Abstract
I identified a new locus responsible for increased yield potential and evaluated its physiological function to understand how to improve potential yield in rice (Oryza sativa) plants. Quantitative trait loci (QTLs) for 1,000-grain weight (TGW) were analyzed under different environments over 3 years in backcross inbred lines of rice japonica cv Nipponbare × indica cv Kasalath. Four QTLs for this trait were detected across environments; rice cv Kasalath had a positive allele only at one QTL on chromosome 6 (tgw6). A near-isogenic line (NILtgw6) that carried a rice cv Kasalath chromosomal segment corresponding to tgw6 in the rice cv Nipponbare genetic background was selected and analyzed to clarify the physiological function of this locus. The carbohydrate storage capacity before heading in NILtgw6 was superior to that in rice cv Nipponbare (control), but other characters (e.g. photosynthetic ability in flag leaf and traits related to plant type) were the same in both plants. In the leaf sheath, the main organ that accumulates carbohydrate before heading in rice, higher contents of carbohydrate and transcripts of genes related to starch synthesis were found in NILtgw6 than in rice cv Nipponbare. Compared with those in rice cv Nipponbare, a high-yield modern cultivar, TGW and yield per plant were significantly higher in NILtgw6, by 10% and 15%, respectively (P[f] < 0.01). These results suggest that tgw6 improves the carbohydrate storage capacity and consequently increases the yield potential in NILtgw6.
That increasing crop yield requires an improvement in carbohydrate production is now recognized and has become a key target in breeding (Mann, 1999a, 1999b). The carbohydrate translated to a sink organ originates from two sources: carbohydrate accumulated during the vegetative stage and photosynthate produced during the reproductive stage. Their proportion was estimated as 3:7 in rice (Oryza sativa) plants (Cook and Yoshida, 1972). To increase yield potential, researchers have tried various genetic or gene-engineering methods to improve photosynthetic ability per leaf area. Unfortunately, it has produced no effects on leaf photosynthesis that would be useful in improving yield potential in cereals (Dunwell, 2000; Horton, 2000). For improvement of photosynthetic ability of the canopy, traits related to plant type have been used as selection markers in most breeding programs (Rasmusson, 1991). The International Rice Research Institute developed a “new plant type” (NPT) under this strategy that has suitable plant height (80–100 cm) and erect leaves that optimize canopy photosynthesis (Duncan, 1971). However, because of poor grain filling, the grain yield of NPT is low. Therefore, another approach is needed to improve yield potential in rice plants (Horton, 2000). The lower carbohydrate storage capacity of NPT is the main reason for the inferior yield of lines with an optimized plant type (Peng et al., 1994).
Dingkuhn et al. (1991) proposed that improvement of the carbohydrate storage capacity before heading could contribute to higher yield in rice. The superior yield potential of japonica × indica F1 hybrid rice came from higher contents of accumulated carbohydrate before heading than in common cultivars (Song et al., 1990). There was a high positive correlation between carbohydrate accumulation and yield among 15 modern indica or japonica cultivars and F1 hybrids (Ishikawa et al., 1993).
A stem (leaf sheath and culm) works as a temporary sink that accumulates carbohydrate before heading and exports it after heading (Yoshida, 1972). The mechanism of carbohydrate storage in rice plants has been studied in leaf sheaths (Perez et al., 1971; Watanabe et al., 1997). Before heading, the second leaf sheath below the flag leaf (–2 leaf sheath) accumulated much higher starch than other leaf sheaths (Watanabe et al., 1997; Hirose et al., 1999). Starch branching enzyme I (BEI) or soluble starch synthase I (SS) catalyzes the elongation of α-1,4-glucosidic bonds on amylose under collaboration (Nakamura, 2002). On chromosome 6, SS and BEI were located at 11 centi-Morgans (cM) from the short arm and 1.1 cM below R1608, respectively (Nakamura et al., 1994; Tanaka et al., 1995). A high correlation was observed between starch content and their activities (Watanabe et al., 1997) and between starch content and the expression of these genes at the transcriptional level (Hirose et al., 1999). The function of carbohydrate storage in the leaf sheath and culm is controlled by complex mechanisms controlled by various genes (Perez et al., 1971). Therefore, the target for improving this capacity has been unclear.
