Abstract
Treatment of the ozone-sensitive tobacco (Nicotiana tabacum L. cv Bel W3) with an ozone pulse (150 nL L–1 for 5 h) induced visible injury, which manifested 48 to 72 h from onset of ozone fumigation. The “classical” ozone symptoms in tobacco cv Bel W3 plants occur as sharply defined, dot-like lesions on the adaxial side of the leaf and result from the death of groups of palisade cells. We investigated whether this reaction had the features of a hypersensitive response like that which results from the incompatible plant-pathogen interaction. We detected an oxidative burst, the result of H2O2 accumulation at 12 h from the starting of fumigation. Ozone treatment induced deposition of autofluorescent compounds and callose 24 h from the start of treatment. Total phenolic content was also strongly stimulated at the 10th and 72nd h from starting fumigation, concomitant with an enhancement in phenylalanine ammonia-lyase a and phenylalanine ammonia-lyase b expression, as evaluated by reverse transcriptase-polymerase chain reaction. There was also a marked, but transient, increase in the mRNA level of pathogenesis-related-1a, a typical hypersensitive response marker. Overall, these results are evidence that ozone triggers a hypersensitive response in tobacco cv Bel W3 plants. We adopted four criteria for detecting programmed cell death in ozonated tobacco cv Bel W3 leaves: (a) early release of cytochrome c from mitochondria; (b) activation of protease; (c) DNA fragmentation by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling of DNA 3′-OH groups; and (d) ultrastructural changes characteristic of programmed cell death, including chromatin condensation and blebbing of plasma membrane. We, therefore, provide evidence that ozone-induced oxidative stress triggers a cell death program in tobacco cv Bel W3.
Ozone (O3) enters the leaf and reacts in the apoplast to produce reactive oxygen species (ROS), such as superoxide anion (•O–2), hydrogen peroxide, hydroxyl radical (OH•) and singlet oxygen (1O2; Mudd, 1997). This triggers the plants to produce an oxidative burst by as yet unknown mechanisms (Schraudner et al., 1996). The effects of O3 on plants are numerous and vary with the intensity and duration of exposure. Short high-peak concentrations induce cell death lesions reminiscent of lesions activated during plant-pathogen interaction (Rao et al., 2000). Among herbaceous plants, tobacco (Nicotiana tabacum) is known to be particularly sensitive to ozone and the ozone-sensitive tobacco cv Bel W3 has been widely used as biomonitor of tropospheric ozone (Heggestad, 1991). The “classical” ozone symptoms in tobacco cv Bel W3 plants occur as sharply defined dot-like lesions on the adaxial side of the leaf resulting from death of group of palisade cells (Loreto et al., 2001).
Plant cell death occurs as programmed cell death (PCD) or necrosis. PCD is genetically controlled and shares some characteristic features with animal apoptosis, such as cell shrinkage, cytoplasmic condensation, chromatin condensation, and DNA fragmentation. Necrosis results from severe persistent trauma and is not considered to be orchestrated genetically (Pennell and Lamb, 1997; Gilchrist, 1998). In plants, PCD is a normal developmental process (Jones and Dangl, 1996; Pennell and Lamb, 1997) involved in anther, megagametophyte, and vascular tissue development as well as in senescence, pollination, and sex determination (Wang et al., 1996b; Groover et al., 1997; Yen and Yang, 1998; Panavas et al., 2000; Wu and Cheung, 2000). Plants also employ PCD as a controlled response to different biotic and abiotic stimuli (Greenberg and Ausubel, 1993; Greenberg et al., 1994; Ryerson and Heath, 1996; McCabe et al., 1997; Solomon et al., 1999; Huh et al., 2002). Several studies have shown that the death of plant cells during the hypersensitive response (HR) in the incompatible plant-pathogen interaction results from the activation of a PCD pathway (Greenberg et al., 1994; Wang et al., 1996a). Many studies have shown that there is an overlap in the signaling pathway and defense-related genes induced by ozone and other stresses, including pathogen infection (Sharma et al., 1996), wounding (Orvar et al., 1997; Koch et al., 1998), UV (Rao et al., 1996), cold, drought, and heavy metal toxicity (Kangasjarvi et al., 1994; Sharma and Davis, 1997; Sandermann et al., 1998). Signaling molecules, including salicylic acid (SA), jasmonic acid, ethylene, and the ROS superoxide and hydrogen peroxide have been shown to modulate plant response to ozone and other stresses. We recently demonstrated that, in the ozone-sensitive tobacco cv Bel W3, high levels of ozone-induced SA can trigger ROS (H2O2) production and subsequent cell death (Pasqualini et al., 2002).
The PCD mechanism has been especially elucidated in vitro on tissue culture subjected to a wide range of biotic (Gao and Showalter, 1999; Heath, 2000) and abiotic stimuli (McCabe et al., 1997; Solomon et al., 1999). Studying PCD in whole plants can however be difficult because it often occurs in a small groups of inaccessible cells buried in a bulk of surrounding healthy cells. Induction of a PCD program by ozone has been reported in the ozone-sensitive mutant rcd1 of Arabidopsis (Overmyer et al., 2000) and the ozone-tolerant poplar (Populus spp.) NE-245 clone (Koch et al., 2000) based on terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) analysis. Unfortunately, TUNEL does not discriminate between random fragmentation and oligonucleosome-sized DNA cleavage and may reveal necrotic cells in certain circumstances (Ben-Sasson et al., 1995). However, the combination of cytological and biochemical studies with TUNEL provides unambiguous evidence for PCD in plants.
The present study attempted to establish whether: (a) ozone induces HR-like lesions in the ozone-sensitive tobacco cv Bel W3, as adjudicated by specific HR markers; and (b) the type of cell death induced by acute ozone fumigation in tobacco cv Bel W3 was attributable to necrosis or the triggering of a cell death program. We therefore examined the early cytological changes that accompany ozone-induced cell death, the DNA fragmentation using TUNEL analysis, and the release of cytochrome c from mitochondria into the cytosol.
RESULTS
Tobacco cv Bel W3 Plants Express Markers Associated with HR
Oxidative Burst
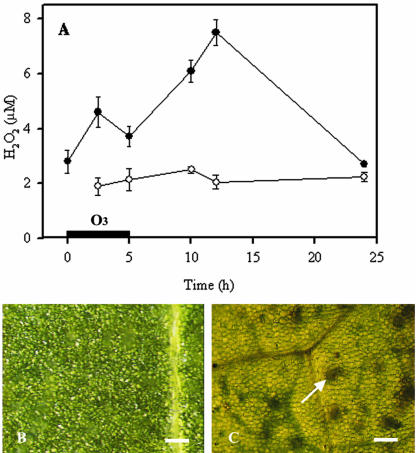
Injured areas appeared in middle-aged leaves of ozone-sensitive tobacco cv Bel W3 10 to 24 h after initiation of a single 5-h ozone exposure (150 nL L–1) and evolved into typical “weather flakes” at 48 to 72 h. Ozone treatment provoked rapid H2O2 accumulation (Fig. 1A), with a first transient peak during exposure and a higher peak (maximum concentration 7.5 μm in the IWF) at 12 h from the onset of treatment. H2O2, as monitored by 3,3′-diaminobenzidine 4 HCl (DAB) staining, was not uniformly distributed (Fig. 1C). Numerous irregular brown stains were visible at 12 h, that is after 7 h of post-cultivation in ozone-free air. DAB failed to stain unfumigated control plants (Fig. 1B).
Figure 1.
A, Accumulation of H2O2 in the intercellular washing fluid (IWF) of ozone-treated tobacco cv Bel W3 plants. Tobacco plants were treated for 5 h with 150 nL L–1 ozone (black symbols) or with pollutant-free air (white symbols) and were further cultivated in ozone-free air. Bars represent ± se, n = 4. B and C, Histochemical localization of H2O2 by DAB staining in leaves of ozone-treated plants at 12 h from the onset of treatment (C) and corresponding control leaves (B) of tobacco cv Bel W3. The arrow points to a brown-stained spot indicative of H2O2 accumulation in treated leaves 12 h after the onset of fumigation. Bars = 200 μm.
