Abstract
p-Chlorophenoxyisobutyric acid (PCIB) is known as a putative antiauxin and is widely used to inhibit auxin action, although the mechanism of PCIB-mediated inhibition of auxin action is not characterized very well at the molecular level. In the present work, we showed that PCIB inhibited BA::β-glucuronidase (GUS) expression induced by indole-3-acetic acid (IAA), 2,4-dichlorophenoxyacetic acid, and 1-naphthaleneacetic acid. PCIB also inhibited auxin-dependent DR5::GUS expression. RNA hybridization and quantitative reverse transcriptase-polymerase chain reaction analyses suggested that PCIB reduced auxin-induced accumulation of transcripts of Aux/IAA genes. In addition, PCIB relieved the reduction of GUS activity in HS::AXR3NT-GUS transgenic line in which auxin inhibits GUS activity by promoting degradation of the AXR3NT-GUS fusion protein. Physiological analysis revealed that PCIB inhibited lateral root production, gravitropic response of roots, and growth of primary roots. These results suggest that PCIB impairs auxin-signaling pathway by regulating Aux/IAA protein stability and thereby affects the auxin-regulated Arabidopsis root physiology.
The plant hormone auxin (indole-3-acetic acid [IAA]) plays an important role in every aspect of plant growth and development (Thimann, 1977; Davies, 1995). Despite its physiological significance, the molecular mechanism of auxin action is not fully understood yet. One of the earliest events in the auxin action involves the changing of expression pattern of some specific genes with a lag period of 5 to 30 min (Abel and Theologis, 1996). Auxin-dependent degradation of Aux/IAA proteins is a key event for early auxin-dependent gene induction (Leyser, 2002). Aux/IAA proteins interact with auxin response factors (ARFs) that bind to auxin-responsive elements (AuxREs) in the auxin-responsive promoters (Guilfoyle, 1998). Aux/IAA proteins are short-lived proteins, and their stability is regulated by auxin through the ubiquitin-mediated protein degradation pathway (Leyser, 2002). In the absence of auxin, large amounts of Aux/IAA proteins are present, bind to ARFs, and prevent ARFs to activate transcription of auxin-induced genes from AuxREs. In the presence of auxin, degradation of Aux/IAA proteins is promoted, and then ARF proteins are released from Aux/IAA proteins and activate transcription of auxin-responsive genes (Tiwari et al., 2003). Although detailed molecular mechanism of the role of ARF and Aux/IAA proteins and their regulation has been revealed in these recent years, the mechanisms of the very early step of auxin signaling, i.e. how auxin is recognized by plant cell and how ubiquitin proteolysis system is activated, still remains unknown (Benfey, 2002).
p-Chlorophenoxyisobutyric acid (PCIB), also called α-(4-chlorophenoxy) isobutyric acid, 2-(p-chlorophenoxy)-2-methylpropionic acid, or clofibric acid, has been most widely used to inhibit auxin action (e.g. Kim et al., 2000; Xie et al., 2000). Because of the structural similarity of PCIB with a synthetic auxin 4-chlorophenoxyacetic acid (Jönsson, 1961) and its competitive inhibition character to auxin-induced physiological responses, it is believed that PCIB inhibits the auxin action by competing with auxin at the binding site of the auxin receptor (MacRae and Bonner, 1953). If this hypothesis is true, PCIB might be a useful tool to elucidate the mechanism of auxin perception and signal transduction and their role in plant growth and development. However, the detailed molecular mechanism of how PCIB inhibits auxin action is almost unknown. Although there are few reports demonstrating that PCIB inhibits auxin-regulated gene expression (Okamoto et al., 1995; Klotz and Lagrimini, 1996), the effects of PCIB have never been thoroughly investigated at the molecular level.
We previously designed BA::β-glucuronidase (GUS) transgenic Arabidopsis containing a GUS reporter gene under the control of the auxin-response domains (AuxRDs) of the promoter from the best-characterized primary auxin-responsive gene, pea (Pisum sativum) PS-IAA4/5 (Oono et al., 1998). GUS activity can be observed in the root elongation zone of the BA::GUS transgenic line in response to auxin. Other plant hormones and auxin antagonists did not induce GUS activity in the roots, indicating that the response is highly specific to auxin. This specific response of the BA::GUS transgenic line against auxin can be exploited to find genetic mutations or biologically active compounds that affect the early auxin gene expression (Oono et al., 1998; 2002; Hayashi et al., 2001). Recently, we found that PCIB inhibits the early auxin gene expression during the examination of auxin-related compounds with BA::GUS line. In this paper, we report characterization of PCIB as an auxin response inhibitor and elucidation of the physiological effects of PCIB on Arabidopsis root growth. We show that PCIB inhibited early auxin gene expression and auxin-dependent protein degradation. Our physiological studies reveal that PCIB inhibited gravitropic response, primary root elongation, and auxin-induced lateral root formation in Arabidopsis roots.