Much information on the genetic relations between yield and agronomic traits of rice (e.g. plant height, photosynthetic ability) is available (Li et al., 1997; Ishimaru et al., 2001b, 2001c, 2001d; Xing et al., 2002). Xiao et al. (1998) identified a locus increasing 1,000-grain weight (TGW) by as much as 8.4% from a wild rice (Oryza rufipogon Griffith). Virtually the entire genomic sequence of a few plant species is now available (e.g. in rice reported by Sasaki et al., 2002), and consequently various DNA markers can be produced. With marker-assisted selection (MAS), allelic variations have been developed within rice (Yano, 2001), Arabidopsis (Nottingham Arabidopsis Stock Centre; http://nasc.nott.ac.uk/), and maize (Zea mays; MaizeDB; http://www.agron.missouri.edu/). With near-isogenic lines (NILs) in which a small chromosomal region is substituted with that of another line, we can characterize the locus underlying a quantitative trait locus (QTL; Lin et al., 2003).
The approach combining physiology with quantitative genetics is a powerful method for elucidation of the factor(s) determining a trait. With this approach, Limami et al. (2002) showed that the Gln synthetase multigene family plays a ubiquitous role in nitrogen metabolism throughout plant development in maize. Cong et al. (2002) clarified the mechanism determining fruit weight in tomato (Lycopersicon esculentum) by using physiological analyses with NILs for alleles at the locus for fw2.2, which accounts for as much as 30% of the difference in fruit size between wild and cultivated tomatoes.
In this paper, I attempted to identify a new locus responsible for increased yield in a high-yielding cultivar and to elucidate its physiological function to understand how to improve potential yield in rice. I analyzed QTLs for yield potential (TGW) and selected an NIL in which the chromosomal region of the targeted QTL was substituted with that of another line. With this NIL line, I clarified the function of the locus controlling TGW in rice plants.
RESULTS
Phenotypic Variation in BILs
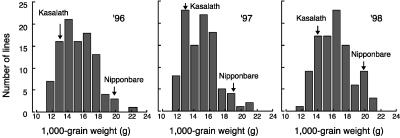
Transgressive segregants were observed in each year, and backcross inbred lines (BILs) showed continuous variation in TGW (Fig. 1). The average TGW of rice cv Nipponbare over 3 years was 20.6 ± 0.4 g, about 1.5 times that of rice cv Kasalath (13.5 ± 0.3 g). The correlation rate (r2) in TGW between 1996 and 1997, 1997 and 1998, and 1996 and 1998 was 0.726, 0.770, and 0.728, respectively (P[f] < 0.001).
Figure 1.
Frequency distributions of BILs for TGW in 1996, 1997, and 1998. Phenotypes of parents (rice cvs Nipponbare and Kasalath) are shown by arrows. Data are the means of six individual plants.
QTL Analysis for TGW over 3 Years
Putative QTLs associated with TGW were localized on a rice genetic map under three different environments for 3 years. QTLs controlling TGW were constantly detected on chromosomes 2, 5, 6, and 10, and each QTL had the same nearest marker throughout 3 years (Fig. 2; Table I). Individual QTLs explained between 7.7% and 18.7% of the total phenotypic variation (R2). On chromosome 11, the QTL for TGW was detected with a strong effect in 1997 and 1998 but not in 1996. One QTL each detected on chromosomes 2 and 6 had a major effect in each year (Table I). Rice cv Kasalath had a positive allele across environments only at a QTL on chromosome 6 (tentatively named tgw6). Other QTLs had positive alleles from rice cv Nipponbare. A comparison with QTLs for other traits derived from the same materials (Ishimaru et al., 2001b, 2001c, 2001d) showed that a QTL for TGW overlapped with QTLs for heading and grain number per panicle on chromosome 2 (Fig. 2). The QTL for heading and the QTL for TGW shared the same nearest marker. QTLs controlling the ratio of Rubisco to soluble protein or nitrogen content, the ratio of chlorophyll a to b content, and seed dormancy were localized near a QTL for TGW on chromosome 5. Among them, the QTL for seed dormancy had the same nearest marker as the QTL for TGW. QTLs controlling TGW did not overlap with QTLs for other traits that I have already reported (Ishimaru et al., 2001b, 2001c, 2001d) on other chromosomes. Similarly, the mapping positions of SS and BEI did not overlap QTLs for TGW.
Figure 2.
Linkage map of BILs showing the putative location of QTLs for TGW in 1996, 1997, and 1998. N and K mean the direction of phenotypic effect caused by rice cvs Nipponbare and Kasalath, respectively. QTLs for other traits were reported by Ishimaru et al. (2001a, 2001b, 2001c). Chl, Chlorophyll. The mapping positions of SS and BEI were reported by Tanaka et al. (1995) and Nakamura et al. (1994), respectively.
Table I.
