Abstract
Two AGL2-like MADS-box genes, Lily MADS Box Gene (LMADS) 3 and LMADS4, with extensive homology of LMADS3 to the Arabidopsis SEPALLATA3 were characterized from the lily (Lilium longiflorum). Both LMADS3 and LMADS4 mRNA were detected in the inflorescence meristem, in floral buds of different developmental stages, and in all four whorls of the flower organ. LMADS4 mRNA is also expressed in vegetative leaf and in the inflorescence stem where LMADS3 expression is absent. Transgenic Arabidopsis, which ectopically expresses LMADS3, showed novel phenotypes by significantly reducing plant size, flowering extremely early, and loss of floral determinacy. By contrast, 35S::LMADS4 transgenic plants were morphologically indistinguishable from wild-type plants. The early-flowering phenotype in 35S::LMADS3 transgenic Arabidopsis plants was correlated with the up-regulation of flowering time genes FT, SUPPRESSOR OF OVEREXPRESSION OF CO 1, LUMINIDEPENDENS, and flower meristem identity genes LEAFY and APETALA1. This result was further supported by the ability of 35S::LMADS3 to rescue the late-flowering phenotype in gigantea-1 (gi-1), constans-3 (co-3), and luminidependens-1 but not for ft-1 or fwa-1 mutants. The activation of these flowering time genes is, however, indirect because their expression was unaffected in plants transformed with LMADS3 fused with rat glucocorticoid receptor in the presence of both dexamethasone and cycloheximide.
The ABCDE model predicts the formation of any flower organs by the interaction of five classes of homeotic genes in plants (Theissen and Saedler, 2001; Theissen, 2001). The A function genes control the sepal formation. The A, B, and E function genes work together to regulate petal formation. The B, C, and E function genes control the stamen formation. The C and E function genes work to regulate carpel formation, whereas the D function gene is involved in ovule development (Theissen and Saedler, 2001; Theissen, 2001). A central role for MADS box genes in flower development can be assumed because most ABCDE genes encode MADS box proteins (Coen and Meyerowitz, 1991; Purugganan et al., 1995; Rounsley et al., 1995; Theissen and Saedler, 1995; Theissen et al., 2000).
In plants, A, B, and C function genes have been studied extensively for many years (Theissen et al., 2000; Theissen, 2001; Theissen and Saedler, 2001). By contrast, the E function genes were established only recently based on relatively few studies (Pelaz et al., 2000; Theissen, 2001). E function genes have been placed into the subclade of AGL2-like genes (Theissen et al., 2000; Theissen, 2001). The best known E function MADS box genes are SEPALLATA1 (SEP1), SEP2, SEP3 (previously described as AGL2, -4, and -9) of Arabidopsis (Theissen, 2001). Although the expression pattern for these three genes are slightly different, for example, SEP1 and SEP2 are expressed in all four whorls of organs whereas SEP3 is only expressed in the inner three whorls of the flower (Rounsley et al., 1995; Savidge et al., 1995; Pelaz et al., 2000), they have been thought as functionally redundant genes (Pelaz et al., 2000; Theissen, 2001). The high sequence similarity and functional redundancy have caused difficulty in identification of single-gene mutations (Savidge et al., 1995). Mutation in any one of these three genes produced only subtle phenotype differences (Pelaz et al., 2000). For example, a sep3 single mutant resulted only in a phenotype similar to that observed in intermediate alleles of APETALA1 (AP1; Pelaz et al., 2001). However, in sep1/sep2/sep3 triple mutants, all of the flower organs were converted into sepals that are noticeably similar to that of double mutants defective in B and C function genes (Pelaz et al., 2000). This result indicates that SEP1, SEP2, and SEP3 are required for the formation of petal, stamen, and carpel (Pelaz et al., 2000; Theissen, 2001). This assumption was further supported by the interaction between SEP and B, C function proteins (Honma and Goto, 2001). For example, AP3 and PI have been shown to regulate petal and stamen development by interacting with SEP1, SEP2, and SEP3 (Pelaz et al., 2000; Honma and Goto, 2001; Theissen, 2001; Theissen and Saedler, 2001). SEP3 has been reported to interact with AG (Honma and Goto, 2001). Further studies indicate the K domain of AG is sufficient to promote interactions with SEP1, SEP2, and SEP3 (Fan et al., 1997). This result strongly indicates that E function genes are necessary for the activities of the B and C function genes. Further analysis indicates that ectopic expression of PI/AP3/SEP3/AG at the same time enables convergence of vegetative leaves into floral organs (Honma and Goto, 2001).
On the basis of the sequence similarity and the expression pattern, putative orthologs for SEP1/2/3 have been identified in other plant species such as FBP2, FBP5, and FBP9 of petunia (Petunia hybrida; Angenent et al., 1992, 1994; Immink et al., 2002; Ferrario et al., 2003), TM5 of tomato (Lycopersicon esculentum; Pnueli et al., 1994), and GRCD1 of Gerbera hybrida (Kotilainen et al., 2000). Mutation of FBP2 and TM5 by antisense or cosuppression in petunia and tomato produces phenotypes resembling Arabidopsis sep1/sep2/sep3 triple mutants (Angenent et al., 1994; Pnueli et al., 1994). FBP2 has been reported to act as the functional complement of the sep mutant of Arabidopsis (Ferrario et al., 2003). These results support the assumption that they are orthologs for E function genes. In addition, similar to the E function genes that are able to interact with another class of MADS box genes, FBP2, FBP5 and FBP9 are able to form heterodimers with D function gene FBP11 in the petunia in regulating ovule development (Immink et al., 2002). GRCD1 has also been shown to interact with C function genes GAGA1 and GAGA2 in G. hybrida in controlling third whorl stamen development (Kotilainen et al., 2000).
In addition to regulating organ differentiation in the three inner whorls of the flowers, E function genes such as SEP3 and its orthologs are thought to act as floral meristem identity genes as well (Angenent et al., 1992, 1994; Pnueli et al., 1994; Rounsley et al., 1995; Mandel and Yanofsky, 1998; Pelaz et al., 2000). The involvement of these E function genes in floral transition and initiation has further been proved by the ectopic expression of these genes in transgenic plants. Ectopic expression of SEP3 produces an extremely early-flowering phenotype and the conversion of inflorescence to the terminal flower structure in Arabidopsis (Honma and Goto, 2001; Pelaz et al., 2001). Similar phenotypes have also been observed in transgenic plants ectopically expressing AP1 or its orthologs (Mandel and Yanofsky, 1995; Kyozuka et al., 1997). Interestingly, the early-flowering phenotype is significantly enhanced in transgenic plants which ectopically express both SEP3 and AP1 (Pelaz et al., 2001). In addition, the interaction between SEP3 and AP1/CAL proteins has been documented (Honma and Goto, 2001; Pelaz et al., 2001). These results strongly indicate that SEP3 interact with AP1 to promote flower initiation in Arabidopsis (Pelaz et al., 2001).
MADS box genes in AGL2 subclade were also identified in monocots (Theissen et al., 2000; Theissen, 2001), for example, ZMM6, ZMM8, and ZMM14 of maize (Zea mays; Cacharrán et al., 1999; Theissen et al., 2000; Theissen, 2001), OsMADS1 of rice (Oryza sativa; Chung et al., 1994; Jeon et al., 2000), and DOMADS1 and DOMADS3 of an orchid hybrid (Dendrobium grex Madame Thong-In; Yu and Goh, 2000). However, relatively heterogeneous expression patterns and less clearly functional analysis for these genes make it difficult to indicate their roles as E function genes. Therefore, the identification and characterization of E function-related genes from monocots has become important. Lilies are monocots with important economic value in the cut flower market around the world. Only a few studies regarding flower formation have been reported for lilies (Theissen et al., 2000; Tzeng and Yang, 2001; Tzeng et al., 2002). Here, we report on the isolation and functional analysis of two putative SEP-like E function MADS box genes from the lily (Lilium longiflorum). Results show that these two lily genes cause different effects on floral formation and floral transition and interact with flowering time genes differently in transgenic Arabidopsis. The exploration of the relationships between these two genes and their closest counterparts in other plant species is discussed.