RESULTS
PCIB Impairs Auxin-Induced Gene Expression
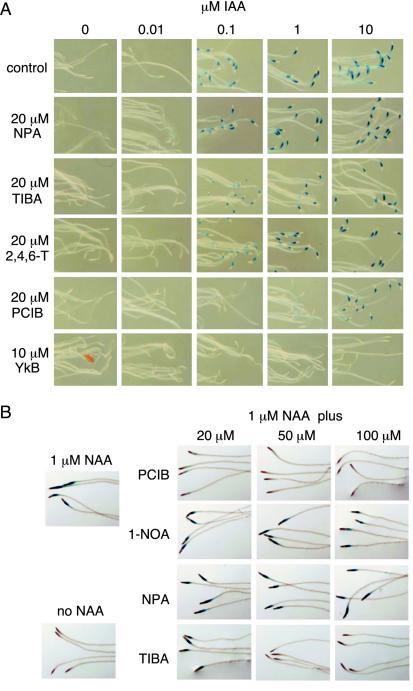
In the preliminary screen, we examined several auxin-related compounds to investigate their effects on auxin-regulated gene expression using the BA::GUS line. Twenty micromolar of N-(1-naphthyl) phthalamic acid (NPA) and 2,3,5-triiodobenzoic acid (TIBA), auxin efflux inhibitors (Lomax et al., 1995), and 2,4,6-trichrolophenoxyacetic acid (2,4,6-T), an inactive or weak auxin (Jönsson, 1961), showed no effect on GUS activity induced by 0.1 μm IAA, whereas 20 μm PCIB effectively inhibited this activity (Fig. 1A). The inhibitory effect of 20 μm PCIB could be overcome with increasing concentrations of IAA (Fig. 1A). Minimum concentration of PCIB to inhibit 0.1 μm IAA-induced GUS activity was between 0.5 and 2 μm (data not shown). Yokonolide B (YkB) isolated from Streptomyces diastatochromogenes has been recently identified as a new type of inhibitor for auxin signal transduction and strongly inhibited IAA-induced BA::GUS expression (Fig. 1A; Hayashi et al., 2001, 2003). In contrast to PCIB, this expression could not be restored with an application of 10 μm IAA (Fig. 1A).
Figure 1.
Effects of PCIB on GUS activity in the root elongation zone of the BA::GUS transgenic line. Five-day-old seedlings were treated with IAA, PCIB, and/or other compounds for 6 h and stained with 5-bromo-4-chloro-3-indolyl β-d-glucuronide (X-gluc). A, Dose response for IAA-induced BA::GUS expression in the presence of 20 μm each of NPA, TIBA, 2,4,6-T, PCIB, and 10 μm YkB. B, Effects of 20, 50, and 100 μm each of PCIB, 1-NOA, NPA, and TIBA on 1 μm NAA-induced BA::GUS expression.
We previously showed that two putative auxin influx inhibitors, Chromosaponin I (CSI) and 1-naphthoxyacetic acid (1-NOA), inhibited BA::GUS expression induced by IAA (Rahman et al., 2002). To investigate whether PCIB inhibition of IAA-induced BA::GUS expression is also a consequence of inhibition of auxin influx or not, we compared the effect of PCIB with the effects of inhibitors for auxin influx as well as efflux on 1-naphthaleneacetic acid (NAA)-induced BA::GUS expression (Fig. 1B). In contrast to IAA, NAA can enter the cells by bypassing the requirement an auxin influx carrier (Delbarre et al., 1996) and NAA-induced BA::GUS expression is not inhibited by either CSI or 1-NOA (Rahman et al., 2002). Figure 1B showed that 20 μm PCIB partially and 50 μm PCIB completely blocked 1 μm NAA-induced BA::GUS expression. Consistent with the previous report (Rahman et al., 2002), 1-NOA even at 100 μm failed to do so, clearly suggesting that the inhibitory effect of PCIB is not due to the blocking of auxin influx. Interestingly, auxin efflux inhibitor TIBA but not NPA inhibited NAA-induced BA::GUS expression at 50 μm (Fig. 1B), although 20 μm TIBA failed to inhibit 0.1 μm IAA- and 1 μm NAA-induced BA::GUS expression (Fig. 1, A and B), suggesting TIBA may have auxin antagonistic activity at high concentration. Twenty micromolar PCIB also blocked 1 μm 2,4-dichlorophenoxyacetic acid (2,4-D)-induced BA::GUS expression (data not shown).