QTLs controlling TGW in 1996, 1997, and 1998 (P[f] < 0.01)
| Year | Chromosome No. (QTL Name) | Nearest Marker | Likelihood Odds Ratio (LOD) | Sourcea | R2b |
|---|---|---|---|---|---|
| 1996 | 2 | C560 | 3.60 | N | 0.172 |
| 5 | R3166 | 2.00 | N | 0.077 | |
| 6(tgw6) | C358 | 2.96 | K | 0.121 | |
| 10 | R1629 | 2.73 | N | 0.127 | |
| 1997 | 2 | C560 | 3.74 | N | 0.167 |
| 5 | R3166 | 2.10 | N | 0.086 | |
| 6(tgw6) | C358 | 4.12 | K | 0.187 | |
| 10 | R1629 | 2.61 | N | 0.123 | |
| 11 | G257 | 2.86 | N | 0.141 | |
| 1998 | 2 | C560 | 3.99 | N | 0.165 |
| 5 | R3166 | 2.22 | N | 0.102 | |
| 6(tgw6) | C358 | 3.71 | K | 0.140 | |
| 10 | R1629 | 2.02 | N | 0.101 | |
| 11 | G257 | 3.56 | N | 0.121 |
a Source of high value for trait: N, Rice cv Nipponbare; K, rice cv Kasalath.
b Phenotypic variation explained by each QTL.
LOD Score of tgw6 on Chromosome 6
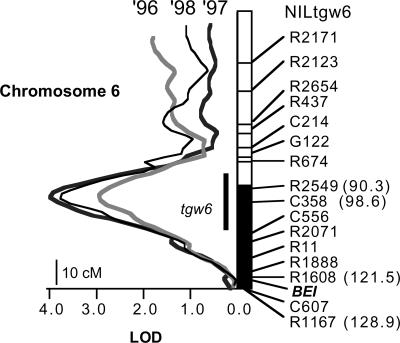
On chromosome 6, both the LOD scores of tgw6 and their peaks matched among years; tgw6 had the highest LOD score in a 100-cM region of the chromosome near C358 (Fig. 3). LOD scores for other QTLs also coincided among years (data not shown). There was a difference of 23.5 cM between the peak LOD score of tgw6 and the location of BEI. Among a series of rice NILs developed by Yano's group (Yano, 2001), I selected an NIL that carries a rice cv Kasalath chromosomal segment containing tgw6 under a rice cv Nipponbare genetic background (NILtgw6) by using MAS according to Lin et al. (2000). NILtgw6 was used for further biochemical and physiological analyses to identify a locus that increases yield in a high-yielding cultivar.
Figure 3.
LOD score of QTL for TGW on chromosome 6 in 3 years. NILtgw6 contains the chromosomal region from rice cv Kasalath (black bar), containing the region of tgw6 (black line) in the background of rice cv Nipponbare. The distance (cM) from the short arm to each marker is shown in parentheses. The position of BEI was reported by Nakamura et al. (1994).
Yield Potential and Amylose Content in NILtgw6
In NILtgw6, TGW was significantly higher by 10% than that in rice cv Nipponbare (P[f] < 0.01; Table II). The ratio of filled grains in NILtgw6 was significantly larger than in rice cv Nipponbare (P[f] < 0.01). Yield per plant was 15% higher in NILtgw6 than in rice cv Nipponbare (P[f] < 0.01). The panicle number per grain and the grain number per plant tended to be higher in NILtgw6 than in rice cv Nipponbare. The amylose content of grains was similar between NILtgw6 and rice cv Nipponbare. There was no difference in the structure of amylopectin in grains between plants (data not shown).
Table II.
Yield components and amylose content in grains of rice cv Nipponbare and NILtgw6
**, Significant difference at P(f) = 0.01. Data are means ± se of samples from 20 different plants.
| Plant Type | TGW | No. Panicle per Plant | Grain No. per Panicle | Ratio of Filled Grains | Yield per Plant | Amylose Content |
|---|---|---|---|---|---|---|
| g | % | g | % | |||
| Rice cv Nipponbare | 20.97 ± 0.15 | 15.90 ± 1.59 | 92.49 ± 1.89 | 84.60 ± 0.98 | 27.01 ± 0.80 | 22.8 ± 0.3 |
| NILtgw6 | 22.97 ± 0.19** | 18.21 ± 1.17 | 97.40 ± 2.36 | 87.04 ± 1.25** | 31.04 ± 0.08** | 22.8 ± 0.2 |
| (110%)a | (114%) | (105%) | (103%) | (115%) | (100%) |
a Percentage relative to rice cv Nipponbare.