RESULTS
Isolation of SEPALLATA-Like Genes from Lily
A combined reverse transcriptase (RT)-PCR and 5′-RACE strategy was used to isolate MADS box genes from lily (Tzeng and Yang, 2001; Tzeng et al., 2002). The full-length cDNA sequence for two putative orthologs of E function genes, Lily MADS Box Gene 3 (LMADS3) and LMADS4, were identified.
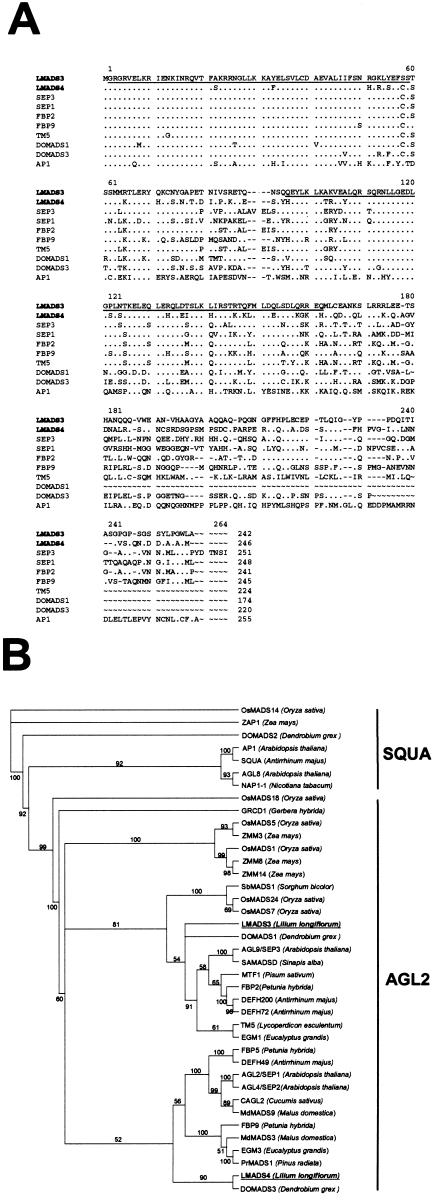
LMADS3 cDNA is 1,012 bp long and encodes a 242-amino acid protein that showed 77% identity and 81% similarity to FBP2 (SEP3 ortholog in petunia; Fig. 1A). LMADS3 also showed high homology to SEP3 (70% identity) of Arabidopsis and DOMADS1 (76% identity) of the orchid (Fig. 1A). In the MADS domain, 98% (57/58) of the amino acids were identical between LMADS3, SEP3 and LMADS3, FBP2 (Fig. 1A). A relatively low identity (93%, 54/58) was observed for LMADS3 and DOMADS1 in the MADS domain (Fig. 1A). In addition to the MADS domain, a putative protein dimerized K domain, which showed 85% (57/67), 78% (52/67), and 75% (50/67) identity to FBP2, SEP3, and DOMADS1, respectively, was found in the middle of the protein (Fig. 1A). The high sequence identity between the LMADS3 and SEP3 orthologs from various species suggests that LMADS3 is the lily SEP3 ortholog.
Figure 1.
Sequence comparison of LMADS3, LMADS4, and related AP1/AGL9 group of MADS box proteins. A, Amino acid sequence alignment between LMADS3, LMADS4, SEP1, SEP3, AP1 (Arabidopsis), FBP2, FBP9 (petunia), DOMADS1, DOMADS3 (Dendrobium grex Madame Thong-In), and TM5 (tomato). The first and second underlined regions represent MADS domain and K domain, respectively. In each alignment, amino acid residues identical to LMADS3 are indicated as dots. To improve alignment, dashes were introduced into the sequence. This sequence alignment was generated by the ClustalW-Multiple Sequence Alignment Program at Biology Work Bench (http://workbench.sdsc.edu). The alignment parameters are indicated as follows: gap open penalty, 10.00; gap extension penalty, 0.20; and percent identity for delay, 30. B, Phylogenetic analysis of SQUA-like and AGL2-like genes in AP1/AGL9 group of MADS box genes. On the basis of the amino acid sequence of full-length protein, LMADS3 is closely related to DOMADS1 and SEP3 whereas LMADS4 is most related to DOMADS3, FBP9, and SEP1/SEP2. Names of the LMADS3 and LMADS4 proteins are bold and underlined. Names of the plant species for each MADS box gene are listed behind the protein names. The tree was generated by the neighbor joining (NJ) method, whereas the distance was calculated based on the Dayhoff Prevent Allowed Mutation matrix (Dayhoff et al., 1983) using the PROTDIST program in PHYLIP software package (v3.5c; Kimura, 1980). Numbers on major branches indicate bootstrap percentages for 1,000 replicate analyses.
LMADS4 cDNA is 1,088 bp long and encodes a 246-amino acid protein that showed 61% identity and 67% similarity to SEP1 and SEP2 (Fig. 1A). LMADS4 also showed high identity to FBP9 (SEP1 ortholog in petunia; 65%) and DOMADS3 (69%) of orchid (Fig. 1A). In the MADS domain, LMADS4 showed a higher identity to the SEP1/SEP2 and DOMADS3 (91%, 53/58) than to FBP9 (90%, 52/58; Fig. 1A). In the K domain, 78% (52/67), 75% (50/67), and 72% (48/67) identity were observed for LMADS4 to FBP9, DOMADS3, and SEP1, respectively (Fig. 1A). The sequence similarity between LMADS4 and SEP indicates that LMADS4 is the lily SEP-like gene. The amino acid sequence alignment shown in Figure 1A and sequence for several other MADS box genes were used to construct a phylogenetic tree for MADS box genes in AGL2 and SQUA subclades (Fig. 1B).
Gene Expression for LMADS3 and LMADS4
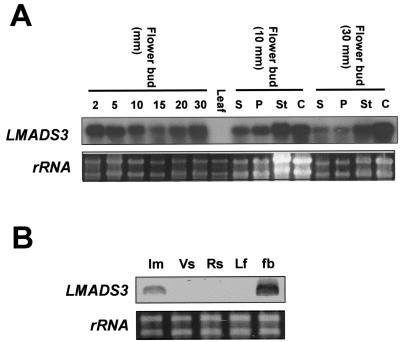
To explore the relationships between sequence similarity and expression pattern for LMADS3 and LMADS4, RNA expression was analyzed. As shown in Figure 2A, LMADS3 mRNA was detected in the flower buds of different developmental stages (from 2 mm to 30 mm in length) yet was absent from vegetative leaves. When the floral organs from 10- and 30-mm floral buds were examined, LMADS3 was found to be highly expressed in all four floral organs. This pattern was slightly different from that observed for SEP3 or its orthologs, which were expressed in the three inner whorls (petal, stamen, and carpel), although absent from the sepal (Angenent et al., 1992; Pnueli et al., 1994; Rounsley et al., 1995). Further analysis indicated that LMADS3 mRNA was also detected in the inflorescence meristem yet was absent from the inflorescence stem (Fig. 2B).