Secondary Metabolite Accumulation
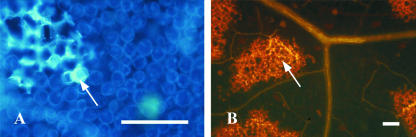
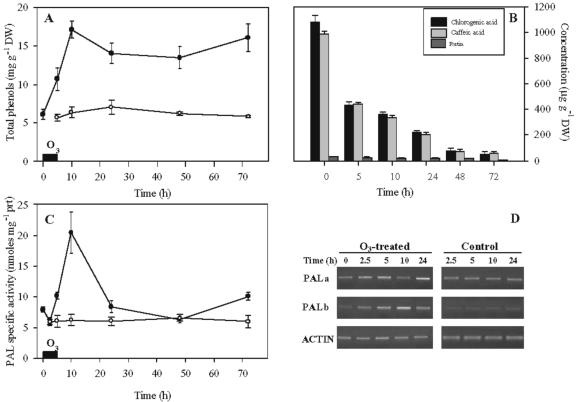
The secondary metabolites produced and deposited by plant cells undergoing hypersensitive cell death can be visualized with autofluorescence. In addition, cell wall materials, including callose and aromatic polymers, are deposited at infection sites (Dietrich et al., 1994). Therefore, we examined tobacco cv Bel W3 leaf discs at different times after ozone fumigation for the presence of autofluorescent materials and stained for callose with aniline blue. Autofluorescence became apparent at 24 h (Fig. 2A), particularly in walls of cells in injured areas that later developed into necrotic spots. Ozone-induced callose accumulation was detected 24 h after the start of 5 h of O3 exposure (Fig. 2B). The leaf content of total phenols was reported in Figure 3. There was already a significant increase at the end of fumigation (Fig. 3A). Levels reached a maximum at 10 h, and then decreased, but rose again at 72 h when necrotic spots were evident. HPLC was used to identify hydroxycinnamic derivates flavonoids in ozonated and control plants. In untreated plants, chlorogenic acid and caffeic acid accounted for 52% and 47%, respectively; rutin, the aglicone of quercitin, for only 1.3%. Both chlorogenic and caffeic acids decreased sharply and rapidly, until at 72 h, they were barely detectable (Fig. 3B). The response of the enzyme Phe ammonia-lyase (PAL), which catalyzes the first committed step of the phenylpropanoid biosynthetic pathway, is shown in Figure 3C. There was a 3-fold increase 5 h after the end of ozone exposure, followed by a decrease to initial levels and then a less pronounced rise after 72 h. Semiquantitative reverse transcriptase (RT)-PCR demonstrated that PALa gene expression was strongly induced by ozone. Induction was maximum 5 h after the initiation of ozone treatment (Fig. 3D); it then fell but rose again at 24 h. PALb was also strongly stimulated; the highest mRNA levels were reached 10 h after the onset of ozone fumigation.
Figure 2.
Examination of UV-stimulated autofluorescence (A) and UV-stimulated fluorescence from leaves stained with aniline blue (B). The fluorescent areas indicated by the arrows represent autofluorescent compounds (A) and callose deposition (B). The observation was carried out on leaves of tobacco cv Bel W3 fumigated plants (150 nL L–1 for 5 h) 24 h after the onset of ozone fumigation. Bars = 200 μm.
Figure 3.
A, Total phenolic content; B, separation by HPLC of the major phenolic compounds; C, PAL specific activity, from tobacco plants treated for 5 h with 150 nL L–1 ozone (black symbols) or maintained in pollutant-free air (white symbols). Regarding the phenolic compounds separation (C), only the control at 0 h time (before the onset of fumigation) is shown. The chlorogenic and caffeic acids and rutin values from untreated plants at the other times were not reported because they did not significantly differ from the value at 0 h. Bars represent ± se, n = 4. D, Semiquantitative RT-PCR. Reverse transcription and PCR were performed with RNA isolated from untreated plants and fumigated plants with primers specific for PALa, PALb, and actin 3 of tobacco.
Pathogenesis-Related (PR)-1a mRNA Accumulation
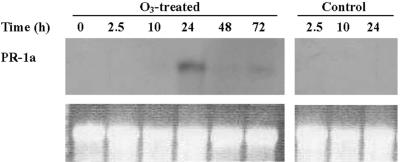
Leaves of ozone-sensitive tobacco cv Bel W3 were assayed for PR-1a mRNA content by northern blotting using a cDNA probe for an acidic PR-1 isoform from tobacco, which hybridized with single RNA species of approximately 800 kb. RNA blots revealed that PR-1a was not expressed constitutively in tobacco cv Bel W3 control plants (Fig. 4). A dramatic increase in PR-1a mRNA level was documented 24 h after the onset of fumigation (Fig. 4) but decreased after 48 and 72 h to become barely detectable.
Figure 4.
Northern-blot analysis of total RNA showing the effect of ozone exposure on transcript levels for PR-1a. Plants were treated with 150 nL L–1 ozone or ozone-free air for 5 h and were then cultivated in pollutant-free air. Samplings were carried out at the indicated times. Ten micrograms of total RNA was loaded in each lane of 1.5% (w/v) agarose gels containing formaldehyde. Equal loading was verified before blotting by visualizing total RNA in the gel stained with ethidium bromide (bottom).
Ozone-Induced Cell Death
Cell Viability
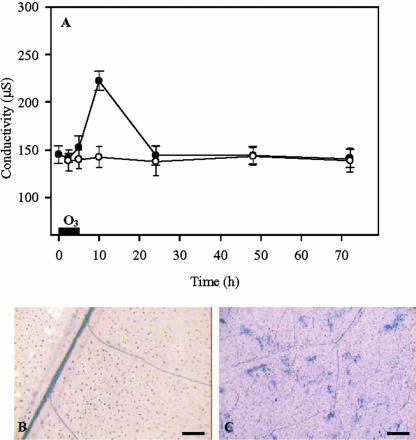
Ozone exposure induced spotty necrotic lesions within 48 h in the sensitive tobacco cv Bel W3, which could have been necrosis due to basic cellular dysfunction or could have resulted from the execution of a cell death program. To distinguish between these two possibilities, it was crucial to establish the time course of cell viability during and after exposure, when plants were left to recover in filtered air. Cell death was first evaluated by measuring ion leakage from leaf discs. As shown in Figure 5A, during ozone fumigation, no increase was observed compared with the value measured in the control plants, indicating that the tobacco cells were still alive at these times. However, between 5 and 10 h after onset of exposure, conductivity and then cell death increased significantly. Cell death in tobacco cv Bel W3 was also demonstrated by staining with trypan blue (Fig. 5, B and C). Ten hours after initiation of O3, staining was not uniformly distributed throughout the leaf, but appeared as spots (Fig. 5C).
Figure 5.
A, Cell death measured as ion leakage from leaf discs of tobacco plants fumigated for 5 h with 150 nL L–1 ozone (black symbols) or with pollutant-free air (white symbols) and further cultivated in ozone-free air. B and C, Histochemical staining with trypan blue from untreated (B) and fumigated plants (C) sampled 10 h from the onset of ozone fumigation. Bars = 200 μm.