We also examined several compounds, 2,4-dichloranisole (DCA), maleic hydrazide, transcinnamic acid, 3-indole-3-butyric acid (3-IBA), and 4,4,4-trifluoro-3-indole-3-butyric acid (TFIBA) that were reported or proposed to have an auxin antagonistic activity (van Overbeek et al., 1951; Leopold and Klein, 1952; MacRae and Bonner, 1953; Katayama et al., 1995; Tomic et al., 1998), and 1-aminocyclopropane-1-carboxylic acid, a precursor of ethylene (McKeon et al., 1995). Among these compounds, 50 μm TFIBA effectively and 100 μm trans-cinnamic acid slightly inhibited BA::GUS expression induced by 1 μm NAA (data not shown). Other compounds did not show any inhibitory effect on BA::GUS expression induced by 1 μm NAA at the concentration up to 100 μm (data not shown).
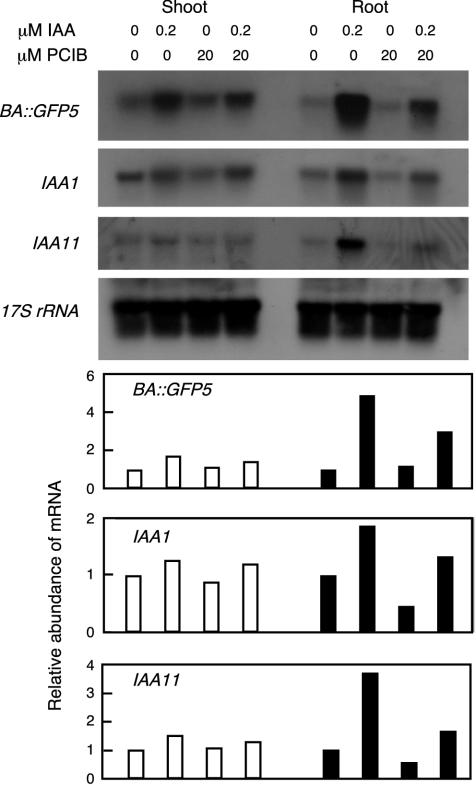
To analyze the effects of PCIB on the steady-state level of transcripts of the marker gene regulated by auxin-responsive promoter as well as that of endogenous early auxin genes, 5-d-old seedlings of the BA::mgfp5-ER line (Aspuria et al., 2002) were treated in liquid germination media (GM) containing IAA and/or PCIB for 2 h, and total RNA was extracted. Twenty and 10 μg of total RNA from shoots and roots, respectively, was subjected to hybridization analysis using DNA fragments of mGFP5-ER, IAA1, and IAA11 (Abel et al., 1995) as probes (Fig. 2). IAA (0.2 μm) increased the accumulation of mRNA for auxin-responsive genes quite effectively in roots and slightly in shoots. Application of 20 μm PCIB reduced the mRNA levels of the BA::mgfp5-ER gene in the presence as well as in the absence of 0.2 μm IAA. The effect of PCIB was much clearer in roots than in shoots. The similar inhibitory effect of PCIB could be observed not only in the mRNA level of transgene with synthetic promoter but also in the mRNA level of the endogenous auxin-inducible genes, suggesting that PCIB impairs early auxin gene expression at transcriptional level (Fig. 2).
Figure 2.
RNA hybridization analysis. Five-day-old seedlings of the BA::mgfp5-ER line were treated with 0 or 0.2 μm IAA and 0 or 20 μm PCIB for 6 h. Twenty micrograms and 10 μg of total RNA for shoots and roots, respectively, was separated by electrophoresis, blotted to a Nytran membrane, and probed with gene-specific probes for the indicated cDNAs. Quantification results were plotted relative to the mRNA level of mock treatment (arbitrary value of 1).
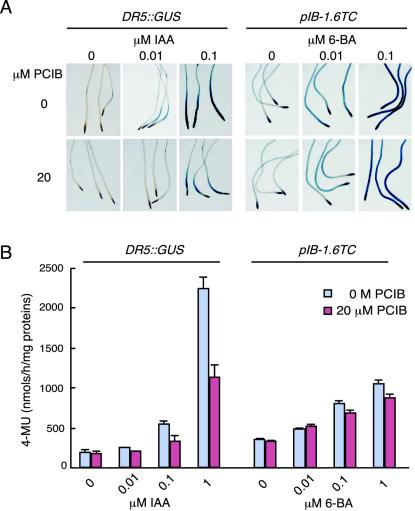
IAA responses in the presence or absence of PCIB were examined by using another auxin-responsive reporter line, DR5::GUS. The DR5::GUS construct consists of seven tandem repeats of an auxin-responsive TGTCTC element, a minimal 35S CaMV promoter, and a GUS coding region. Exogeneously applied auxin induces GUS accumulation strongly in all cells in the primary root meristem of the transgenic DR5::GUS Arabidopsis line (Ulmasov et al., 1997; Sabatini et al., 1999). Therefore, auxin-induced GUS activity in DR5::GUS line can be analyzed quantitatively with higher sensitivity than in BA::GUS line. Application of 20 μm PCIB in the IAA incubation media effectively inhibited auxin-induced GUS activity in roots of DR5::GUS transgenic line (Fig. 3A). Quantitative GUS analysis using whole seedlings also showed that PCIB inhibited IAA-induced GUS activity, although the detectable level of auxin response was obtained at much higher concentrations of IAA than it was required in histochemical experiments (Fig. 3B). This may be because root tissue is much more sensitive to auxin than the aerial tissues that provide the majority of proteins in quantitative GUS analysis.