Figure 2.
Detection of the LMADS3 expression. A, Total RNA was isolated from flower buds at six different developmental stages (2, 5, 10, 15, 20, and 30 mm long, respectively), from four flower organs of 10- and 30-mm floral buds, and from vegetative leaves. For northern hybridization, LMADS3-specific DNA probes (without MADS box domain) were used. The results indicate that a 1-kb band of the RNA for LMADS3 is specifically detectable during all stages of flower development and is expressed in all four floral organs. No signals could be detected in vegetative leaves for LMADS3. An ethidium bromide (EtBr)-stained gel before blotting and hybridization is shown as a control. S, Sepal; P, petal; St, stamen; and C, carpel. B, Total RNA isolated from inflorescence meristem (Im), inflorescence stem before flowering (Vs) and after flowering (Rs), vegetative leaves (Lf), and 2-mm-long flower buds (fb) were used as templates for RT-PCR. The results indicate that LMADS3 mRNA is detectable in the inflorescence meristem and in flower buds yet was absent in leaf and stem. This experiment was repeated twice with similar results. rRNA stained in an EtBr gel was used to show the amount of RNA used for each RT-PCR reactions.
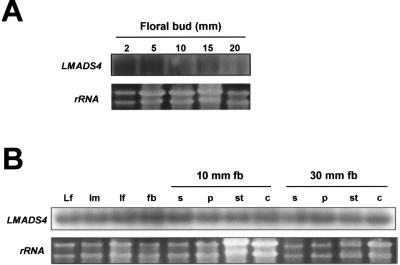
Similar to LMADS3, LMADS4 mRNA was detected in the inflorescence meristem, in flower buds of different developmental stages (Fig. 3A), and in all four floral organs (Fig. 3B). However, LMADS4 mRNA was also detected in other organs such as vegetative leaf and the inflorescence stem (Fig. 3B). This expression pattern was different from that observed for SEP1/SEP2, which was expressed only in four flower organs (Flanagan and Ma, 1994; Rounsley et al., 1995; Savidge et al., 1995; Pelaz et al., 2000), or DOMADS3, which was only detected in the pedicel of the flower (Yu and Goh, 2000).
Figure 3.
Detection of the LMADS4 expression. A, Total RNA was isolated from flower buds of different developmental stages (2, 5, 10, 15, and 20 mm long, respectively). For northern hybridization, LMADS4-specific DNA probes (without MADS box domain) were used. The results indicate that a 1.1-kb band of the RNA for LMADS4 is detectable in all stages of flower development. An EtBr-stained gel before blotting and hybridization is shown as a control. B, Total RNA isolated from leaves (Lf), inflorescence meristem (Im), inflorescence stem (If), 2-mm-long flower buds (fb), flower organs, sepal (s), petal (p), stamens (st), and carpel (c) of 10-mm and 30-mm-long floral buds were used as templates for RT-PCR and Southern analysis. The results indicate that LMADS4 mRNA is detectable in all organs tested. This experiment was repeated twice with similar results. rRNA stained in an EtBr gel was used to show the amount of RNA used for each RT-PCR reaction.
Ectopic Expression of LMADS3 and LMADS4 Causes Different Effects in Transgenic Arabidopsis Plants
To further investigate the function of LMADS3 and LMADS4, ectopic expression of these two genes in transgenic plants is necessary. cDNAs for LMADS3 or LMADS4 driven by the cauliflower mosaic virus (CaMV) 35S promoter were transformed into Arabidopsis plants for functional analysis.
Ten independent 35S::LMADS3 transgenic Arabidopsis T1 plants were obtained. Two plants were phenotypically indistinguishable from wild-type plants, whereas the other eight plants showed the identical novel phenotypes described below. These eight plants were small and produced petioleless, oval-shaped cotyledons and small curled leaves after germination (Fig. 4A), which were very similar to that observed in extremely early-flowering emf mutants (Fig. 4B; Sung et al., 1992; Yang et al., 1995). Conversely, wild-type plants of the same age produce round cotyledons with long petioles and expanded leaves (Fig. 4C). These 35S::LMADS3 plants flowered significantly earlier (Fig. 4D) than wild-type plants (Fig. 4E) by producing only two to three small curled rosette leaves and two small curled cauline leaves on the inflorescence. The sepal, petal, stamen, and carpel structures in the mature flowers of these eight plants were apparently normal without any floral organ conversion. However, the 35S::LMADS3 plants lost inflorescence indeterminacy by producing terminal flowers at the end of the inflorescence (Fig. 4, F and G). These terminal flowers were composed of two to three carpels, similar to those observed in Arabidopsis terminal flower 1 mutants.
Figure 4.
Phenotypic analysis of transgenic Arabidopsis plants ectopically expressing LMADS3. A, A 7-d-old 35S::LMADS3 Arabidopsis plant grown on Murashige and Skoog agar medium produced oval-shaped cotyledon (c) and small curled rosette leaves (cr). B, A 7-d-old emf2–3 Arabidopsis mutant plant grown on Murashige and Skoog agar medium produced oval-shaped cotyledon (c) and small curled rosette leaves (cr) similar to 35S::LMADS3 Arabidopsis plant in (A). C, A 7-d-old wild-type Arabidopsis plant grown on Murashige and Skoog agar medium produced round-shaped cotyledon (c) and expanded rosette leaves (r). D, A 15-d-old 35S::LMADS3 Arabidopsis plant grown on Murashige and Skoog agar medium flowered significantly early by producing only three small curled rosette leaves (cr) and two small curled cauline leaves (cc) on the inflorescence. This plant was only 1 cm tall during this stage. E, A 15-d-old wild-type Arabidopsis plant grown on Murashige and Skoog agar medium did not flower and only produced round rosette leaves (r), which were distinct from curled rosette leaves of a 35S::LMADS3 Arabidopsis plant in D. F, Terminal flower consisting of three flowers was produced in a 35S::LMADS3 plant. Three carpels (cp) were observed in this terminal flower. G, Terminal flower (right) produced at the end of the inflorescence in a 35S::LMADS3 plant. Two carpels (cp) were observed in this terminal flower, and the flower organs were phenotypically normal in this terminal flower. A single normal flower (left) was also observed. H, A 35S::LMADS3 ft-1 transgenic plant produced only numerous small rosette leaves. I, A 35S::LMADS3 ft-1 transgenic plant produced elongated inflorescence with only leaf-like structures. J, A 35-d-old ft-1 mutant (left) did not bolt and produced rosette leaves only. In the same stage, a wild-type Columbia plant (right) has already flowered by producing about 10 rosette leaves. K, A 45-d-old ft-1 mutant flowered later and produced more rosette and cauline leaves than wild-type plant. L, Only leaves were produced in the primary and secondary (arrowed) inflorescences of a 35S::LMADS3 fwa-1 transgenic plant. M, A leaf-like flower produced in a 35S::LMADS3 fwa-1 transgenic plant. In this flower, petals were absent and sepals were converted into leaves. Stamen-like (sl) and carpel-like (cl) structures were observed. N, A cluster of leaf-like flower produced at the inflorescence apex of a 35S::LMADS3 ft-1 transgenic plant. Carpel-like (cl) structures were observed. O, A cluster of flower buds produced at the inflorescence apex of an ft-1 mutant. These flower buds are indistinguishable from that observed in wild-type plants.