Physical and Morphological Characters and Contents of Accumulated Carbohydrate in NILtgw6
The photosynthetic rate in flag leaves or the area of flag leaf was similar between NILtgw6 and rice cv Nipponbare (Table III). Plant height was 110.2 ± 0.7 cm in NILtgw6 and 110.2 ± 0.9 cm in rice cv Nipponbare. There was no difference in heading date or the length of the senescence stage between NILtgw6 and rice cv Nipponbare, as indicated by the decrease in chlorophyll content of flag leaves and in dark respiration rate (data not shown). Two days before heading, the content of accumulated starch was measured in the canopy and a –2 leaf sheath. In NILtgw6, the starch content was significantly higher by 18% as high as in rice cv Nipponbare canopy and by 80% than in Nipponbare –2 leaf sheath. The length of the –2 leaf sheath was 24.5 ± 0.4 cm in NILtgw6 and 24.8 ± 0.4 cm in rice cv Nipponbare.
Table III.
Photosynthetic rate and leaf area in flag leaves and the contents of accumulated starch in a canopy and — 2 leaf sheath before heading
**, *, Significant difference at P(f) = 0.01, 0.05, respectively. Data are means ± se of samples from 10 different plants.
| Plant Type | Photosynthetic Rate | Flag Leaf Area | Starch in a Canopy | Starch in — 2 Leaf Sheath |
|---|---|---|---|---|
| mmol CO2 | cm2 | μmol hexose | μmol hexose | |
| m-2 s-1 | g-1 dry wt | g-1 fresh wt | ||
| Rice cv Nipponbare | 21.2 ± 2.4 | 40.5 ± 2.1 | 152.2 ± 4.3 | 14.8 ± 1.8 |
| NILtgw6 | 20.6 ± 0.6 | 40.3 ± 3.2 | 180.8 ± 6.2* | 27.0 ± 4.2** |
| (97%)a | (100%) | (118%) | (182%) |
a Percentage relative to rice cv Nipponbare.
Northern-Blot Analysis of –2 Leaf Sheaths
The expression of genes for SS and BEI, key enzymes in starch synthesis in rice, was examined by northern-blot analysis of total RNA extracted from –2 leaf sheaths before heading in NILtgw6 and rice cv Nipponbare using 3′-untranslated regions of these genes as probes (Fig. 4). Three individual plants in rice cv Nipponbare or NILtgw6 were used for northern-blot analysis. mRNA for SS and BEI was detected in all samples. Higher levels of SS and BEI mRNA were detected in NILtgw6.
Figure 4.
Expression of genes related to starch synthesis—SS and BEI—in the –2 leaf sheath of rice cv Nipponbare (control) and NILtgw6. Total RNA (10 μg) from the –2 leaf sheath was used for northern-blot analysis. The ethidium bromide-stained gel of the same samples is shown at the bottom. Three individual plants in rice cv Nipponbare or NILtgw6 were used for this analysis.
DISCUSSION
I have identified a new locus that increased yield in a high-yielding cultivar and evaluated its physiological function in rice. A trait is determined by a combination of genetic and environmental factors (Tanksley, 1993; Sari-Gorla et al., 1997). To identify a gene for TGW across environments, I analyzed QTL for TGW for 3 years. Across environments, QTLs for TGW were detected consistently on chromosomes 2, 5, 6, and 10. Their LOD scores were exactly coincident in each year (e.g. on chromosome 6; Fig. 3), and they had the same positive allele (Table I). There was a high correlation in phenotypic variation in BILs over 3 years. These results show that the gene underlying each QTL might affect TGW across environments. Among QTLs for TGW, only tgw6 had a positive allele from rice cv Kasalath with a major effect in each year (Table I). Comparison of the chromosome locations showed that tgw6 did not overlap with other QTLs already reported by my group (e.g. plant height, heading date; Ishimaru et al., 2001b, 2001c, 2001d). Two other groups analyzed QTLs for grain weight by using various cross-combinations of rice cultivars and detected one in a similar region to tgw6 (Li et al., 1997; Xing et al., 2002). These results suggest that tgw6 might function broadly across various cultivars, and should therefore be the main target for identifying a gene that increases yield in high-yielding cultivars in this study. From the NILs produced by Yano's group (Yano, 2001), I selected NILtgw6, in which only the chromosomal region corresponding to tgw6 was substituted with that of rice cv Kasalath in a rice cv Nipponbare background with MAS (Fig. 3).
Among detected QTLs for TGW, four QTLs with the same nearest marker in each year were detected on chromosomes 2, 5, 6, and 10 (Fig. 2; Table I). Those controlling the ratio of filled grains were strongly affected by environment under the same conditions (K. Ishimaru, unpublished data). Among yield components (number of panicles per plant, number of grains per panicle, TGW, and ratio of filled grains), TGW is barely influenced by environmental changes, whereas the ratio of filled grains is most strongly influenced (Matsushima et al., 1966). These results suggest that the sensitivity of a trait to an environment might be explained by that of the genes controlling the trait, if QTLs are involved.