Figure 3.
Effects of PCIB on GUS activity in the DR5::GUS and pIB-1.6TC transgenic lines. Five-day-old seedlings were treated for 6 h. A, Histochemical GUS analysis in roots of DR5::GUS and pIB-1.6TC seedlings treated with or without 20 μm PCIB in the presence of various concentrations of IAA or 6-BA. B, Quantitative GUS analysis using whole seedlings. Bars represent se of the average (n = 6). Absence of error bars indicates error less than thickness of the line.
We also examined pIB-1.6TC transgenic line, which contains a GUS reporter gene with 1.6-kb upstream sequences of cytokinin-responsive gene ARR5 (D'Agostino et al., 2000). GUS expression in 5-d-old pIB-1.6TC seedlings treated with various concentrations of 6-benzyladenine (6-BA) and with or without 20 μm PCIB for 6 h is shown in Figure 3. The intensity of GUS staining was not significantly different with 20 μm PCIB treatment or without (Fig. 3A). The quantitative GUS assay using whole plant extract showed that PCIB had little or no effect on 6-BA-induced GUS activity in pIB-1.6TC transgenic line (Fig. 3B).
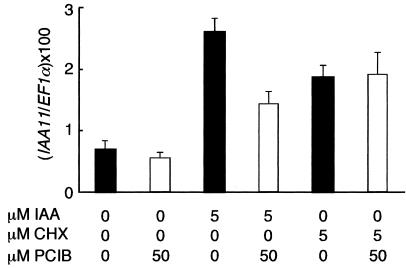
Cycloheximide (CHX), a well-characterized protein synthesis inhibitor, is known to stimulate mRNA expression of the primary auxin-responsive genes such as the Aux/IAA genes (Abel et al., 1995). In contrast to auxin, the mRNA induction by CHX is hypothetically caused by the inhibition of synthesis of short-lived transcription repressor proteins (Ballas et al., 1993). To examine whether PCIB affects CHX-induced mRNA induction, real-time reverse transcriptase (RT)-PCR with IAA11 primers were performed. The results clearly showed that PCIB inhibited IAA-induced but not CHX-induced mRNA expression of IAA11 gene, suggesting that PCIB specifically affects the auxin-signaling pathway (Fig. 4).
Figure 4.
Real-time PCR analysis of the expression of IAA11 gene. Seven-day-old seedlings were treated with IAA or CHX with or without PCIB for 2 h. Relative copy number of IAA11 transcripts was calculated by normalizing with number of copies of EF1αA4 transcript. The average of five independent treatments, RNA extraction, and RT-PCR was shown. Bars represent se of the average.
PCIB Prevents Auxin-Mediated Aux/IAA Protein Degradation
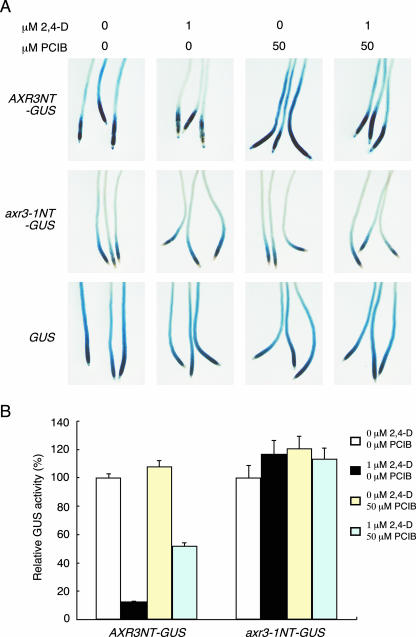
Recently, it was proposed that auxin promotes degradation of Aux/IAA proteins through ubiquitin-proteasome pathway and thereby induces the early auxin response (Zenser et al., 2001; Ramos et al., 2001; Gray et al., 2001; Tiwari et al., 2001). To address PCIB effect on ubiquitin-proteasome-dependent Aux/IAA degradation, we examined GUS activity in the HS::AXR3NT-GUS transgenic Arabidopsis line, which produces GUS protein fused translationally with the N-terminal half of AXR3. Because the N-terminal half of AXR3 is a target of ubiquitin-proteasome-dependent protein degradation system, the GUS activity in the HS::AXR3NT-GUS transgenic line is reduced by incubating with auxin after heat treatment (Gray et al., 2001). HS::AXR3NT-GUS seedlings treated with 1 μm 2,4-D showed decreased GUS activity in root compared with the control seedlings (Fig. 5, A and B). Our results showed stronger GUS activity than those reported by Gray et al. (2001), and more time was required for detecting the degradation of GUS activity. This may be due to the difference of the method to give the heat treatment, a most critical step for controlling basal GUS activity. The degradation of GUS activity by 1 μm 2,4-D was partially relieved by the addition of 50 μm PCIB (Fig. 5, A and B). To confirm that PCIB-induced increase in GUS activity in the presence of 2,4-D is related to the protein stability of the AXR3NT-GUS protein, we examined two control lines, HS::axr3-1NT-GUS, in which the mutation in domain II of AXR3 resulting in an increased stability of the protein, and HS::GUS transgenic lines (Gray et al., 2001). Neither 2,4-D nor PCIB significantly affected GUS activity in these transgenic lines (Fig. 5, A and B). These results suggested that PCIB partially inhibits the auxin-mediated degradation of Aux/IAA protein through ubiquitin-proteasome pathway.