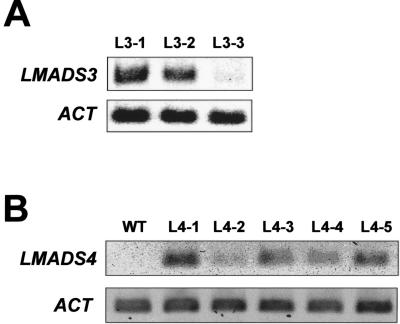
To explore whether the severe phenotype correlated to LMADS3 expression in 35S::LMADS3 transgenic plants, RT-PCR analysis was performed. As shown in Figure 5A, high LMADS3 expression was observed in the severe phenotype transgenic plants, whereas expression was nearly undetectable in 35S::LMADS3 transgenic plants which are indistinguishable from wild-type plants. This result clearly indicated that the abnormal phenotypes observed in 35S::LMADS3 transgenic Arabidopsis were due to the ectopic expression of the lily LMADS3 gene.
Figure 5.
The detection of LMADS3 and LMADS4 expression in transgenic Arabidopsis plants. A, Total RNA isolated from three 14-d-old 35S::LMADS3 transgenic Arabidopsis plants were used as a template. L3-1 and L3-2 are severed, whereas L3-3 is wild-type-like 35S::LMADS3 transgenic plant. The expression of LMADS3 was clearly high in L3-1 and L3-2, whereas its expression was almost undetectable in L3-3. A fragment of ACTIN (ACT) gene was amplified as an internal control. B, Total RNA isolated from five wild-type-like 14-d-old 35S::LMADS4 transgenic Arabidopsis (L4-1 to L4-5) and one untransformed wild-type plant were used as a template. The results indicated that the level of LMADS4 expression was higher in L4-1, L4-3, L4-5 than in L4-2, L4-4 transgenic plants. A fragment of ACTIN (ACT) gene was amplified as an internal control.
Nine independent 35S::LMADS4 transgenic Arabidopsis T1 plants were obtained. In contrast to 35S::LMADS3 transgenic plants, these nine plants were phenotypically indistinguishable from wild-type plants. No abnormal phenotype in leaf, inflorescence, or flower development was observed. To determine that this wild-type-like phenotype was not due to the lack of stable LMADS4 mRNA, the expression of LMADS4 in these transgenic plants was analyzed. The result indicated that both high and low LMADS4 expression was observed among these transgenic plants (Fig. 5B). This result clearly indicates that ectopic expression of LMADS4 has no effect in transgenic Arabidopsis.
35S::LMADS3 Was Not Able to Rescue Late-Flowering Phenotype in ft-1 and fwa-1 and Caused Severe Defects in the Floral Meristem Identity
Similar to those observed in 35S::LMADS3 transgenic plants, ectopic expression of flowering time genes such as CONSTANS (CO) and FT also significantly promote flowering in transgenic Arabidopsis plants (Kardailsky et al., 1999; Kobayashi et al., 1999; Samach et al., 2000). To explore the relationship between LMADS3 and flowering time genes in the floral induction pathway of transgenic Arabidopsis plants, LMADS3 cDNA driven by the CaMV 35S promoter were transformed into Arabidopsis lateflowering mutants, co-3, gi-1, ft-1, fwa-1, and luminidependens (ld)-1.
All 35S::LMADS3 transgenic gi-1, co-3, or ld-1 plants flowered significantly early, similar to those observed in 35S::LMADS3 transgenic plants (Fig. 4, A and D). Only a few small curled rosette and cauline leaves were produced on the inflorescence of these plants. This result indicated that GI, CO, and LD are not likely the targets for LMADS3 in 35S::LMADS3 transgenic plants. In contrast, 35S::LMADS3 transgenic ft-1 or fwa-1 plants showed novel phenotypes that were distinct from 35S::LMADS3 transgenic plants. 35S::LMADS3 ft-1 transgenic plants produced significantly more small rosette leaves (Fig. 4H) than that in 35S::LMADS3 transgenic plants (Fig. 4D) previous to showing inflorescence elongation (Fig. 4I). Interestingly, only leaves were produced at these elongated inflorescences (Fig. 4I). Despite the late-flowering phenotype, these 35S::LMADS3 ft-1 transgenic plants are smaller than untransformed ft-1 mutants (Fig. 4, J and K). 35S::LMADS3 fwa-1 transgenic plants (Fig. 4L) looked very similar to 35S::LMADS1 ft-1 transgenic plants by producing leaves only. Leaves were also generated in the position normally occupied by secondary inflorescence (Fig. 4L). Leaf-like flowers (Fig. 4, M and N) distinct from flowers observed in wild-type plants or ft-1 mutants (Fig. 4O) were occasionally observed in 35S::LMADS3 ft-1 and 35S::LMADS3 fwa-1 transgenic plants. In these flowers, petals were absent and sepals were always converted into leaves, whereas carpel- and stamen-like structures were observed in the inner two whorls (Fig. 4, M and N). These results indicate that ectopic expression of LMADS3 in ft-1 and fwa-1 not only fails to rescue the late-flowering phenotype but also causes an enhanced defect in floral meristem identity of ft-1 and fwa-1. As LMADS3 fails to rescue the late-flowering phenotype of ft-1 and fwa-1, FT likely was the target for LMADS3 in transgenic Arabidopsis plants.
Flowering Time Genes Are Induced Differentially in 35S::LMADS3 Transgenic Arabidopsis Plants
To further confirm the relationship between LMADS3 and flowering time genes in transgenic plants, the expression of GI, CO, FT, SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1), LD, LEAFY (LFY), and AP1 in 35S::LMADS3 transgenic plants was analyzed using quantitative RT-PCR.
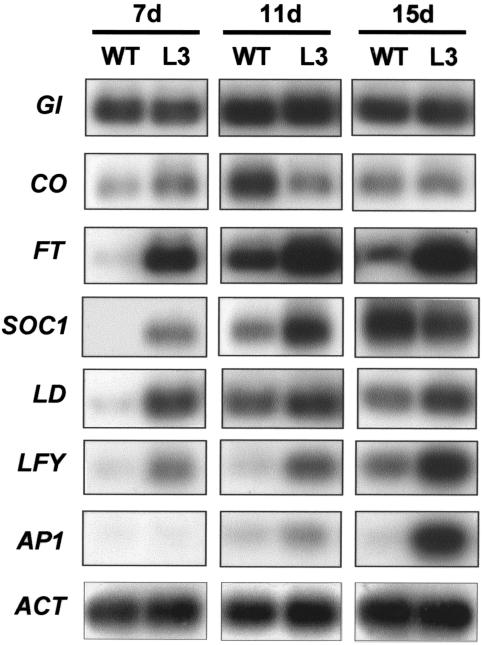
Total RNA isolated from 35S::LMADS3 transgenic or wild-type Columbia plants at 7, 11, and 15 d after germination were used as templates. As shown in Figure 5, the expression of GI and CO were unaffected in 35S::LMADS3 transgenic plants at these three stages tested (Fig. 6). By contrast, the level of FT, SOC1, LD, and LFY expression was significantly up-regulated in 35S::LMADS3 transgenic plants 7 d after germination and remained at a higher level than that in wild-type plants after 11 d of germination (Fig. 6). The level of AP1 expression was also clearly up-regulated in 35S::LMADS3 transgenic plants, although the induction timing was later than that observed for FT, SOC1, LD, and LFY (Fig. 6). The AP1 transcripts were initially up-regulated at 11 d after germination and were significantly up-regulated at 15 d after germination. The result indicated that the promotion of flowering in 35S::LMADS3 transgenic Arabidopsis plants was correlated with the up-regulation of FT, SOC1, LD, LFY, and AP1 transcripts by LMADS3.
Figure 6.