Compared with rice cv Nipponbare, a high-yielding cultivar (Saitou et al., 1993), TGW and yield per plant in NILtgw6 were significantly higher by 10% and 15%, respectively (Table II). Morphological traits (plant height, heading date, and period of senescence) were the same between NILtgw6 and rice cv Nipponbare (data not shown). These results show that tgw6 can increase yield potential in a high-yield cultivar rice without any effect on plant type, which has been the main target in breeding for high-yield rice. Xiao et al. (1998) identified a QTL that increased total yield in elite line by 26% from a wild cultivar (O. rufipogon), which was not localized on chromosome 6. These results indicate that the gene identified in this study differs from the gene underlying QTL reported by Xiao et al. (1998), and that plural genes might exist that would allow the barriers of yield potential in rice to be overcome. TGW of rice cv Kasalath is about 75% lower than that of rice cv Nipponbare (Fig. 1). Nevertheless rice cv Kasalath, a low-yield cultivar, contains a gene that can substantially increase the yield potential in rice cv Nipponbare, a high-yield cultivar. These results show the potential for using not only wild relatives but also modern cultivars to improve traits of agronomic importance.
Grain yield of rice is mainly determined by the sum of carbohydrate accumulated before heading and produced after heading. After heading, the flag leaf contributes strongly to grain filling (Cook and Evands, 1983), and its photosynthetic ability is determined by two main factors: photosynthetic ability per leaf area and leaf area (Mann, 1999a). The photosynthetic rate in flag leaves in NILtgw6 was the same as in rice cv Nipponbare (Table III). At 2 d before heading, the content of accumulated starch in NILtgw6 was significantly higher in the canopy and in the –2 leaf sheath, the main sink organ before heading, than in rice cv Nipponbare (Table III). After heading, the content of accumulated starch decreased to almost zero in the canopy and the –2 leaf sheath, and there was no difference in dark respiration between NILtgw6 and rice cv Nipponbare (data not shown). These results indicate that the greater accumulated carbohydrate in NILtgw6 might be translated into panicles and contribute to the higher yield than in rice cv Nipponbare. Our results support the proposal by Dingkuhn et al. (1991) that improvement of the carbohydrate storage capacity before heading could increase rice yield, presenting the route for improvement of yield potential.
Carbohydrate storage capacity is a target for improvement in lines with an optimized plant type (Peng et al., 1994). The locus identified in this study, tgw6, did not affect other traits of plant type besides TGW. Therefore, the introduction of tgw6 into new cultivars with optimal plant type like NPT may enhance the yield potential of rice plants. When photosynthesis is limited by adverse conditions such as drought after heading, the carbohydrate accumulated before heading becomes more important in grain filling (Gallagher et al., 1976). The improvement of carbohydrate accumulation capacity by tgw6 may contribute to stable yield independent of environmental factors.
To elucidate the physiological function of tgw6, I analyzed the characteristics of starch accumulation in NILtgw6 leaf sheaths, which accumulate carbohydrate before heading as a temporary sink organ. Before heading, the content of accumulated starch in NILtgw6 was 1.8 times as high as in rice cv Nipponbare (Table III). The morphological features of the –2 leaf sheaths did not differ between plants (data not shown). In starch synthesis in leaf sheaths, SS or BEI plays a rate-limiting role (Watanabe et al., 1997), and a high correlation was observed between the expression of these genes at the transcriptional level and starch content in the –2 leaf sheath (Hirose et al., 1999). In northern-blot analysis with the 3′-untranslated region of SS or BEI, the results showed higher transcriptional expression of SS and BEI in NILtgw6 than in rice cv Nipponbare (Fig. 4). Both SS and BEI were located on chromosome 6 (Nakamura et al., 1994; Tanaka et al., 1995), but tgw6 did not contain either (Fig. 2). NILtgw6 carries the chromosomal segment from rice cv Kasalath for the location of BEI. In progeny of a backcross of NILtgw6/rice cv Nipponbare containing rice cv Kasalath segments between R2549 and R2071, outside the location for BEI, on chromosome 6, their characters were similar to those of NILtgw6 (K. Ishimaru, unpublished data). These results suggest that the higher carbohydrate accumulation in NILtgw6 might be determined not by morphological alteration but by the superior sink capacity shown by the higher expression of related genes (SS and BEI) and, moreover, that tgw6 might be responsible for the higher expression of these genes in NILtgw6.
Starch is composed of two types of polysaccharide molecules, amylose and amylopectin, and amylose content and structure of amylopectin largely determine the taste of rice (Okuno et al., 1983). There was no difference in amylose content in grains between NILtgw6 and rice cv Nipponbare (Table II), and the structure of amylopectin was similar (data not shown). These results suggest that tgw6 could increase the yield of rice without reducing the quality or taste of grains and could have a high utility in breeding.