Figure 5.
PCIB impairs auxin-dependent degradation of Aux/IAA protein stability. A, Seven-day-old HS::AXR3NT-GUS, HS::axr3-1NT-GUS, and HS::GUS transgenic seedlings were incubated in GM liquid media for 120 m at 37°C followed by transferring seedlings into GM liquid media at 23°C. After 20 m, seedlings were transferred into new GM liquid media containing 2,4-D and/or PCIB and incubated for another 120 m. The seedlings were then incubated in GUS-staining buffer containing X-gluc for 18 h for the HS::AXR3NT-GUS line or 1 h for the HS::axr3-1NT-GUS and HS:: GUS transgenic lines. B, Excised roots from 7-d-old seedlings were treated with 2,4-D and/or PCIB as in A. Relative GUS activity was measured quantitatively and expressed as percentage of the mock incubation.
Inhibition Kinetics of PCIB
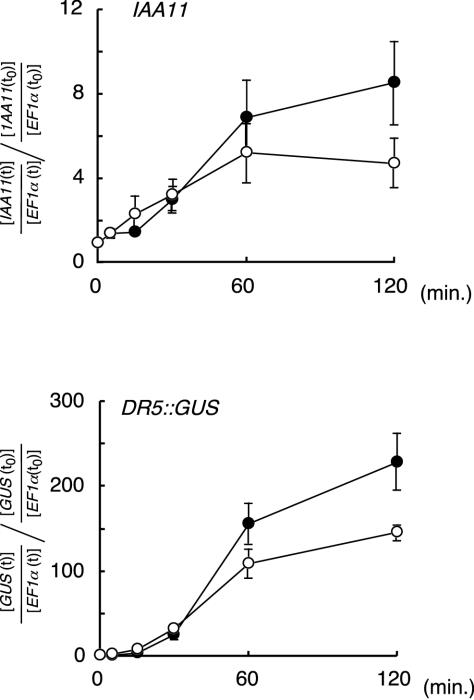
It has been hypothesized that PCIB inhibits the auxin action by competing with auxin at the binding site of the auxin receptor (MacRae and Bonner, 1953). There are several auxin-binding proteins identified in different subcellular locations (Jones, 1994). If auxin and PCIB simply compete at the extracellular binding site on the cell surface, it is logical to assume that PCIB would inhibit auxin action immediately. To assess this possibility, time course of IAA-induced mRNA level in the presence or absence of PCIB was examined by real-time RT-PCR (Fig. 6). It has been reported that IAA induces mRNA of primary auxin responsible genes very rapidly (Theologis et al., 1985; Koshiba et al., 1995). The response of most Aux/IAA members in Arabidopsis occurs within 5 to 30 min (Abel et al., 1995). Consistent with the previous reports, our results also showed that level of IAA11 and DR5::GUS mRNA were increased by 1 μm IAA within 30 min of incubation (Fig. 6). However, the inhibitory effect of PCIB could not be clearly detected until 1 h after starting the incubation. The presence of the lag period to see the PCIB effect suggests that IAA and PCIB do not simply compete at their extracellular binding site.
Figure 6.
Time course of the induction of IAA11 and DR5::GUS gene expression by 1 μm IAA in the presence (white circle) or absence (black circle) of 20 μm PCIB. Seven-day-old DR5::GUS transgenic seedlings were treated for the time indicated. Three independent sets of experiments were done and the averages of the values relative to the expression level of the zero time control are shown. Bars represent se of the average.