Analysis of flowering time gene expression in 35S::LMADS3 transgenic Arabidopsis plants. mRNA accumulation for GI, CO, LD, FT, SOC1, LFY, and AP1 was determined by RT-PCR and Southern analysis. Total RNA isolated from 7-, 11-, and 15-d-old wild-type Columbia (WT) and 35S::LMADS3 (L3) transgenic plants was used as a template. A fragment of ACTIN (ACT) gene was amplified as an internal control. Each experiment was repeated three times with similar results. The results indicated that the levels of expression for FT, SOC1, LD, LFY, and AP1 were up-regulated in 35S::LMADS3 transgenic plants at different developmental stages.
Up-Regulation of Flowering Time Genes in 35S::LMADS3 Plants Is Indirect
Because the evidence indicated that FT, SOC1, LD, LFY, and AP1 were possible targets for LMADS3 in LMADS3-overexpressing plants (Fig. 6), it was necessary to find out whether LMADS3 acted directly or indirectly to activate the transcription of these genes. To explore this question, a construct containing the fusion of LMADS3 and the hormone-binding domain of the rat glucocorticoid receptor (GR) was constructed and transformed into Arabidopsis. The induction of these genes by dexamethasone (DEX) in 7-d-old transgenic plants in the presence or absence of the protein synthesis inhibitor cycloheximide (CYC) was analyzed.
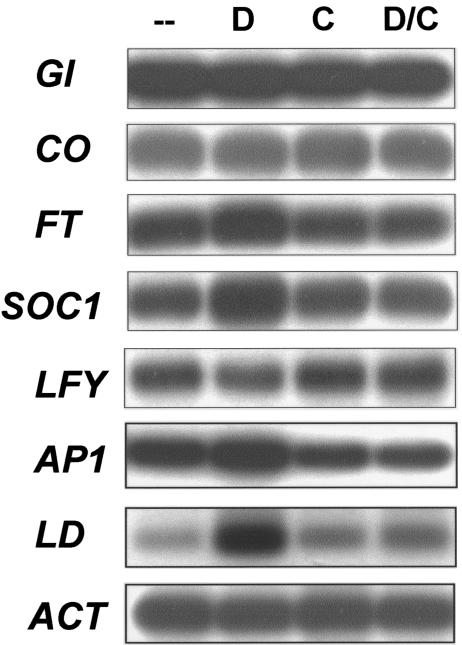
As shown in Figure 7, the expression of GI and CO was unaffected in 35S::LMADS3-GR transgenic plants at the two conditions tested (Fig. 7). By contrast, the level of FT, SOC1, and LD expression was up-regulated in the presence of DEX (Fig. 7). This result was similar to the data observed in Figure 6. The level of expression for these genes was however unaffected in the presence of both DEX and CYC (Fig. 7). This result indicates that the transcriptional induction of flowering time genes by 35S::LMADS3 in transgenic Arabidopsis plants is likely indirect.
Figure 7.
Analysis of flowering time gene expression in 35S::LMADS3-GR transgenic Arabidopsis plants. mRNA accumulation for GI, CO, LD, FT, SOC1, LFY, and AP1 was determined by RT-PCR and Southern analysis. Total RNA used as a template was isolated from 7-d-old 35S::LMADS3-GR transgenic plants treated with DEX (D) and/or CYC (C). A fragment of ACTIN (ACT) gene was amplified as an internal control. Each experiment was repeated twice with similar results. The results indicated that the levels of expression for FT, SOC1, LD, and AP1 were up-regulated by DEX treatment yet were unaffected in the presence of both DEX and CYC.
DISCUSSION
In investigating the role of MADS box genes in regulating lily (L. longiflorum) flower development, two MADS box genes were cloned and characterized in this study. On the basis of protein sequence alignment, LMADS3 is closely related to SEP3 and its orthologs within the E function genes (Fig. 1B). There is only one amino acid difference between LMADS3 and SEP3 in the MADS domain. Moreover, LMADS3 proved to be highly similar to SEP3 (78% identity) in the K domain. The mRNA expression pattern for LMADS3 was slightly different from SEP3. SEP3 and its orthologs, FBP2 from the petunia and TM5 from the tomato, specifically expressed in the inner three whorls, have been thought to regulate organ differentiation for petals, stamens, and carpels (Angenent et al., 1992, 1994; Pnueli et al., 1994; Rounsley et al., 1995). Conversely, mRNA for LMADS3 was detected in all four floral organ whorls and was similar to that observed for SEP1 and SEP2 (Rounsley et al., 1995; Mandel and Yanofsky, 1998; Pelaz et al., 2000). One explanation for this difference is the degree of similarity between the sepals and petals within flowers. Sepals and petals are completely different organs in plants such as Arabidopsis, tomatoes, tobacco (Nicotiana tabacum), and petunias, whereas these two organs are nearly identical in the lily, known as tepals. Therefore, the genes that control petal formation in the lily are very likely expressed in the sepal. Alternatively, because SEP1, SEP2, and SEP3 are functionally redundant genes (Pelaz et al., 2000; Theissen, 2001), the slight difference between LMADS3 and SEP3 may reflect the diversity in the E group genes in various plant species during evolution.
The early-flowering phenotype and the production of terminal flowers observed in 35S::LMADS3 transgenic Arabidopsis plants were no doubt similar to Arabidopsis plants ectopically expressing SEP3, AP1, or their orthologs from heterologous plants (Mandel and Yanofsky, 1995; Kyozuka et al., 1997; Honma and Goto, 2001; Pelaz et al., 2001). These results strongly indicate that LMADS3 is also involved in floral initiation and is closely related to SEP3. This assumption was supported by the detection of LMADS3 expression in the inflorescence meristem (Fig. 2B).
LMADS4, the second gene characterized here was found to be closely related to SEP in the AGL2 subclade of MADS box genes based on its protein sequence (Fig. 1B). This suggests that LMADS4 is a putative SEP-like gene in the lily. However, expression of LMADS4 differs from SEP1/SEP2/SEP3 or their orthologs (Angenent et al., 1992; Pnueli et al., 1994; Rounsley et al., 1995). Different from SEP1/SEP2 mRNA, which was only detected in four flower organs (Pnueli et al., 1994; Rounsley et al., 1995), LMADS4 mRNA is not floral specific and is expressed in all four floral organs as well as in vegetative leaf and the inflorescence stem. The different expression pattern between LMADS4 and SEP of E function genes may also reflect the diversity in the E group genes in various plant species during evolution. Alternatively, LMADS4 may have a different function in the orchid. Interestingly, different from that observed in 35S::LMADS3 transgenic plants, ectopic expression of LMADS4 in Arabidopsis did not produce early-flowering or terminal flower phenotypes. By contrast, 35S::LMADS4 transgenic plants are almost indistinguishable from wild-type plants. Because no result of functional analysis was available for 35S::SEP1 or 35S::SEP2, whether they also produce a similar phenotype as seen for 35S::LMADS4 is unclear and remains under investigation. Therefore, LMADS4 may represent a class of MADS box gene that shows high sequence homology to E function MADS box genes but with a different function in the orchid.
Because 35S::LMADS3 significantly promoted flowering in Arabidopsis, the exploration of the relationship between LMADS3 and the Arabidopsis flowering time genes is interesting. Our results indicate that the expression of flowering time genes FT and SOC1 in the photoperiod flowering pathway and floral meristem identity genes LFY and AP1 were up-regulated in 35S::LMADS3 transgenic Arabidopsis plants (Fig. 6). This result indicates that these genes are possible targets for LMADS3. Because FT and SOC1 were involved in the positive regulation of LFY (Nilsson et al., 1998; Onouchi et al., 2000; Reeves and Coupland, 2000; Blázquez, 2000; Samach et al., 2000), the up-regulation of LFY may be due to the activation of FT and SOC1 by LMADS3. According to our data, up-regulation of AP1 occurs several days later than that for FT, SOC1, and LFY (Fig. 6). Because LFY was able to bind to the AP1 promoter in regulating AP1 directly (Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995; Parcy et al., 1998; Wagner et al., 1999), the up-regulation of AP1 in 35S::LMADS3 transgenic plants is therefore possibly due to the activation of FT, SOC1, and/or LFY by LMADS3.