In this study, I have identified a locus (tgw6) that increased the yield of a high-yield rice cultivar, rice cv Nipponbare. Through analyses of its function, I conclude that tgw6 might improve carbohydrate storage and yield potential without any effects on plant type or grain quality. The results of this study suggest a high possibility of the increase of rice yield potential by the improvement of carbohydrate storage capacity with tgw6.
MATERIALS AND METHODS
Plant Materials for QTL Analysis
Ninety-eight BC1F5 lines (hereafter referred to as BILs) were developed from a backcross of rice (Oryza sativa) cvs Nipponbare/Kasalath//Nipponbare by the single-seed descent method at the National Institute of Agrobiological Sciences, Japan (http://www.rgrc.dna.affrc.go.jp). Seeds of these BILs and their two parental lines were sown at the beginning of May 1996, 1997, and 1998 in a greenhouse. The seedlings were transplanted at the beginning of June and were grown under natural conditions in Tsukuba (latitude 38°N) with three replications of 10 plants in a randomized complete design. Mean air temperature and solar radiation were measured in Tsukuba (Hayashi et al., 1998). In 1997, the mean air temperature in September was about 5°C lower and solar radiation was about 20% lower than in 1996 (20.4°C, 335.7 MJ m–2 in 1996). At other times, they were similar to values in 1996. In 1998, the mean temperatures were similar to those in 1996, although the solar radiation in September was 70% of that in 1996.
QTL Analysis for TGW and Selection and Growth Conditions of NILtgw6
Chromosomal locations of putative QTLs were determined by single-point analysis and interval analysis with QGENE (Nelson, 1997). I used a probability level of 0.01 as a threshold for detecting significant differences in a trait between the two genotypes (Ishimaru et al., 2001d). Among a series of NILs that were developed by Yano's group at the National Institute of Agrobiological Sciences (Yano, 2001), I selected NILtgw6, which carries the chromosomal segment from rice cv Kasalath for a QTL controlling TGW (tgw6) in the genetic background of rice cv Nipponbare, by using MAS. NILtgw6 and rice cv Nipponbare (control) were grown under natural conditions in Tsukuba with three replications of 20 plants in a randomized complete design in 2000.
Carbohydrate and Amylose Contents and Amylopectin Structure
Before heading (2 d before heading), –2 leaf sheaths in rice cv Nipponbare and NILtgw6 were sampled around noon, frozen immediately in liquid nitrogen, and stored at –80°C. At the same time, canopy in rice cv Nipponbare and NILtgw6 was sampled and dried at 80°C for 2 d. Samples of approximately 50 mg fresh weight in –2 leaf sheath or dry weight in canopy were powdered in liquid nitrogen in a mortar with a pestle and extracted twice with 80% (v/v) ethanol at 80°C. Each sample was centrifuged at 12,000g for 10 min. To determine starch content, the pellets were boiled in distilled water for 2 h and then digested with amyloglucosidase for 15 min at 55°C. Starch contents were measured enzymatically as described by Ishimaru et al. (2001a). All enzymes used in these procedures were obtained from Boehringer Mannheim GmbH (Mannheim, Germany). For chlorophyll measurements, one or two fresh leaf discs (0.28 cm2) were taken from the flag leaf and soaked in 1 mL of 96% (v/v) ethanol. Chlorophyll content was measured by the method of Wintermans and De Mots (1965). The contents of amylose were measured by the method of Okuno et al. (1983). The fine structure of amylopectin was determined by high-performance anion-exchange chromatography coupled to pulsed amperometric detection, as described by Nakamura et al. (1989).
Photosynthetic Activity and Dark Respiration Rate
The rates of photosynthetic CO2 assimilation and dark respiration were measured with a portable gas-exchange system (LI-6400, LI-COR, Lincoln, Nebraska). Measurements were made on intact flag leaf blades between 11 am and noon on September 18. Light was provided by an LED source (red/blue, 6400-02 LED source, LI-COR). For measurements of photosynthetic CO2 assimilation rates, the photon flux density was 1,200 μmol photons m–2 s–1, leaf temperature was 25°C, and the reference CO2 concentration was 350 μL L–1.