Physiological Effects of PCIB on Arabidopsis Root Growth
The results shown above suggested that PCIB inhibited an early step of auxin signaling and prompted us to examine the effects of PCIB at physiological level. Because we found severe physiological effects of PCIB in root but not in aerial part of Arabidopsis seedlings, we focused our study on analysis of root growth.
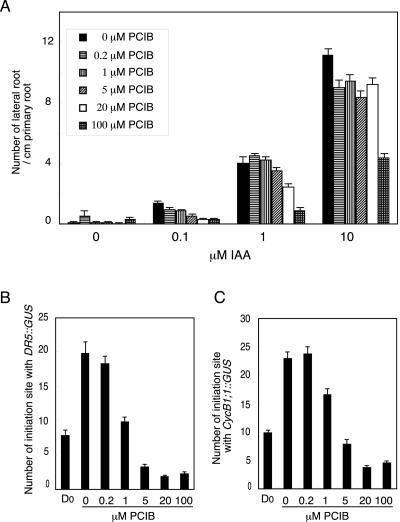
Figure 7A shows the density of lateral roots (the number of lateral root per centimeter of primary root length) in the seedlings treated for 5 d in the medium containing IAA and/or PCIB. PCIB inhibited exogenous IAA-induced emergence of lateral roots. Higher concentration of PCIB was required to inhibit lateral root emergence that was induced by higher concentration of IAA. During the early lateral root initiation, auxin is involved in determination of the position and frequency of lateral root, initiation of cell division in pericycle, and maintenance of active lateral root meristem (Celenza et al., 1995; Himanen et al., 2002). To determine which stage PCIB inhibits, we examined two transgenic lines harboring the DR5::GUS and the CycB1;1::GUS genes. The DR5::GUS gene is frequently used to visualize auxin accumulation and auxin response at cellular resolution (Sabatini et al., 1999; Rashotte et al., 2001; Friml et al., 2002). On the other hand, the transcription from the CycB1;1 promoter is closely associated with the G2 and M phases of cell division. The CycB1;1::GUS gene can be used as an indicator for early mitotic event associated with lateral root initiation (Ferreira et al., 1994). Both markers are expressed in the dividing pericycle cells of lateral root initiation and indicate the initial cells where lateral roots initiation occurs (Ferreira et al., 1994; Casimiro et al., 2001; A. Rahman and Y. Oono, unpublished data). Figure 7, B and C, show the number of the lateral root sites stained with GUS in DR5::GUS and CycB1;1::GUS lines, respectively. In both markers, the number of GUS-staining sites was significantly reduced with increasing concentration of PCIB. These results suggested that PCIB inhibited auxin response and subsequent initiation of cell division in lateral root initiation site.
Figure 7.
Effects of different concentrations of IAA and PCIB on lateral root production. A, Five-day-old seedlings grown vertically were transferred onto GM media containing various concentrations of IAA and/or PCIB and incubated vertically for additional 5 d. The number of emerging lateral roots (average ± se) per centimeter of primary root was calculated in each seedling (n = 30). B and C, Five-day-old seedlings grown vertically were transferred onto GM media containing various concentrations of PCIB and incubated vertically for additional 5 d. DR5::GUS (B) or CycB1;1::GUS (C) seedlings were subjected to histochemical GUS staining. Number of GUS-staining sites in pericycle cells was counted as the number of lateral root initiation site under the microscope. Bars represent se of the average (n = 10). Number of GUS-staining sites in the seedling before transfer is presented as D0.
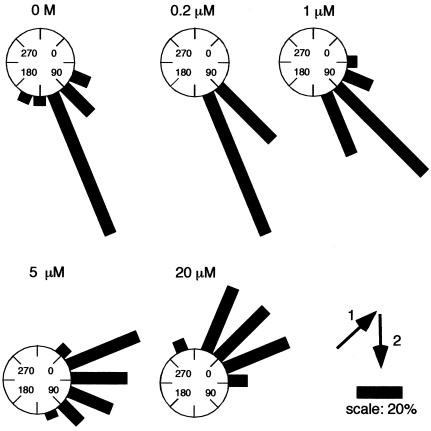
Because auxin was suggested to be a signal for controlling gravitropic response (Muday, 2001), we examined gravitropic response of Arabidopsis roots in the presence of various concentrations of PCIB. Five-day-old seedlings grown on GM medium were transferred to fresh GM medium containing various concentrations of PCIB and continued to grow in the dark for 3 d with changing the gravity orientation through 135°. Figure 8 shows the distributions of root growth directions of the seedlings. PCIB significantly reduced gravitropic response of Arabidopsis roots from 1 μm and completely inhibited gravitropic response at 20 μm.
Figure 8.
The distributions of root growth directions of the seedlings after 3 d of stimulation at 135° to the vertical (n = 24). Five-day-old seedlings were transferred onto GM media containing various concentrations of PCIB and incubated in the dark for 3 d after rotating plates to 135°. The arrows indicate the vector of gravity before (1) and after (2) the commencement of gravistimulation. Zero degrees represents roots that showed nil response. The angles were grouped into 16° classes and plotted as circular histograms.