The activation of FT and SOC1 by 35S::LMADS3 is further supported by the inability of LMADS3 to rescue the late-flowering phenotype for ft-1 and fwa-1 in this study. This data strongly supports that LMADS3 activate FT and SOC1 in the photoperiod flowering pathway. Because 35S::LMADS3 was not able to up-regulate the expression for GI and CO, two upstream genes for FT and SOC1 in the photoperiod flowering pathway (Blázquez, 2000; Onouchi et al., 2000; Samach et al., 2000), LMADS3 possibly functions in parallel with CO in regulating flowering time in transgenic plants. This assumption is supported by the result that 35S::LMADS3 is able to compensate for the late-flowering defect in gi-1 and co-3 mutants.
In addition to the induction of FT and SOC1, ectopic expression of LMADS3 also caused early induction of LD in transgenic plants. This result suggests that along with the target genes FT and SOC1 in the photoperiod flowering pathway, another flowering pathway was also activated by the ectopic expression of LMADS3. If LD is the downstream gene of 35S::LMADS3 in transgenic plants, 35S::LMADS3 should not be able to rescue the late-flowering phenotype for ld-1 mutants. However, this was not the case in our result. 35S::LMADS3 transgenic ld-1 plants showed an early-flowering phenotype. It has been thought that pathways other than the CO/GI pathway are also involved in the activation of FT, because the expression of FT remains detectable in co mutants during late development (Kardailsky et al., 1999; Kobayashi et al., 1999). In addition, it has been shown that FT is the common component of both photoperiod and autonomous flowering pathways (Blázquez, 2000; Onouchi et al., 2000; Samach et al., 2000). Therefore, FT might be a downstream gene for LD in autonomous flowering pathways. It is possible that even in the absence of LD activity, FT will still be activated by 35S::LMADS3 and CO in 35S::LMADS3 transgenic ld-1 plants and result in a early-flowering phenotype as seen in our result.
One interesting question that needs to be answered is whether FT, SOC1, and LD are the direct targets for 35S::LMADS3. Apparently, the answer is no. Although the expression of these genes in 35S::LMADS3-GR transgenic plants is also up-regulated in the presence of DEX, their expression is clearly unaffected in the presence of both DEX and CYC. This result reveals that the transcriptional induction of FT, SOC1, and LD by 35S::LMADS3 in transgenic Arabidopsis plants is indirect. Therefore, we propose that the ectopic expression of LMADS3 is able to activate one or more unknown genes in regulating FT/SOC1/LD and the floral transition. This mechanism may also play a role for many MADS-box genes when overexpressed in Arabidopsis. However, results did not reveal that LMADS3 is more involved in flowering time control of lily than in specifying flower organ formation. Our result is not necessary to indicate that LMADS3 could also interact with flowering time gene orthologs in the wild-type lily. One might suspect that ectopic expression of LMADS3 in the lily may also promote flowering by activating the lily flowering time gene orthologs. However, this assumption needs to be tested in the future once a transformation system and the flowering time gene orthologs are available for the lily.
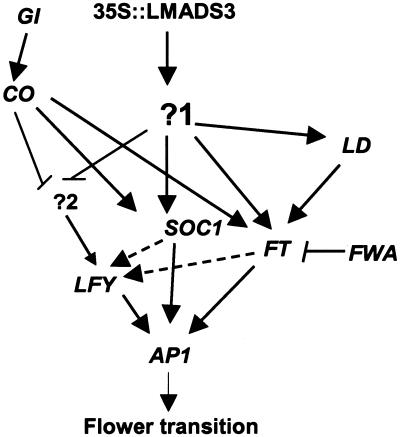
One more interesting result obtained in this study was the generation of leaf-like flowers in ft-1 and fwa-1 mutants ectopically expressing LMADS3. This severe defect in floral meristem identity is also observed in fwa lfy, ft lfy double mutants (Ruiz-Garcia et al., 1997), suggesting suppression of LFY by LMADS3 in transgenic plants. However, this is not the case observed in 35S::LMADS3 transgenic plants in which LFY was clearly up-regulated (Fig. 6). One possible explanation is that 35S::LMADS3 affect LFY expression through two different pathways. The induction of LFY in 35S::LMADS3 transgenic plants is mainly due to the activation of FT or SOC1 by LMADS3 in one pathway. LFY can be activated by the second pathway which was however, suppressed by 35S::LMADS3. In ft-1 or fwa-1 single mutants, LFY can still be activated by this second pathway, resulting in the normal flower formation. Conversely, ectopic expression of LMADS3 in ft-1 and fwa-1 mutants suppresses both FT and the second pathway and causes severe LFY suppression and the generation of leaf-like flowers similar to that observed in ft lfy, fwa lfy double mutants. A similar defect in floral meristem identity was also reported in 35S::CO fwa transgenic plants (Onouchi et al., 2000). Interestingly, similar to LMADS3, CO also activated FT and SOC1 expression in the photoperiod flowering pathway (Onouchi et al., 2000; Samach et al., 2000). A similar explanation for the possible suppression of LFY by CO through a pathway different from FT in regulating floral meristem identity has also been proposed (Onouchi et al., 2000). This result provides further evidence to support that LMADS3 may have a function similar to CO once ectopically expressed in Arabidopsis. A model depicting the affect caused by 35S::LMADS3 in transgenic Arabidopsis and its possible interaction with genes that affect flowering transitions is presented in Figure 8.
Figure 8.
Possible model for the affect caused by 35S::LMADS3 in transgenic Arabidopsis. Ectopic expression of LMADS3 promoted flowering by activating (→) an unknown gene (?1) and caused indirect activation of flowering time genes FT, SOC1, and LD and floral meristem identity genes such as LFY and AP1 during early development. Ectopic expression of LMADS3 may also cause the suppression (→) of a pathway (?2) involved in the activation (→) of LFY during floral initiation. This pathway was also possibly suppressed (→) by the 35S::CO as described by Onouchi et al. (2000). On the basis of this model, LFY activity will be abolished in ft-1 or fwa-1 mutants ectopically expressing LMADS3 and results in the formation of leaf-like flowers as seen in our results.
In summary, putative E function MADS box genes LMADS3 and LMADS4 specifying flower development were characterized from the lily (L. longiflorum). Ectopic expression of LMADS3 and LMADS4 causes different effects on floral formation and floral transition in heterologous Arabidopsis plants. 35S::LMADS3 significantly promotes flowering by indirectly activating the flowering time genes FT, SOC1, and LD whereas 35S::LMADS4 has no effects in transgenic Arabidopsis plants. These characteristics for LMADS3 and LMADS4 provide useful information in understanding E function MADS box genes in flower development. Because the genome size of the lily (L. longiflorum) is about 500 times greater than that for Arabidopsis (Joseph et al., 1990; Fosket, 1994), it is possible that other SEP-like genes beside LMADS3 and LMADS4 may also exist in the lily genome. Further characterization of more SEP-like MADS box genes from the lily are in progress and should lead to a deeper understanding of the diverse roles for the E functional MADS box genes during evolution.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Plants of lily (L. longiflorum Thunb. cv Snow Queen) used in this study were grown in the field in Tein Wei County, Chang Haw, Taiwan. Late-flowering mutant lines (fwa-1, ft-1, co-3, gi-1, and ld-1) used in this study were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Seeds for Arabidopsis were sterilized and placed on agar plates containing one-half times Murashige and Skoog medium (Murashige and Skoog, 1962) at 4°C for 2 d. The seedlings were then grown in growth chambers under long-day conditions (16-h light/8-h dark) at 22°C for 10 d before being transplanted to soil. The light intensity of the growth chambers was 150 μE m–2 s–1.
