil 2007;21:698–705 [DOI] [PubMed] [Google Scholar]
- 66.Ebersbach G, Edler D, Kaufhold O, Wissel J. Whole body vibration versus conventional physiotherapy toimprove balance and gait in Parkinson's disease. Arch Phys Med Rehabil 2008;89:399–403 [DOI] [PubMed] [Google Scholar]
- 67.Pohl M, Rockstroh G, Rückriem S, et al. Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson's disease. Arch Phys Med Rehabil 2003;84:1760–1766 [DOI] [PubMed] [Google Scholar]
- 68.Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther 2010;90:157–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther 2010;90:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, et al. How does explicit prioritization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther 2010;90:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kizony R, Levin MF, Hughey L, et al. Cognitive load and dual-task performance during locomotion poststroke: a feasibility study using a functional virtual environment. Phys Ther 2010;90:252–260 [DOI] [PubMed] [Google Scholar]