Northern-Blot Analysis of SS and BEI in –2 Leaf Sheaths
Samples of approximately 50 mg in mass were powdered in liquid nitrogen with a mortar and pestle and mixed in 2× cetyl-trimethyl-ammonium bromide solution. For northern-blot analysis, total RNA was isolated as described by Ishimaru et al. (1998). Total RNA of 10 μg lane–1, was separated on a 1.15% (w/v) agarose gel containing formaldehyde, transferred to a nylon membrane (Hybond-N, Amersham Biosciences, Buckinghamshire, UK), and hybridized with [32P]cDNA probes. The 3′-untranslated region of BEI (Nakamura et al., 1994; D38221) or SS (Tanaka et al., 1995; D16202) was used for northern-blot analysis. The hybridized membrane was washed twice with 2× SSC (150 mm NaCl and 15 mm sodium citrate) plus 0.1% (w/v) SDS at room temperature for 5 min and with 0.2× SSC plus 0.1% (w/v) SDS at 55°C for 1 h, and then visualized in a Bio Imaging System (BAS 2000, Fujix, Tokyo).
Acknowledgments
I thank Dr. M. Yano (National Institute of Agrobiological Sciences) for giving rice materials and kind suggestions and Dr. S. Fukuoka (National Institute of Agrobiological Sciences) and Prof. Y. Nakamura (Akita Prefectural University), for measuring amylose contents and doing the high-performance anion-exchange chromatography coupled to pulsed amperometric detection analyses. I also thank the staff of the Farm Management Division, National Institute of Agrobiological Sciences (Japan), for their care of the plants used in these experiments.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027607.
This work was supported in part by a Grant-in-Aid (Bio Cosmos Program) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
References
- Cong B, Liu J, Tanksley SD (2002) Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc Natl Acad Sci USA 99: 13606–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JH, Yoshida S (1972) Accumulation of 14C-labelled carbohydrate before flowering and its subsequent redistribution and respiration in the rice plant. Proc Crop Sci Soc Jpn 41: 226–234 [Google Scholar]
- Cook MG, Evands LT (1983) Some physiological aspects of the domestication and improvement of rice (ORYZA spp.). Field Crops Res 6: 219–238 [Google Scholar]
- Dingkuhn M, Penning de Vries FWT, de Datta SK, van Laar HH (1991) Concepts for a New Plant Type for Direct Seeded Flooded Tropical Rice. International Rice Research Institute, Los Baños, Philippines, pp 17–38
- Duncan W (1971) Leaf angle, leaf area and canopy photosynthesis. Crop Sci 11: 482–485 [Google Scholar]
- Dunwell JM (2000) Transgenic approaches to crop improvement. J Exp Bot 51: 487–496 [DOI] [PubMed] [Google Scholar]
- Gallagher JN, Biscoe PV, Hunter B (1976) Effects of drought on grain growth. Nature 264: 541–542 [Google Scholar]
- Hayashi Y, Toritani H, Goto S, Yokozawa M, Seino H (1998) Climatic Recorders of the National Institute of Agro-Environmental Sciences, Vol. 23. Miscellaneous Publication of the National Institute of Agro-Environmental Sciences, Tsukuba, Japan, pp 53–60 [Google Scholar]
- Hirose T, Endler A, Ohsugi R (1999) Gene expression of enzymes for starch and sucrose metabolism and transport in leaf sheaths of rice (Oryza sativa L.) during the heading period in relation to the sink to source transition. Plant Prod Sci 2: 178–183 [Google Scholar]
- Horton P (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51: 475–485 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Akita S, Li Q (1993) Relationship between contents of non-structural carbohydrates before panicle initiation stage and grain yield in rice (Oryza sativa L.). Jpn J Crop Sci 62: 130–131 [Google Scholar]
- Ishimaru K, Hirose T, Aoki N, Takahashi S, Ono K, Yamamoto S, Wu J, Saji S, Baba T, Ugaki M et al. (2001a) Antisense expression of a rice sucrose transporter OsSUT1 in rice (Oryza sativa L.). Plant Cell Physiol 42: 1181–1185 [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Kobayashi N, Ono K, Yano M, Ohsugi R (2001b) Are contents of Rubisco, soluble protein and nitrogen in flag leaves of rice controlled by the same genetics? J Exp Bot 52: 1827–1833 [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Ohkawa Y, Ishige T, Tobias DJ, Ohsugi R (1998) Elevated pyruvate, orthophosphate dikinase (PPDK) activity alters carbon metabolism in C3 transgenic potatoes with a C4 maize PPDK gene. Physiol Plant 103: 340–346 [Google Scholar]
- Ishimaru K, Shirota K, Higa M, Kawamitsu Y (2001c) Identification of quantitative trait loci for adaxial and abaxial stomatal frequencies in Oryza sativa. Plant Physiol Biochem 39: 173–177 [Google Scholar]
- Ishimaru K, Yano M, Aoki N, Ono K, Hirose T, Lin SY, Monna L, Sasaki T, Ohsugi R (2001d) Toward the mapping of physiological and agronomic characters on a rice function map: QTL analysis and comparison between QTLs and expressed sequence tags. Theor Appl Genet 102: 793–800 [Google Scholar]