Cloning of cDNA for LMADS3 and LMADS4 from Lily
Total RNA was isolated from floral buds of lily using ULTRASPEC RNA Isolation System (BIOTECX Company, Houston). cDNA was synthesized from 500 μg of total RNA using cDNA synthesis kit 200401 (Stratagene, La Jolla, CA). One- to 1.5-kb synthesized cDNA fragments were collected and used as templates in PCR experiments as described by Tzeng and Yang (2001). PCR products were cloned and sequenced. Partial sequence for LMADS3 and LMADS4 that showed high sequence identity to SEP-like MADS box genes were identified. Internal gene-specific primers were designed for LMADS3 and LMADS4 for 5′-RACE by using the SMART RACE cDNA amplification kit (BD Biosciences Clontech, Palo Alto, CA). The gene-specific primer for 5′-RACE of LMADS3 was 5′-GTACTGGGTGCTAAGTTTGA-3′. The gene-specific primer for 5′-RACE of LMADS4 was 5′-GAGGGCATGCTCGGTCCGCTA-3′. The cDNAs for LMADS3 and LMADS4 were obtained by PCR amplification using the following 5′ primers: LMADS3, 5′-CACTTGGGATCCACCAGAAGAGGAGCT-3′; LMADS4, 5′-CGGATCCAATAAAGCATCTACCTCT-3′; and the same 3′ primer, 5′-CACTTGGGATCC(T)18-3′. The specific 5′ and 3′ primers for LMADS3 and LMADS4 contained the generated BamHI recognition site (5′-GGATCC-3′, underlined) to facilitate the cloning of the cDNAs.
35S::LMADS3-GR Gene Fusion Construct
An XbaI-BamHI fragment containing cDNA of LMADS3 was amplified by PCR. This fragment was introduced into binary vector PBI-GR between CaMV 35S promoter and the coding region of rat GR, and the recombinant plasmid, 35S::LMADS3-GR, was generated. The vector PBI-GR (Samach et al., 2000) was kindly supplied by Dr. G. Coupland (Max-Planck-Institute, Köln, Germany). Primers used to generate this XbaI-BamHI fragment are PLX (5′-GTCTAGATGGGGCGGGGGAGA-3′) and PLB (5′-GGATCCGCAAGCCATCCAGGC-3′). These two primers contained the generated XbaI (5′-TCTAGA-3′, underlined) or BamHI recognition site (5′-GGATCC-3′, underlined) to facilitate the cloning of the cDNA.
Plant Transformation and Transgenic Plants Analysis
A BamHI fragment containing the cDNA for LMADS3 or LMADS4 gene was cloned into binary vector PBI121 (BD Biosciences Clontech) under the control of CaMV 35S promoter. These constructs and 35S::LMADS3-GR were transformed into Arabidopsis plants using vacuum infiltration method as described elsewhere (Bechtold et al., 1993). Transformants were selected in the medium containing 50 μg mL–1 kanamycin and were further verified by PCR and RT-PCR analyses.
Northern-Blot Analysis
Ten micrograms of total RNA isolated from various organs or tissues of lily was electrophoresed in formaldehyde-agarose gels and transferred to Hybond N+ membranes (Amersham Biosciences, Buckinghamshire, UK). The membranes were prehybridized for 30 min and hybridized with a 32P-labeled DNA probes overnight at 65°C in the same solution (0.25 m Na2HPO4, pH 7.2, and 7% [w/v] SDS) and then washed twice each in solution 1 (20 mm Na2HPO4, pH 7.2, and 5% [w/v] SDS) and solution 2 (20 mm Na2HPO4, pH 7.2, and 1% [w/v] SDS) for 30 min per wash. The blots were then air dried, covered with plastic wrap, and autoradiographed. The DNA probes specific for LMADS3 or LMADS4 were partial cDNA fragments (without MADS box) amplified from cDNA clone through PCR.
RT-PCR and Southern Analysis
Total RNA was isolated from various organs of lily or from leaves of 35S::LMADS3 and 35S::LMADS3-GR transgenic Arabidopsis plants. For cDNA synthesis, total RNA (1 μg) was reverse-transcribed in a 20-μL reaction mixture using the BcaBEST RNA PCR system (TaKaRa Shuzo, Shiga, Japan). Five microliters of cDNA sample from RT reaction was used for a 25 cycles of PCR reaction as follow: denaturation at 94°C (45 s), annealing at 60°C (45 s), and extension at 72°C (90 s). The final 5 min at 72°C was performed as extension. Total PCR product (25 μL) in each reaction was analyzed by electrophoresis in 1.5% (w/v) agarose gels. For further Southern analysis, the agarose gels were transferred to Hybond N+ membranes (Amersham Biosciences) for highly stringent hybridization as described above. Primers specific for LMADS3, LMADS4, GI, CO, FT, SOC1, LD, LFY, and AP1 used in RT-PCR and in the generation of DNA probes were listed below. LMADS3, L3-1 (5′-GAGCTCCTGAGACAAATATA-3′) and L3-2 (5′-GTACTGGGTGCTAAGTTTGA-3′). LMADS4, L4-1 (5′-GAGGTACCATAAGTGCAGCTATAAT-3′) and L4-2 (5′-GAGGGCATGCTCGGTCCGCTA-3′). GI, GI-5-A (5′-GAGCTGTCTTTCTCCGTTGTTT-3′) and GI-3-A (5′-CTTCAATAGATTGGATAAACCGTC-3′). CO, CO-5–1 (5′-AACAGTGACAGATCCAGAGAACAG-3′) and CO-3-1 (5′-TTCTCTGCATACGCTTTCCTTGAA-3′). FT, FT5–3 (5′-CCTGCTACAACTGGAACAACCTTT-3′) and FT3-2 (5′-GCTATATAGGCATCATCACCGTTCGTTACTCG-3′). SOC1, SOC1–1 (5′-GTTTCTGAAGAAAATATGCAGCATT-3′) and SOC1–2 (5′-GAACAAGGTAACCCAATGAACAA-3′). LD, LD-5–1 (5′-ATGGACGCGTTCAAGGAGGAGATA-3′) and LD-3-1 (5′-ACATGCCTCCGGATGTATAGAGTT-3′). LFY, LFY-1-A (5′-TCATTTGCTACTCTCCGCCGCT-3′) and LFY-1-B (5′-CATTTTTCGCCACGGTCTTTAG-3′). AP1, AP1–3A (5′-GCTCCAAAAAAAGGAGAAGGC-3′) and AP1–3B (5′-GCCAAAATATATTAATTGGATGAAA-3′). Primers specific for ACTIN (ACT) used in RT-PCR reaction as internal control were ACT-1 (5′-ATGAAGATTAAGGTCGTGGCA-3′) and ACT-2 (5′-TCCGAGTTTGAAGAGGCTAC-3′).