The Release of Cytochrome c from Mitochondria into Cytosol
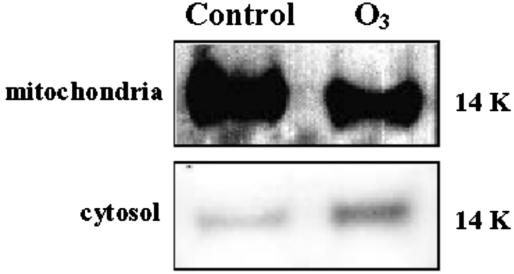
Western blotting showed that cytochrome c was present in the mitochondrial fraction of unfumigated tobacco cv Bel W3 plants (Fig. 6). There was a severe reduction in the concentration of cytochrome c in the mitochondria after 2.5 h of ozone, whereas a band relative to the cytochrome c released in the cytosol was evident at this time.
Figure 6.
Cytochrome c immunodetection in mitochondrial and cytosolic protein extracts (60 μg) from fumigated leaves of tobacco cv Bel W3 plants 2.5 h after onset of ozone fumigation and from corresponding control leaves.
Induction of Protease Activity
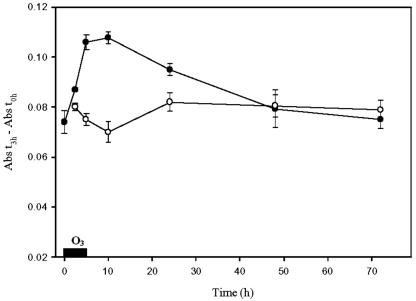
With azocasein as substrate, extracts from ozone-treated tobacco cv Bel W3 leaves displayed a significant increase in protease activity, which started to increase already during the ozone fumigation (Fig. 7). The protease activity increased about 40% at the end of fumigation, remained high until 10 h, and then decreased significantly. Addition of phenylmethane-sulfonyl fluoride (PMSF) and leupeptin to the homogenate significantly inhibited protease activity (data not shown).
Figure 7.
Analysis of the effect of ozone treatment on protease activity in tobacco cv Bel W3 plants using azocasein as substrate. Tobacco plants were treated for 5 h with 150 nL L–1 ozone (black symbols) or with pollutant-free air (white symbols) and were further maintained in ozone-free air. Bars represent ± se, n = 4.
In Situ Detection of DNA Fragmentation by TUNEL Analysis
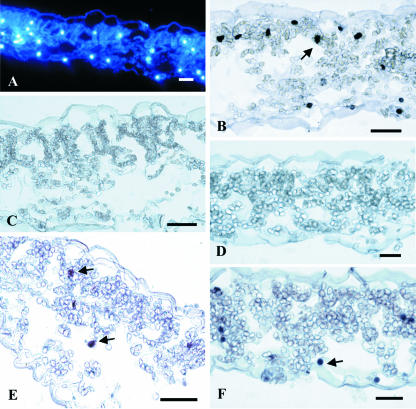
TUNEL was employed to establish whether ozone treatment induced nuclear DNA fragmentation. To rule out possible false positive/negative staining, results were interpreted by comparing TUNEL-positive signals with internal positive (Fig. 8B) and negative (Fig. 8C) controls and DAPI-stained sections (Fig. 8A). DNA fragmentation was detected in ozone-treated leaf tissue at both 2.5 h (Fig. 8E) and 5 h (Fig. 8F) after starting treatment, as indicated by incorporation of digoxigenin-labeled dUTP. There was no evidence of digoxigenin incorporation in controls (Fig. 8D). We also subjected DNA from tobacco leaves to agarose gel electrophoresis and to gel-blot analysis. This electrophoresis shows whether DNA is processed and fragmented into regularly sized pieces. However, no accumulation of discrete low-Mr DNA fragments corresponding to multimers of 180 bp was observed in ozone-treated plants (data not shown). Lack of DNA laddering was further confirmed by Southern analysis (data not shown).
Figure 8.
In situ detection of DNA degradation by TUNEL analysis in tobacco cv Bel W3 leaves. A through D, Leaf samples from untreated control plants (2.5 h). E and F, Leaf samples from ozone-treated tobacco cv Bel W3 plants (150 nL L–1 for 5 h). A, Section was stained with 4′-6-diamino-2-phenylindole (DAPI) for DNA; B, as positive control, section was treated with DNase to stain nuclei TUNEL positive; C, as negative control, the TdT enzyme was omitted from the labeling mixture; D, section from untreated control plants stained by using TUNEL method; E and F, sections from ozonated plants after 2.5 and 5 h from the onset of fumigation and stained by using TUNEL method. Arrows indicate TUNEL positive nuclei. Bars = 20 μm.
Ozone-Induced Ultrastructural Changes
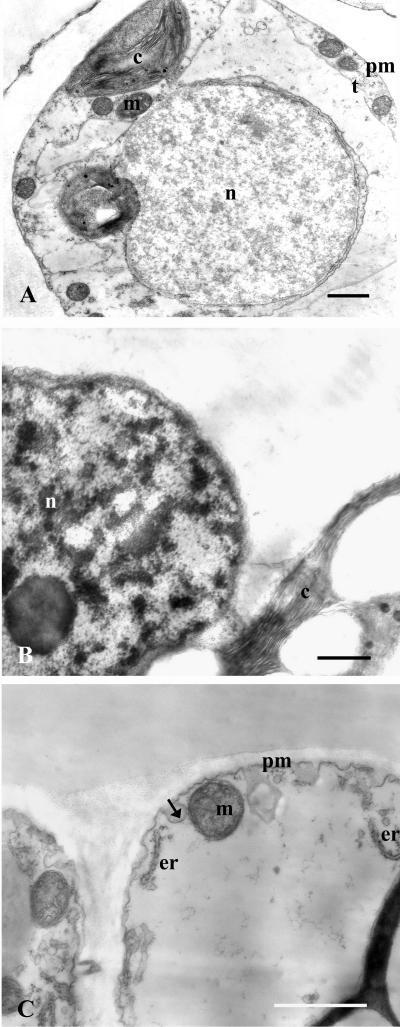
Because plant PCD often involves morphological changes, including chromatin condensation and cytoplasm shrinkage as seen in mammalian apoptosis (McCabe et al., 1997), transmission electron microscopy was used to identify early ultrastructural changes induced by ozone. Figure 9A shows normal-looking nucleus and chloroplast from a cell of untreated control leaf. After 2.5 h of ozone treatment, a nuclear degeneration pattern was observed in mesophyll cells. Large masses of an electron-dense material, strongly suggestive of chromatin condensation and aggregation, were seen in degenerating nuclei (Fig. 9B). The nuclear envelope persisted almost unchanged (Fig. 9B). Up to 5 h of fumigation, the plasma membrane also appears to be integer, despite evident invagination and apparent formation of cytoplasmic vesicles from the plasma membrane (Fig. 9C). No cytoplasm condensation or shrinkage of the cytoplasm was seen at this stage.
Figure 9.
Electron micrographs from sections of untreated control leaves (2.5 h, A) and ozone-treated leaves, after 2.5 h (B) and 5 h (C) from the onset of ozone fumigation (150 nL L–1 for 5 h), showing morphological changes in nuclei and plasma membrane alterations of cells undergoing PCD. A, Normal-looking nucleus and chloroplast from a cell from untreated control leaf. B, Apparent condensation of nuclear material into discrete patches distributes throughout the nucleus from cell of ozone-treated leaf (2.5 h after the onset of fumigation). C, invaginations and apparent formation of cytoplasmic vesicles from the plasma membrane in cell undergoing PCD. Arrow indicates plasma membrane invagination. The sections were obtained from leaves of tobacco plants subjected to 5 h of ozone fumigation. c, Chloroplast; er, endoplasmic reticule; m, mitochondria; n, nucleus; pm, plasma membrane; t, tonoplast. Bars = 1 μm.