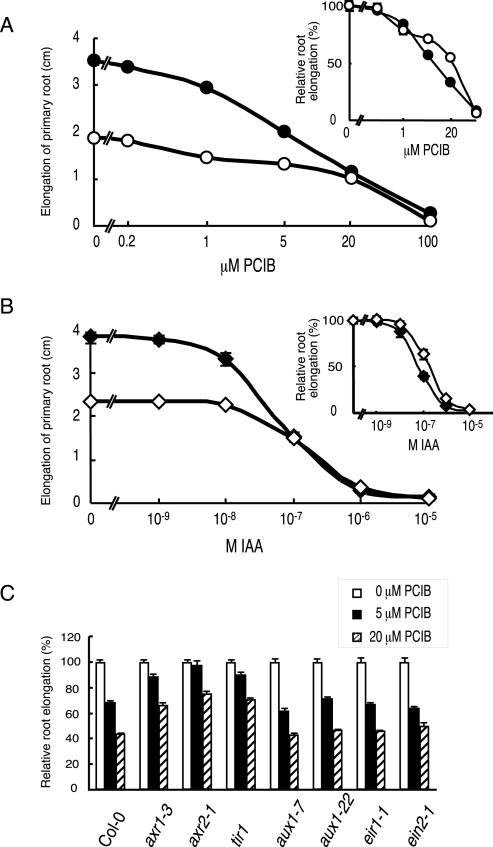
Figure 9 shows the length of the primary root of the seedlings treated for 5 d on GM medium containing various concentrations of PCIB. In contrast to counteractive effect of PCIB in lateral root emergence shown in Figure 7A, PCIB inhibited primary root elongation by itself and failed to counteract the effect of 0.1 μm IAA on root elongation (Fig. 9A). We also investigated dose response of IAA in the presence and absence of PCIB and found IAA did not counteract the inhibition of primary root elongation caused by 5 μm PCIB (Fig. 9B). Regarding the relative primary root elongation, 20 μm PCIB reduced primary root elongation to 33% of control in the absence of IAA but 55% of control in the presence of 0.1 μm IAA (Fig. 9A, inset). Furthermore, 0.1 μm IAA reduced primary root elongation to 40% of control in the absence of PCIB but 64% in the presence of 5 μm PCIB (Fig. 9B, inset). These data suggested that primary roots were more resistant to IAA or PCIB in the presence of PCIB or IAA, respectively. The mutants axr1-3, axr2-1, and tir1-1, which are defective in auxin signaling (Leyser et al., 1993; Ruegger et al., 1998; Nagpal et al., 2000; del Pozo et al., 2002), showed partial resistance to PCIB compared with wild type on primary root elongation (Fig. 9C).
Figure 9.
Effects of different concentrations of IAA and PCIB on primary root elongation. Five-day-old seedlings grown vertically were transferred to fresh medium containing IAA and/or PCIB at different concentrations and cultured vertically for another 5 d. A and B, Dose response of PCIB in the presence (white circle) or absence (black circle) of 0.1 μm IAA (n = 40; A), and dose response of IAA in the presence (white diamond) or absence (black diamond) of 5 μm PCIB (n = 20; B). The average of elongated length with se during 5 d of incubation was presented. Absence of bars indicates error less than size of symbol. The same data were plotted as a percentage of root elongation and shown in the upper right corner. C, Relative root elongation in the auxin-related mutants (n > 30). Mean values (cm) ± se in the absence of PCIB for 100% root growth were 3.54 ± 0.07 (Col-0), 4.82 ± 0.12 (axr1-3), 3.44 ± 0.08 (axr2-1), 3.38 ± 0.01 (tir1-1), 3.89 ± 0.11 (aux1-7), 4.11 ± 0.11 (aux1-22), 3.34 ± 0.11 (eir1-1), and 3.73 ± 0.12 (ein2-1).
On the other hand, the mutants defective in auxin influx and efflux carrier, aux1-7, aux1-22, eir1-1 (Bennett et al., 1996; Luschnig et al., 1998), and ethylene insensitive mutant ein2-1 (Guzman and Ecker, 1990), showed the same response as wild type, suggesting that auxin transport and ethylene are not but auxin signaling is involved in the PCIB-mediated inhibition of the primary root elongation (Fig. 9C).
DISCUSSION
PCIB Inhibits Auxin Response
More than 50 years ago, it was proposed that PCIB acts as an antiauxin (Burström, 1950). PCIB inhibits many auxin-induced physiological effects. For example, PCIB antagonizes IAA-induced inhibition of wheat (Triticum aestivum) root growth (Burström, 1950) and competitively inhibits IAA and 2,4-D induced growth of Avena sp. coleoptile (MacRae and Bonner, 1953).