- Li ZK, Pinson SRM, Park WD, Paterson AH, Stansel JW (1997) Epistatic for three grain yield components in rice (Oryza sativa L.). Genetics 145: 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limami AM, Rouillon C, Glevarec G, Gallais A, Hirel B (2002) Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase. Plant Physiol 130: 1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HX, Liang ZW, Sasaki T, Yano M (2003) Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice. Breed Sci 53: 51–59 [Google Scholar]
- Lin HX, Yamamoto T, Sasaki T, Yano M (2000) Characterization and detection of epistatic interaction of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theor Appl Genet 101: 1021–1028 [Google Scholar]
- Mann CC (1999a) Crop scientists seek a new revolution. Science 283: 310–314 [Google Scholar]
- Mann CC (1999b) Genetic engineers aim to soup up crop photosynthesis. Science 283: 314–316 [DOI] [PubMed] [Google Scholar]
- Matsushima S, Wada G, Matsuzaki A (1966) Analysis of yield-determining process and its application to yield-prediction and culture improvement of lowland rice. Proc Crop Sci Soc Jpn 34: 321–328 [Google Scholar]
- Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43: 718–725 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nagamura Y, Kurata N, Minobe Y (1994) Linkage localization of the starch branching enzyme I (Q-enzyme I) gene in rice. Theor Appl Genet 89: 859–860 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yuki K, Park SY, Ohya T (1989) Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol 30: 833–839 [Google Scholar]
- Nelson JC (1997) QGENE: software for marker-based genomics analysis and breeding. Mol Breed 3: 239–245 [Google Scholar]
- Okuno K, Fuwa H, Yano M (1983) A new mutant gene lowering amylose content in endosperm starch of rice, Oryza sativa L. Jpn J Breed 39: 387–394 [Google Scholar]
- Peng S, Khush KG, Cassman KG (1994) Evolution of a new plant ideotype for increased yield potential. In KG Cassman, ed, Proceedings of a Workshop on Rice Yield Potential in Favorable Environments. International Rice Research Institute, Los Baños, Philippines, pp 5–20
- Perez CM, Palmiano EP, Baun LC, Juliano BO (1971) Starch metabolism in the leaf sheaths and culm of rice. Plant Physiol 47: 404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson DC (1991) A plant breeder's experience with ideotype breeding. Field Crop Res 26: 191–200 [Google Scholar]
- Saitou K, Shimoda H, Ishihara K (1993) Characteristics of dry matter production process in high-yield rice varieties. Jpn J Crop Sci 62: 509–517 [Google Scholar]
- Sari-Gorla M, Calinski T, Kaczmarek Z, Krajewski P (1997) Detection of QTL × environment interaction in maize by a least squares interval mapping method. Heredity 78: 146–157 [Google Scholar]
- Sasaki T, Matsumoto T, Yamamoto K, Sakata K, Baba T, Katayose Y, Wu J, Niimura Y, Cheng Z, Nagamura Y et al. (2002) The genome sequence and structure of rice chromosome 1. Nature 420: 312–316 [DOI] [PubMed] [Google Scholar]
- Song X, Agata W, Kawamitsu Y (1990) Studies on dry matter and grain production of F1 hybrid rice in China. Jpn J Crop Sci 59: 107–112 [Google Scholar]
- Tanaka K, Ohnishi S, Kishimoto N, Kawasaki T, Baba T (1995) Structure, organization, and chromosomal location of the gene encoding a form of rice soluble starch synthase. Plant Physiol 108: 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley SD (1993) Mapping polygenes. Anal Rev Genet 27: 205–233 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakamura Y, Ishi R (1997) Relationship between starch accumulation and activities of the related enzymes in the leaf sheath as a temporary sink organ in rice (Oryza sativa). Aust J Plant Physiol 24: 563–569 [Google Scholar]
- Wintermans JFGM, De Mots A (1965) Spectrophotometric characteristics of chlorophylls and their pheophytins in ethanol. Biochim Biophys Acta 109: 448–453 [DOI] [PubMed] [Google Scholar]
- Xiao J, Li J, Grandillo S, Ahn SN, Yuan L, Tanksley SD, McCouch SR (1998) Identification of trait-improving quantitative trait loci alleles from a wild rice relative, Oryza rufipogon. Genetics 150: 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing YZ, Tan YF, Hua JP, Sun XL, Xu CG, Zhang Q (2002) Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor Appl Genet 105: 248–257 [DOI] [PubMed] [Google Scholar]
- Yano M (2001) Genetic and molecular dissection of naturally occurring variation. Curr Opin Plant Biol 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Yoshida S (1972) Physiological aspects of grain yield. Anal Rev Plant Physiol 23: 437–464 [Google Scholar]