DISCUSSION
Ozone Induces HR Markers in Tobacco cv Bel W3 Plants
After its entry through leaf stomata, ozone is rapidly converted into ROS in the apoplastic fluid surrounding leaf mesophyll cells (Rao et al., 2000). Thus, ozone-derived ROS and subsequent oxidative products have long been assumed to be the causal agents of plant cell death and necrotic lesion development. During recent years, however, a new picture has emerged that indicates that ozone acts as an abiotic elicitor of a typical HR and systemic acquired resistance in pathogen-infected plants (Sandermann et al., 1998). The HR is a rapid, localized cell death that occurs at sites of invasion by an incompatible pathogen and acts to restrict the pathogen to the immediate area (Heath, 2000). The ubiquity of the HR, its requirement for plant development, and a few features shared with a form of mammalian PCD known as apoptosis support the assumption that hypersensitive cell death is endogenously programmed (Heath, 2000).
Tobacco cv Bel W3 is particularly sensitive to ozone and responds to ambient ozone concentrations by developing hypersensitive-like reaction consisting of typical necrotic spots on expanded leaves. The macroscopic injuries induced by ozone are similar to the necrotic lesions induced by the incompatible pathogen tobacco mosaic virus in tobacco cv Bel W3 (Rossetti and Bonatti Medeghini, 2001). The aims of our research were to establish whether ozone induces HR-specific markers in tobacco cv Bel W3 and to investigate whether ozone-induced cell death is attributable to necrosis or PCD.
One of the earliest events in the HR is an oxidative burst leading to the generation of superoxide anion radicals (•O–2) and hydrogen peroxide (H2O2; Lamb and Dixon, 1997). Our results show that in ozone-fumigated plants, there was a strong accumulation of H2O2. It exhibited two peaks; one during exposure and a second massive and persistent peak during the recovery period in ozone-free air 7 h after terminating fumigation. DAB staining revealed that ozone-induced H2O2 was not homogeneously distributed over the leaf surface, but concentrated in discrete areas, where after 48 h, necrotic lesions developed. Schraudner et al. (1998) and Wohlgemuth et al. (2002) reported similar findings in tobacco and tomato (Lycopersicum esculentum) plants. The biphasic nature of the oxidative burst in ozone-exposed tobacco plants is reminiscent of the oxidative burst in incompatible plant-pathogen interaction (Draper, 1997). It was postulated by Levine et al. (1994) that biphasic or prolonged ROS generation is necessary for hypersensitive cell death to occur. In tobacco plants, the source of the oxidative burst is considered to be partly due to a NAD(P)H-oxidase complex (Schraudner et al., 1998) and two isoforms of oxalate oxidase detected in apoplastic washing fluid of tobacco cv Bel W3 cells (Thalmair, 1996). On the basis of subcellular localization of CeCl3 staining, we recently demonstrated H2O2 accumulation in the walls of palisade and spongy parenchyma cells, particularly those proximal to intercellular spaces, and in the plasma membrane of epidermal cells (Pasqualini et al., 2002). We also documented high accumulation of both free and conjugated SA, which occurred after 7 h of the post-fumigation period and corresponded to the second H2O2 peak (Pasqualini et al., 2002).
The HR is characterized by well-known markers; for example, induction of cellular barriers, like callose and lignin deposition, and activation of the phenylpropanoid pathway (Dietrich et al., 1994). Both fluorescent phenolic compounds and callose deposition were recorded within the necrotic areas of tobacco cv Bel W3 leaves 24 h after initiating O3 treatment. Similar results have been reported in tobacco (Schraudner et al., 1992) and in parsley (Petroselinum crispum; Eckey-Kaltenbach et al., 1994) subjected to ozone treatment. A similar reaction is known for pathogen-induced HR (Greenberg et al., 1994). We documented a 3-fold increase in total phenolic content 10 h after the onset of ozone fumigation, followed by a late and less pronounced secondary one at 72 h. Like kinetics were also observed for PAL enzyme activity. Transcript values for PALa and PALb showed a different pattern of induction. Transcript value for PALa increased at 5 h and again at 24 h after initiating treatment, whereas the maximum increase in PALb, which was weakly expressed in control plants, occurred at 10 h. The biochemical/physiological processes that PALb is involved in remain unknown. However, the weak expression in untreated leaf and the strong increase in PALb mRNA levels suggest that it is crucial in the activation of PAL enzymatic activity under ozone stress. Our results also indicate that PALb transcript accumulation could be dependent on SA accumulation, which starts to accumulate 8 h after onset of ozone exposure (Pasqualini et al., 2002).
Qualitative analysis of the phenolic compounds by HPLC revealed that the hydroxycinnamic acid derivates, chlorogenic and caffeic acids, were the most abundant compounds in tobacco cv Bel W3 leaves. Among phenolic acids, chlorogenic and caffeic acids were the most active antioxidants, and chlorogenic acid protects against ascorbic acid destruction (Larson, 1988). We detected a significant decrease in the accumulation of hydroxycinnamic acid derivates already at the end of ozone treatment, which agrees with the results of Kanoun et al. (2001) in bean (Phaseolus vulgaris) leaves under chronic ozone pollution. Such a decrease could be due to the depletion of phenolic compounds by reaction with ROS and the formation of conjugated polyamines. Both free and insoluble conjugated putrescine was detected in tobacco cv Bel W3 leaves after an acute ozone fumigation by van Buuren et al. (2002). Conjugated to caffeic, ferulic, or p-coumaric acids, putrescine is a highly efficient radical scavenger (Bors et al., 1989). The ozone-induced decrease of three HPLC-identified phenolic compounds could be contradictory compared with the increase of total phenolic compounds. However, the protocol that we used to extract and purificate phenols before HPLC injection was strongly selective because it enables the recovery of only hydroxycinnamic acid derivates and some flavonoids. Therefore, phenols such as anthocyanins and phytoalexins were not measured, and particularly phytoalexins are reported to be strongly stimulated by an ozone treatment (Eckey-Kaltenbach et al., 1994).
PR genes are used as molecular markers for local activation of host defense mechanisms during HR (Linthorst, 1991). Among the PR proteins, the expression of acidic PR genes was shown to be induced by SA (Yalpani et al., 1994). Our experiments show that PR-1a was not constitutively expressed in tobacco cv Bel W3 unfumigated plants. Although ozone caused a marked rise in PR-1a expression at 24 h, it was transient and barely detectable at 48 and 72 h from the start of fumigation. A similar late induction after ozone fumigation in tobacco cv Bel W3 plants was reported by Ernst et al. (1992) for PR-1b.
Electrolyte leakage measurements and trypan blue incorporation indicated that ozone-induced cell death occurred at about 10 h from the start of fumigation, that is at the same time as H2O2 enhancement in tobacco cv Bel W3. Therefore H2O2 in the HR could, as suggested by Alvarez (2000), be the diffusible signal that triggers a HR in the neighboring cells, so directly or indirectly boosting the SA level. The HR was documented by the greatest PR-1a induction and callose deposition found at 24 h. The oxidative burst, the induction of phenylpropanoid metabolism, the formation of cellular barriers, and the SA-mediated induction of PR-1a are testimony that ozone induces HR-like lesions in tobacco cv Bel W3 plants.
Ozone Induces Characteristic Features of PCD in Tobacco cv Bel W3 Leaves
Several lines of evidence suggest that the death of plant cells during HR results from the activation of a PCD pathway. To test this hypothesis, we analyzed eventual early modifications at mitochondrial level. In animal, mitochondria are considered the control point of apoptosis, which involves the caspase cascade and a series of regulatory molecules that trigger or prevent apoptotic cell death (Desagher and Martinou, 2000). Our western-blot analysis detected a decrease in mitochondrial cytochrome c, accompanied by an increase in cytosolic cytochrome c, already at 2.5 h of ozone exposure. This is, we believe, the first report on the behavior of cytochrome c in ozone-induced PCD.