The Induction of LMADS3-GR by Chemical Treatments
Seedlings were grown for 7 d on one-half times Murashige and Skoog medium (Murashige and Skoog, 1962) and then transferred to medium containing the combination of either 1 μm DEX (Sigma-Aldrich, St. Louis) or 10 μm CYC (Sigma-Aldrich) for 16 h as described by Lamb et al. (2002). Total RNA was then isolated from whole seedlings for RT-PCR and Southern analysis as described above.
Acknowledgments
We thank Dr. G. Coupland for providing vector PBI-GR.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026997.
This work was supported by the Council of Agriculture and National Science Council, Taiwan, Republic of China (grant nos. 90AS–2.1.1–FD–Z1 and NSC90–2317–B–005–002 to C.-H.Y.).
References
- Angenent GC, Busscher M, Franken J, Mol JNM, van Tunen AJ (1992) Differential expression of two MADS box genes in wild-type and mutant petunia flowers. Plant Cell 4: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, Weiss D, van Tunen AJ (1994) Co-suppression of the petunia homeotic gene fbp2 affects the identity of the generative meristem. Plant J 5: 33–44 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III Sci Vie 316: 1194–1199 [Google Scholar]
- Blázquez MA (2000) Flower development pathways. J Cell Sci 113: 3547–3548 [DOI] [PubMed] [Google Scholar]
- Cacharrán J, Saedler H, Theissen G (1999) Expression of MADS-box genes ZMM8 and ZMM14 during inflorescence development of Zea mays discriminates between the upper and the lower floret of each spikelet. Dev Genes Evol 209: 411–420 [DOI] [PubMed] [Google Scholar]
- Chung Y-Y, Kim S-R, Finkel D, Yanofsky MF, An G (1994) Early flowering and reduced apocal dominance result from ectopic expression of a rice MADS box gene. Plant Mol Biol 26: 657–665 [DOI] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353: 31–37 [DOI] [PubMed] [Google Scholar]
- Dayhoff MO, Barker WC, Hunt LT (1983) Establishing homology in protein sequences. Methods Enzymol 91: 524–545 [DOI] [PubMed] [Google Scholar]
- Fan H-Y, Hu Y, Tudor M, Ma H (1997) Specific interactions between the K domains of AG and AGLs, members of the MADS domain family of DNA binding proteins. Plant J 11: 999–1010 [DOI] [PubMed] [Google Scholar]
- Ferrario S, Immink RGH, Shchennikova A, Busscher-Lange J, Angenent GC (2003) The MADS box gene FBP2 is required for SEPALLATA function in petunia. Plant Cell 15: 914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan CA, Ma H (1994) Spatially and temporally regulated expression of the MADS-box gene AGL2 in wild-type and mutant Arabidopsis flowers. Plant Mol Biol 26: 581–595 [DOI] [PubMed] [Google Scholar]
- Fosket DE (1994) The size and complexity of plant genomes. In DE Fosket, ed, Plant Growth and Development: A Molecular Approach. Academic Press, San Diego, pp 79–152
- Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529 [DOI] [PubMed] [Google Scholar]
- Immink RGH, Gadella TWJ, Ferrario S, Busscher M, Angenent GC (2002) Analysis of MADS box protein-protein interactions in living plant cells. Proc Natl Acad Sci USA 99: 2416–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J-S, Jang S, Lee S, Nam J, Kim C, Lee S-H, Chung Y-Y, Kim S-R, Lee YH, Cho Y-G, An G (2000) leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell 12: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JL, Sentry JW, Smyth DR (1990) Interspecies distribution of abundant DNA sequences in Lilium. J Mol Evol 30: 146–154 [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kotilainen M, Elomaa P, Uimari A, Albert VA, Yu D, Teeri TH (2000) GRCD1, an AGL2-like MADS-box gene, participates in the C function during stamen development in Gerbera hybrida. Plant Cell 12: 1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka J, Harcourt R, Peacock WJ, Dennis ES (1997) Eucalyptus has functional equivalents of the Arabidopsis AP1 gene. Plant Mol Biol 35: 573–584 [DOI] [PubMed] [Google Scholar]
- Lamb RS, Hill TA, Tan QK-G, Irish VF (2002) Regulation of APETALA3 floral homeotic gene expression by meristem identity genes. Development 129: 2079–2086 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF (1995) A gene triggering flower formation in Arabidopsis. Nature 377: 522–524 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF (1998) The Arabidopsis AGL9 MADS box gene is expressed in young flower primordia. Sex Plant Reprod 11: 22–28 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–479 [Google Scholar]
- Nilsson O, Lee I, Blázquez MA, Weigel D (1998) Flowering time genes modulate the response to LEAFY activity. Genetics 150: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onouchi H, Igeño MI, Périlleux C, Graves K, Coupland G (2000) Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell 12: 885–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D (1998) A genetic framework for floral patterning. Nature 395: 561–566 [DOI] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203 [DOI] [PubMed] [Google Scholar]
- Pelaz S, Gustafson-Brown C, Kohalmi AE, Crosby WL, Yanofsky MF (2001) APETALA1 and SEPALLATA3 interact to promote flower development. Plant J 26: 385–394 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Hareven D, Broday L, Hurwitz C, Lifschitz E (1994) The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan MD, Rounsley SD, Schmidt RJ, Yanofsky MF (1995) Molecular evolution of flower development: diversification of the plant MADS-box regulatory gene family. Genetics 140: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PH, Coupland G (2000) Response of plant development to environment: control of flowering by daylength and temperature. Curr Opin Plant Biol 3: 37–42 [DOI] [PubMed] [Google Scholar]
- Rounsley SD, Ditta GS, Yanofsky MF (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7: 1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Garcia L, Madueno F, Wilkinson M, Haughn G, Salinas J, Martinez-Zapater JM (1997) Different role of flowering time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell 9: 1921–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Savidge B, Rounsley SD, Yanofsky MF (1995) Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 7: 721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung ZR, Belachew A, Bai S, Bertrand-Garcia R (1992) EMF, an Arabidopsis gene required for vegetative shoot development. Science 258: 1645–1647 [DOI] [PubMed] [Google Scholar]
- Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Biol 4: 75–85 [DOI] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, Winter K-U, Saedler H (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42: 115–149 [PubMed] [Google Scholar]
- Theissen G, Saedler H (1995) MADS-box genes in plant ontogeny and phylogeny: Haeckel's “biogenetic law” revisited. Curr Opin Genet Dev 5: 628–639 [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H (2001) Floral quartets. Nature 409: 469–471 [DOI] [PubMed] [Google Scholar]
- Tzeng T-Y, Chen H-Y, Yang C-H (2002) Ectopic expression of carpelspecific MADS box genes from lily and lisianthus causes similar homeotic conversion of sepal and petal in Arabidopsis. Plant Physiol 130: 1827–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng T-Y, Yang C-H (2001) A MADS box gene from lily (Lilium longiflorum) is sufficient to generate dominant negative mutation by interacting with PISTILLATA (PI) in Arabidopsis thaliana. Plant Cell Physiol 42: 1156–1168 [DOI] [PubMed] [Google Scholar]
- Wagner D, Sablowski RW, Meyerowitz EM (1999) Transcriptional activation of APETALA1 by. LEAFY Sci 285: 582–584 [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377: 495–500 [DOI] [PubMed] [Google Scholar]
- Yang C-H, Cheng L-J, Sung ZR (1995) Genetic regulation of shoot development in Arabidopsis: the role of EMF genes. Dev Biol 169: 421–435 [DOI] [PubMed] [Google Scholar]
- Yu H, Goh C-J (2000) Identification and characterization of three orchid MADS-box genes of the AP1/AGL9 subfamily during floral transition. Plant Physiol 123: 1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]