We tested the effect of ozone fumigation on aspecific proteases using azocasein as substrate and registered a significant protease activation during 5 h of ozone exposure. Addiction to homogenate of PMSF, an inhibitor of Ser proteases and certain Cys proteases (papain family; Alonso et al., 1996), and leupeptin, an inhibitor of cystein proteases, induced a significant inhibition of ozone-induced protease activity, suggesting that the tested protease activity could be caspase-like. Activation of caspases causes the internucleosomal cleavage of genomic DNA typically found in apoptosis (Enari et al., 1998). Activation of DNA endonucleases provokes the appearance of oligonucleosomal-sized DNA fragments revealed by agarose gel electrophoresis (Eastman et al., 1994). However, this is not always the case; PCD cannot involve the fragmentation of DNA into oligonucleosome-sized pieces in either animal and plant cells (Schwartz et al., 1993; Groover et al., 1997; Panavas et al., 2000). When tobacco cv Bel W3 plants were treated with ozone, we did not observe any evidence of DNA laddering during ozone treatment or in the following recovery period (data not shown). It has been shown that in the case of plant PCD in cultured cells containing only a small number of dead cells, DNA laddering could not be detected by agarose gel electrophoresis (McCabe et al., 1997). This could also occur in our case because the cell death in tobacco cv Bel W3 ozonated leaves occurred in a small group of cells buried in a bulk of surrounding healthy cells. However, nuclear DNA degradation can be investigated by an in situ TUNEL assay (Gavrieli et al., 1992). Our TUNEL experiments demonstrated that nuclear DNA fragmentation occurred early in leaf mesophyll cells of tobacco cv Bel W3 plants, because positive TUNEL staining was observed after 2.5 h of ozone-treatment and at the end of the fumigation period (5 h). Nevertheless, this nuclear feature did not generate oligonucleosomal DNA fragments.
Transmission electron microscopy ultrastructural studies showed that mesophyll cell nuclei maintained their structural integrity during the ozone fumigation even if they assume a granular appearance. Nuclear material appeared to be condensated into discrete patches distributed through the nucleus. These ultrastructural changes were evident very early and particularly after 2.5 h of ozone fumigation, that is at the same time as the positive TUNEL staining. We further investigated the morphological changes occurring in the cytosol of mesophyll cells of plants fumigated for 5 h with 150 nL L–1 ozone. We observe that mitochondria maintained their structural integrity, but the plasma membrane presented invaginations and the formation of vesicles. This was also reported during PCD in the HR (Mittler et al., 1997).
CONCLUSIONS
Our detection of the first signals of altered mitochondrial functionality already during the ozone treatment is evidence that PCD is triggered early during ozone treatment in tobacco cv Bel W3 plants. But which signal was responsible for the triggering? Several signals, including H2O2, nitric oxide, SA and ethylene are all implicated in the regulatory network that controls PCD in plants (Beers and McDowell, 2001). Because in our plant system, hallmarks of PCD preceded both H2O2 and SA accumulation (both were induced 7 h after terminating fumigation), these molecules must be involved in the activation of defense gene induction and the establishment of a typical HR, but not in PCD induction. The signal molecule that has been demonstrated to be induced very early in ozonated tobacco cv Bel W3 leaves is ethylene (Langebartels et al., 1991). Langebartels et al. (1991) showed a rapid production of ethylene in tobacco cv Bel W3 plants after 1 to 2 h of exposure. Ethylene production or action has also been linked to endosperm PCD (Young and Gallie, 2000), aerenchyma formation in root cortex cells (Drew et al., 2000) and epidermal cell death associated with adventitious root growth (Mergemann and Sauter, 2000). A critical role for ethylene in H2O2 accumulation has been demonstrated in ozone-induced (Moeder et al., 2002) and in camptothecin-induced cell death (De Jong et al., 2002). So ethylene may well also be the signal molecule that triggers PCD in tobacco cv Bel W3 leaves. Taken together these results demonstrate that the ozone response in the sensitive tobacco cv Bel W3 is very complex and mediated by three signal molecules: ethylene, H2O2, and SA. How nitric oxide cross-talks with the other signal molecules to mediate the ozone response of tobacco cv Bel W3 plants remains to be explained.
MATERIALS AND METHODS
Plant Material
The tobacco (Nicotiana tabacum L. cv Bel W3) seeds were kindly provided by Dr. V. Sisson of the Oxford Tobacco Research Station (Oxford, NC). Growth chamber conditions were: 14-h photoperiod, photosynthetic photon fluence rate of 120 μmol m–2 s–1, day/night air temperature of 25°/20°C, and relative humidity 60% to 75%. The fourth leaf from the apex of four treated and four untreated (controls) 12-week-old plants were used in all experiments, and all were run in triplicate.
Ozone Treatment
Plants were exposed for 5 h (from 8 am to 1 pm) to 150 nL L–1 O3 or to filtered air in plexiglass chambers (0.32 m3) under growth light intensity. The ozone produced by UV irradiation (OEG50L lamp, Helios Italquartz s.r.l., Milan) was continuously monitored by a UV-Photometric O3 analyzer (Thermo Electron Corporation, Franklin, MA). Sampling was done 2.5 h after the start of treatment, immediately after the ozone-fumigation (5 h), and after a further 10, 24, 48, and 72 h of initiating exposure. The H2O2 measurement was also sampled at 12 h. Plants were left in growth chamber to recover.
IWF Preparation and H2O2 Measurement
IWF was obtained as previously reported (Pasqualini et al., 2002). H2O2 concentration in the IWF was determined by the method of Jiang et al. (1990) based on the peroxidase-mediated oxidation of Fe+2, followed by the reaction of Fe+3 with xylenol orange o-cresolsulfonephtalein 3′-3′-bis[methylimino] diacetic acid, sodium salt. The method is extremely sensitive and used to measure low levels of water-soluble hydroperoxide present in the aqueous phase. H2O2 was determined by adding 300 μL of H2O2 to 200 μL of IWF and 500 μL of assay reagent (500 μm ammonium ferrous sulfate, 50 mm H2SO4, 200 μm xylenol orange, and 200 mm sorbitol). Absorbance of the Fe+3-xylenol orange complex (A560) was detected after 45 min. The specificity for H2O2 was tested by eliminating H2O2 in the reaction mixture with catalase. Standard H2O2 curves were obtained for each independent experiment by adding variable amounts of H2O2 to 500 μL of assay reagent. Data were normalized and expressed as micromolar in the IWF.
Hystochemistry and Microscopy
Autofluorescent materials and callose deposition were detected by the Dietrich et al. (1994) method. In brief, leaf discs were cleared by boiling in lactophenol and rinsed first in 50% (v/v) ethanol and then in water. To visualize autofluorescence, cleared leaf discs were examined with a UV epifluorescence microscope (DM RHC, Leica, Wetzlar, Germany). Callose deposition was analyzed by staining cleared leaves for 1 h at room temperature in 0.01% (w/v) aniline blue in 0.15 m K2HPO4 and examining them with epifluorescence microscope. Autofluorescence was visualized with excitation filter BP 450–490 (450–490 nm) and suppression filter LP 515 (515 nm), and callose with excitation filter BP 340–380 (340–380 nm) and suppression filter LP 425 (425 nm). For histochemical detection of H2O2, leaf discs were infiltrated with phosphate-buffered saline buffer, pH 7.4, containing 0.5% (w/v) DAB, examined under the light microscope, and photographed after at least 2 to 3 h. Samples for electron microscopy were prepared as previously described (Loreto et al., 2001).
Extraction and Assay of Phenolic Compounds
Total phenolic concentration was determined by the Folin-Ciocalteu method (Singleton and Rossi, 1965), in which 1 g of liquid N2-frozen leaves was macerated in 10 mL of ice-cold 95% (v/v) methanol. The mixture was kept for 48 h in the dark at room temperature. After filtration and adjustment of the volume (10 mL), the aqueous-methanolic extract was used for the phenolic assay. One milliliter of extract was added to 2 mL of Folin-Ciocalteu reagent (1:10 [v/v] Folin-Ciocalteu:water) and after mixing with a Vortex, was added to 8 mL of 700 mm Na2CO3 solution. A765 was measured after 120 min. Gallic acid was used as a standard.
Separation and Identification of Phenolic Compounds
Leaves (2 g of –80°C frozen material) were ground in a mortar with liquid nitrogen. The ground material was extracted in the dark for 48 h with 30 mL of an aqueous-alcoholic mixture (30:70 [v/v] water:MeOH). After filtration, the sample was evaporated at 45°C using a rotary evaporator. The aqueous sample was then partitioned into an equal volume of chloroform to eliminate pigments. The chloroform phase was discarded whereas the aqueous phase (9 mL) was resuspended in 9 mL of 4 n HCl and hydrolyzed for 45 min at 85°C. The mixture was then partitioned into 20 mL of ethyl acetate, the upper organic phase was evaporated at 45°C using a rotary evaporator, and the dried sample was resuspended into 2 mL of MeOH and after filtration (0.2-μm filter), was analyzed by HPLC. The sample was fractionated by reverse-phase gradient HPLC using a Hypersil column (250 mm long × 4 mm i.d., 5 μm; Teknokroma, Barcelona). Gradient conditions were: Solvent A, 3% (v/v) acetic acid; Solvent B, acetonitrile; 16% to 30% (v/v) B over 20 min; flow rate of 0.7 mL min–1; eluate was monitored at 340 nm. Identification of the major phenolic compounds was done by comparison of the retention time and UV spectra with those of reference compounds.
PAL Activity
Preparation of extracts for determination of PAL activity was done according to Tholakalabavi et al. (1997). The reaction mixture consisted of 6 mm Phe, 0.1 m sodium-borate buffer (pH 8.7), and 100 μL of enzyme in a total volume of 1.5 mL. The reaction was initiated after 10 min of preincubation by adding the substrate. After 70 min at 37°C, the reaction was stopped by the addition of 50 μL of 5 n HCl, and the reaction mixture was centrifuged at 13,000g for 10 min to remove precipitates. Enzyme activity was determined spectrophotometrically by measuring the amount of t-cinnamic acid formed at 290 nm. A calibration curve was prepared using a range of 2.5 and 25 mg of t-cinnamic acid.
Protein Assay
The soluble protein concentration in the desalted crude enzyme extract was measured by the dye-binding method of Bradford (1976) using bovine serum albumin (BSA) as standard.
Northern Analysis and Semiquantitative RT-PCR
Leaves were frozen in liquid nitrogen and ground to a fine powder. RNA was extracted and subjected to RNA gel-blot analysis as previously described (Pasqualini et al., 2001). RNA gel blots were hybridized with tobacco PR-1a cDNA (Novartis, Research Triangle Park, NC). Two micrograms of total RNA was DNaseI digested (Ambion, Austin, TX) and reverse transcribed for 1 h at 42°C by 200 units of SuperscriptII RT (Invitrogen, Carlsbad, CA) with 1× corresponding buffer, 10 mm dithiothreitol, 0.4 mm each dNTPs, 0.5 μg μL–1 oligo(dT) (5′-T(25)-VX-3′) primer (Invitrogen). The cDNA was used for PCR with 1 unit of Taq polymerase (Amersham Biosciences, Uppsala), 1× corresponding buffer, 0.2 mm each dNTP, and 10 μm of the actin, PALa, and PALb primers (Invitrogen). The PAL primers were designed on the published sequences (Taguchi et al., 1998) and deposited on the National Center for Biotechnology Information database with the accession numbers AB008199 (PALa) and AB008200 (PALb): PALa forward primer, 5′-CTGGTGTTGCACAAAATGGTCACC-3′; PALa reverse primer, 5′-GCGTTGAGGGTTTCGCCATTAGGT-3′; PALb forward primer, 5′-GGCATCAAATGGTCATGTTAATGGAGG-3′; and PALb reverse primer, 5′-GCATTAAGTGTCTCACCATTGGGAC-3′. The cDNA were standardized against actin 3 of tobacco, which was amplified using ACT1 primer (forward primer, 5′ TCTCGAGTTCCTGTTCATAGTC 3′) and ACT2 (reverse primer, 5′ GGCCCGCGATACTGGTGTGAT 3′). The thermal cycle conditions were 1 min of initial denaturation followed by a variable number of cycles (26 for actin and PALa and 30 for PALb) of denaturation at 94°C for 5 s, annealing at 60°C (for the PAL genes) or at 54°C (for actin gene) for 20 s, and extension at 72°C for 45 s; and then a final extension at 72°C for 7 min. The authenticity of the PCR products was checked by two directional sequencing using ABI Prism 310 Genetic Analyzer (PerkinElmer Life Sciences, Boston).
Cell Viability
Cell death was evaluated by (a) histochemical analysis using trypan blue; and (b) measuring ion leakage from leaf discs. For trypan blue staining, samples were covered with an alcoholic lactophenol trypan blue mixture (30 mL of ethanol, 10 g of phenol, 10 mL of water, 10 mL of glycerol, 10 mL of lactic acid, and 10 mg of trypan blue), placed in a boiling water bath for 2 to 3 min, left at room temperature for 1 h, transferred into a chloral hydrate solution (2.5 g mL–1), and boiled for 20 min to destain. After multiple changes of chloral hydrate solution to reduce the background, samples were equilibrated with 50% (w/v) glycerol, mounted, and observed with a stereomicroscope. For each ion leakage measurement, 10 leaf discs (1.1 cm in diameter) were floated, abaxial side up, on 5 mL of distilled water for 3 h at room temperature. After incubation, the conductivity of the bathing solution was measured with a conductivity meter (model MC1, Bibby Science, Stone, UK).
Mitochondria Isolation
Mitochondria were isolated according to Vianello et al. (1997). In brief, 50 g of leaves was cut and homogenized by a mortar in 120 mL of a medium composed of 20 mm HEPES-Tris, pH 7.6, 0.4 m Suc, 5 mm EDTA, 25 mm potassium metabisulfite, 0.3% (w/v) BSA, and 0.6% (w/v) polyvinilpolypyrrolidone. The homogenate was then filtered through eight gauze layers. Debris was again homogenized in 100 mL of the medium and filtered once more. The filtrate was centrifuged at 3,500g for 5 min at 4°C (1st centrifugation) to avoid plastid contamination. The supernatant was then centrifuged at 28,000g for 5 min (2nd centrifugation). The pellet was resuspended in 120 mL of homogenization medium in a Potter homogenizer. This fraction was centrifuged at 2,500g for 3 min (3rd centrifugation), and the supernatant was centrifuged at 28,000g for 5 min (4th centrifugation). The pellet (crude mitochondrial fraction) was resuspended in a final volume of 1 mL of 20 mm HEPES-Tris (pH 7.5), 0.4 m Suc, and 0.1% (w/v) BSA. The suspension was stored on ice at about 10 mg protein mL–1. The intactness of the outer mitochondrial membrane was monitored by KCN-sensitive, succinatecytochrome c oxidoreduttase activity as described by Douce et al. (1973), resulting in an improvement in intactness from about 80% to approaching 90%. The supernatant obtained after the 2nd centrifugation (cytosolic fraction) was centrifuged at 100,000g for 40 min, and the supernatant was fractionated with ammonium sulfate. Solid ammonium sulfate was added at 4°C with stirring to give 40% saturation. The precipitate was removed by centrifugation at 10,000g for 10 min, and the supernatant was brought to 90% saturation with ammonium sulfate. After 30 min at 4°C, the suspension was centrifuged at 10,000g for 10 min. The precipitate, dissolved in 15 mL of 20 mm HEPES-Tris (pH 7.5) and 0.4 m Suc, was dialyzed against 10 mm HEPES-Tris (pH 7.5) for 12 h and represents cytoplasmic fraction. To assess an eventual mitochondrial contamination, cytoplasmic fraction was tested for the activity of cytochrome oxidase (Storrie and Madden, 1990) and were also examined by western blotting (see below) for the presence of cytochrome oxidase subunit IV. Both analyses demonstrated that no mitochondria were detectable in cytoplasmic fraction.
Cytochrome c Release Measurements
Samples of mitochondrial and cytoplasmic fractions were separated by SDS-PAGE (15% [w/v] acrylamide/4% [w/v] acrylamide stacker) to detect the presence of cytochrome c, according to the method described by Mather and Rottenberg (2001) with minor changes. The resolved polypeptides were electroblotted overnight for 30 V to nitrocellulose membrane in 25 mm Tris-HCl-0.2 m Gly-20% (v/v) methanol transfer buffer. The blots were saturated for 1 h in a blocking buffer (5% [w/v] skim milk in Tris saline buffer) and then for 2 h at room temperature with mouse monoclonal antibodies for denatured cytochrome c (1/500, Pharmingen, San Diego) or cytochrome oxidase subunit IV (0.1 μg mL–1, Molecular Probes, Eugene, OR). After washing, the membrane was incubated with antimouse peroxidase-conjugated IgG (1/80,000; Sigma-Aldrich, St. Louis) for 1 h at room temperature. Labeling was detected using the “SuperSignal West Dura” substrate, chemioluminescent reagent, according to supplier's manual (Pierce, Rockford, IL). The membrane was then exposed to an x-ray film for 1 min.
Protease Activity Assay
Proteolytic activity was performed according to Prestidge et al. (1971) using azocasein as substrate. Leaf samples were homogenized at 4°C with liquid nitrogen in a mortar with 5 volumes of 250 mm citrate buffer (pH 5.0) and 2 mm β-mercaptoethanol. The homogenate was centrifuged at 15,000g for 30 min at 4°C, and the extract was tested for proteolytic activity. Samples (50 μL) were mixed with 250 μL of the extraction buffer and 150 μL of azocasein (10 mg mL–1), and the reaction was stopped with 10% (w/v) trichloroacetic acid (TCA). Four test tubes were prepared for each sample; two, designated as toh, were immediately blocked with TCA, and the other two were incubated for 3 h at 37°C and then blocked with TCA (t3 h). After the addition of blocking solution, the samples were incubated at 4°C for 45 min and then were centrifuged at 14,000g for 5 min. The supernatants were read at 366 nm, and the relative protease activity was calculated as Abst3 h – Abst0 h. To test whether aspecific protease activity had a caspase-like activity, 1 mm PMSF, an inhibitor of the Ser proteases and of certain Cys proteases (papain family; Alonso et al., 1996), and 0.1 mm leupeptin, which is an established inhibitor of Cys proteases, were separately added to homogenate.
DNA Extraction and Analysis
DNA was extracted with DNeasy Plant Mini Kit (Qiagen USA, Valencia, CA). Ten micrograms of DNA was analyzed by 1% (w/v) agarose gel electrophoresis. DNA was visualized by UV light after ethidium bromide staining. To improve sensitivity, the DNA was transferred to a nylon membrane (Hybond N Plus, Amersham Biosciences) and hybridized by total tobacco DNA (cut with Sau3A) 32P-labeled by the random priming method.
In Situ Detection of Nuclear DNA Fragmentation
In situ detection of nuclear DNA fragmentation was performed according to Caccia et al. (2001) after minor modifications. Tissue was vacuum infiltrated for 10 min with 4% (w/v) formaldehyde in phosphate-buffered saline and then incubated overnight at room temperature. After dehydration in ethanol, the samples were embedded in paraffin. Twelve-micrometer-thick sections were deparaffinated and rehydrated in a graded ethanol series. For TUNEL analysis (Gavrieli et al., 1992), DIG-11-dUTP and an anti-digoxigenin antibody conjugated to alkaline phosphatase were used to visualize DNA 3′-OH groups. Sections were digested for 20 min at room temperature with proteinase K (0.5 μg mL–1 in 0.05 m Tris-HCl, pH 7.6), briefly rinsed with cold water and after with TdT buffer (25 mm Tris-HCl, pH 6.6, 200 mm sodium-cacodylate, and 5 mm CoCl2), and incubated in a humid atmosphere with labeling mix (10 units of TdT and 0.5 nmol of dig-11dUTP in 50 μL of TdT buffer per slide) for 1 h at 37°C. The reaction was terminated by rinsing the slides with 300 mm NaCl, 30 mm sodium-citrate for 30 min at room temperature. The slides were washed again for 10 min with 100 mm Tris-HCl, pH 7.5, and 150 mm NaCl and then for 50 min with the same buffer containing 1% (w/v) blocking reagent. The immunological detection was performed using DIG DNA labeling and detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Positive and negative controls were obtained by treating sections with DNase I (23 units mL–1) for 10 min at room temperature before TdT incubation and by incubating sections with labeling mix lacking TdT, respectively. Stained sections were observed under a bright-field light microscope and immediately photographed. For nuclear staining, the fluorescent dye DAPI at final concentration of about 1 mg mL–1 in phosphate buffer (pH 7.0) was used. The samples were examined with light and epifluorescence microscope (Leica DM RHC). For observation of DAPI staining, filters enabling the use of an excitation wavelength between 334 and 365 nm and an emission wavelength of 420 nm were used.
Acknowledgments
In the year of her retirement, this work is dedicated to Professor Marisa Antonielli to acknowledge her continuous support and commitment to plant physiology studies.
We thank Dr. Novello Pocceschi for the separation and identification of phenolic compounds, Dr. Andrea Mazzucato for valuable suggestions about TUNEL method, and Dr. Nicola Tosti for assistance with PCR analysis of PALa and PALb.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026591.
References
- Alonso M, Hidalgo J, Hendricks L, Velasco A (1996) Degradation of aggrecan precursors within a specialized subcompartment of the chicken chondrocyte endoplasmic reticulum. Biochem J 316: 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44: 429–442 [DOI] [PubMed] [Google Scholar]
- Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogen, stress and developmental cues. Curr Opin Plant Biol 4: 561–567 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson SA, Sherman Y, Gavrieli Y (1995) Identification of dying cells: in situ staining. Methods Cell Biol 46: 29–39 [PubMed] [Google Scholar]
- Bors W, Langebartels C, Michel C, Sandermann H Jr (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28: 1589–1595 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Caccia R, Delledonne M, Levine A, De Pace C, Mazzucato A (2001) Apoptosis-like DNA fragmentation in leaves and floral organs precede their developmental senescence. Plant Biosys 135: 183–189 [Google Scholar]
- De Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214: 537–545 [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou J-C (2000) Mitochondria as the control point of apoptosis. Trends Cell Biol 10: 369–377 [DOI] [PubMed] [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals J, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77: 565–577 [DOI] [PubMed] [Google Scholar]
- Douce R, Mannella CA, Bonner WD Jr (1973) The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta 292: 105–116 [DOI] [PubMed] [Google Scholar]
- Draper J (1997) Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci 2: 162–165 [Google Scholar]
- Drew MC, He CI, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5: 122–127 [DOI] [PubMed] [Google Scholar]
- Eastman A, Barry MA, Demarcq C, Li J, Reynolds JE (1994) Endonucleases associated with apoptosis. In E Mihich, R T Schimke, eds, Apoptosis. Plenum Press, New York, pp 249–259
- Eckey-Kaltenbach H, Ernst D, Heller W, Sandermann H Jr (1994) Biochemical plant responses to ozone: IV. Cross-induction of defensive pathways in parsley (Petroselinum crispum L.) plants. Plant Physiol 104: 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50 [DOI] [PubMed] [Google Scholar]
- Ernst D, Schraudner M, Langebartels C, Sandermann H Jr (1992) Ozone-induced changes of mRNA of β-1,3 glucanase, chitinase and “pathogenesis-related” protein 1b in tobacco plants. Plant Mol Biol 20: 673–682 [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J 18: 321–331 [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist DG (1998) Programmed Cell death in plant disease: the purpose and promise of cellular suicide. Annu Rev Phytopathol 39: 393–414 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Ausubel FM (1993) Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 4: 327–341 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Guo A, Klessig DF, Ausubel FM (1994) Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense reactions. Cell 77: 551–563 [DOI] [PubMed] [Google Scholar]
- Groover A, De Witt N, Heidel A, Jones A (1997) Programmed cell death of plant tracheary elements differentiating in vitro. Protoplasma 196: 197–211 [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Heggestad HE (1991) Origin of Bel-W3, Bel-C and Bel-B tobacco varieties and their use as indicators of ozone. Environ Pollut 74: 264–291 [DOI] [PubMed] [Google Scholar]
- Huh G-H, Damaz B, Matsumoto T, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29: 649–659 [DOI] [PubMed] [Google Scholar]
- Jiang Z-Y, Wollard ACS, Wolff SP (1990) Hydrogen peroxide production during experimental protein glycation. FEBS Lett 268: 69–71 [DOI] [PubMed] [Google Scholar]
- Jones AM, Dangl JL (1996) Logjam at the Styx: programmed cell death in plants. Trends Plant Sci 1: 114–119 [Google Scholar]
- Kangasjarvi J, Talvinen J, Utriainen M, Karjalainen R (1994) Plant defence systems induced by ozone. Plant Cell Environ 17: 783–794 [Google Scholar]
- Kanoun M, Goulas MJP, Biolley J-P (2001) Effect of a chronic and moderate ozone pollution on the phenolic pattern of bean leaves (Phaseolus vulgaris L. cv Nerina): relations with visible injury and biomass production. Biochem Syst Ecol 29: 443–457 [DOI] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR (2000) Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol 123: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Sherzer AJ, Eshita SM, Davis KR (1998) Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol 118: 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Langebartels C, Kerner K, Leonardi S, Scharaudner M, Trost M, Heller W, Sandermann H Jr (1991) Biochemical plant response to ozone: I. Differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol 95: 882–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27: 969–978 [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Linthorst HJM (1991) Pathogenesis-related proteins of plants. Crit Rev Plant Sci 10: 123–150 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Rottenberg H (2001) Polycations induce the release of soluble intermembrane mitochondrial proteins. Biochim Biophys Acta 1053: 357–368 [DOI] [PubMed] [Google Scholar]
- McCabe PF, Levine A, Meijer P-J, Tapon NA, Pennell RI (1997) A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J 12: 267–280 [Google Scholar]
- Mergemann H, Sauter M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 124: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Simon L, Lam E (1997) Pathogen-induced programmed cell death in tobacco. J Cell Sci 110: 1333–1344 [DOI] [PubMed] [Google Scholar]
- Moeder W, Barry CS, Tauriainen AA, Betz C, Toumainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjarvi J (2002) Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol 130: 1918–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd JB (1997) Biochemical basis for the toxicity of ozone. In M Yunus, M Iqba, eds, Plant Response to Air Pollution. Wiley & Sons, New York, pp 267–284
- Orvar BL, McPherson J, Ellis BE (1997) Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J 11: 203–212 [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H Jr, Kangasjarvi J (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panavas T, LeVangie R, Mistler J, Reid PD, Rubinstein B (2000) Activities of nucleases in senescing daylily petals. Plant Physiol Biochem 39: 837–843 [Google Scholar]
- Pasqualini S, Della Torre G, Ferranti F, Ederli L, Piccioni C, Reale L, Antonielli M (2002) Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Physiol Plant 115: 204–212 [DOI] [PubMed] [Google Scholar]
- Pasqualini S, Ederli L, Piccioni C, Batini P, Bellucci M, Arcioni S, Antonielli M (2001) Metabolic regulation and gene expression of root phosphoenolpyruvate carboxylase by different nitrogen sources. Plant Cell Environ 24: 439–447 [Google Scholar]
- Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9: 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L, Gage V, Spizizen J (1971) Protease activities during the course of sporulation in Bacillus subtilis. J Bacteriol 107: 815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Koch JR, Davis KR (2000) Ozone: a tool for probing programmed cell death in plants. Plant Mol Biol 44: 345–358 [DOI] [PubMed] [Google Scholar]
- Rao MV, Pallyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110: 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti S, Bonatti Medeghini P (2001) In situ histochemical monitoring of ozone- and TMV-induced reactive oxygen species in tobacco leaves. Plant Physiol Biochem 39: 433–442 [Google Scholar]
- Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8: 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H Jr, Ernst D, Heller W, Langebartels C (1998) Ozone: an abiotic elicitor of plant defense reactions. Trends Plant Sci 3: 47–50 [Google Scholar]
- Schraudner M, Ernst D, Langebartels C, Sandermann H Jr (1992) Biochemical plant responses to ozone: III. Activation of the defence-related proteins β-1,3-glucanase and chitinase in tobacco. Plant Physiol 99: 1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Langebartels C, Sandermann H (1996) Plant defence systems and ozone. Biochem Soc Trans 24: 456–461 [DOI] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van Camp W, Inzé D, Langebartels C, Sandermann H Jr (1998) Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J 16: 235–245 [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Smith SW, Jones MEE, Osborne BA (1993) Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci USA 90: 980–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YK, Davis KR (1997) The effects of ozone on antioxidant responses in plants. Free Radic Biol Med 23: 480–488 [DOI] [PubMed] [Google Scholar]
- Sharma YK, Leon J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA 93: 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticul 16: 144–158 [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11: 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Madden EA (1990) Isolation of subcellular organelles. Methods Enzymol 182: 203–225 [DOI] [PubMed] [Google Scholar]
- Taguchi G, Sharan M, Gonda K, Yanagisawa K, Shimosaka M, Hayashida N, Okazaki M (1998) Effect of methyl jasmonate and elicitor on PAL gene expression in tobacco cultured cells. J Plant Biochem Biotechnol 7: 79–84 [Google Scholar]
- Thalmair M (1996) Reaktion von streβ-proteinaen auf ozon und UV-B strahlung bei tabak (Nicotiana tabacum L. cv Bel W3 und Bel B). PhD thesis. University of Munchen, Germany
- Tholakalabavi A, Zwtazek JJ, Thorpe TA (1997) Osmotically-stressed poplar cell cultures: anthocyanin accumulation, deaminase activity, and solute composition. J Plant Physiol 151: 489–496 [Google Scholar]
- van Buuren ML, Guidi L, Fornalè S, Ghetti F, Francescheti M, Soldatini GF, Bagni N (2002) Ozone-response mechanisms in tobacco: implications of polyamine metabolism. New Phytol 156: 389–398 [DOI] [PubMed] [Google Scholar]
- Vianello A, Braidot E, Petrussa E, Macrì F (1997) ATP synthesis driven by a a-keto acid-stimulated alternative oxidase in pea leaf mitochondria. Plant Physiol 38: 1368–1374 [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG (1996a) Apoptosis: a functional paradigm for programmed cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu HM, Cheung AY (1996b) Pollination induces mRNA poly (A)tail-shortening and cell deterioration in flower transmitting tissue. Plant J 9: 715–727 [DOI] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel H-J, Overmyer K, Kangasjarvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25: 717–726 [Google Scholar]
- Wu HM, Cheung AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44: 267–281 [DOI] [PubMed] [Google Scholar]
- Yalpani N, Enjedi A, Leon J, Raskin I (1994) Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193: 372–376 [Google Scholar]
- Yen CH, Yang CH (1998) Evidence for programmed cell death during leaf senescence in plants. Plant Cell Physiol 39: 923–927 [Google Scholar]
- Young TE, Gallie DR (2000) Programmed cell death during endosperm development. Plant Mol Biol 44: 283–301 [DOI] [PubMed] [Google Scholar]