Because of its structure and inhibition kinetics, PCIB has been believed to inhibit auxin action by competing with auxin at its binding site of the receptor (MacRae and Bonner, 1953). On the other hand, the effect of PCIB was often attributed to the inhibition of auxin transport (Katekar and Geissler, 1980; Tsai and Arteca, 1984), the regulation of auxin metabolism (Frenkel and Haard, 1973), or nonspecific toxic effect (Yip and Yang, 1986). Although the effects of PCIB presented in this study such as inhibition of lateral root development, loss of gravitropic response, and inhibition of primary root growth are also caused by auxin influx or efflux inhibitors (Fujita and Syono 1996; Luschnig et al., 1998; Casimiro et al., 2001; Malamy and Ryan, 2001; Rahman et al., 2001, 2002), some of the PCIB effects are crucially different from the effects of auxin influx and efflux inhibitors. First, PCIB inhibited not only IAA-induced, but also both NAA- and 2,4-D-induced BA::GUS expression. NAA and 2,4-D do not require influx and efflux carrier for moving in and out of the cells, respectively (Delbarre et al., 1996). Second, root growth in Arabidopsis mutants aux1-7, aux1-22 and eir1-1, which are defective in auxin influx or efflux carriers and resistant to auxin transport inhibitors such as NPA and TIBA (Morris, 2000; Luschnig et al., 1998), were not resistant to PCIB. In addition, auxin transport activity in the Arabidopsis inflorescence axis was inhibited by 9-hydroxyfluorene-9-carboxylic acid, NPA, and TIBA but not by PCIB (Okada et al., 1991). PCIB did not affect the auxin influx or efflux during the auxin transport in suspension cultured tobacco cells (Imhoff et al., 2000). All these evidences also support our observation that PCIB does not function as auxin transport inhibitor.
Another explanation for the PCIB-induced inhibition of early auxin gene expression could be that PCIB enhances destruction of auxin. In fact, Frenkel and Haard (1973) reported that PCIB decreased the Km of IAA oxidase extracted from Barlett pear in vitro and hypothesized that the antiauxin action of PCIB is due to the acceleration in the breakdown of auxin. However, this hypothesis is not appropriate to explain the result shown in this work, because PCIB inhibited the BA::GUS expression induced by NAA and 2,4-D, which are very stable and are not substrates for enzymatic oxidation (Thimann, 1977).
Yip and Yang (1986) proposed that a high concentration of PCIB is not a specific inhibitor of auxin-mediated ethylene production. They observed that 1 mm PCIB effectively inhibits ethylene production caused by 100 μm IAA, benzyladenine, and thidiazuron, a cytokinin-active urea derivative. In our experiments, all the PCIB responses such as inhibition of early auxin gene expression, lateral root production, gravitropic response, and primary root growth are demonstrated at lower concentrations (5–50 μm) than they used in their study. In addition to this, we have several lines of evidence to suggest that PCIB-mediated inhibition is not simply due to the nonspecific toxic effects. First, applying as little as 10 μm IAA counteracts PCIB-induced inhibition of early auxin gene expression and lateral root emergence. Second, GUS activity, which was observed by nonauxin-specific cytokinin inducible promoter, was not extensively inhibited in the presence of PCIB. Third, 2,4-D-induced reduction of GUS activity in HS::AXR3NT-GUS plants was counteracted by PCIB treatment. All of these results suggest that PCIB-mediated effects that we observed are not a toxic effect but rather support our idea that PCIB specifically inhibits auxin response in Arabidopsis roots.
PCIB Disturbs Auxin Signaling at the Upstream of Auxin-Mediated Aux/IAA Protein Degradation
It is well known that expression of the primary auxin-induced genes is enhanced by not only auxin but also CHX, a protein synthesis inhibitor (Theologis et al., 1985; Abel et al., 1995). This CHX-mediated increase of mRNA expression is explained by decreasing short-lived transcriptional repressor in a consequence of CHX-directed inhibition of protein synthesis (Ballas et al., 1993). Recent evidence suggests that Aux/IAA proteins are the short-lived repressors that regulate expression of primary auxin-induced genes including Aux/IAA genes themselves, and auxin promotes the degradation of Aux/IAA proteins by ubiquitin-mediated process (Leyser, 2002). Thus, although both auxin and CHX induce primary auxin-responsive genes by reducing amount of repressor, their acting mechanisms are quite different. Our observation that PCIB inhibited IAA-induced but not CHX-induced expression of IAA11 gene suggested that PCIB specifically acts on auxin signaling.
Several proteins, such as AXR1 and TIR1, which are involved in the ubiquitin-related Aux/IAA degradation and auxin response, are well characterized (Leyser 2002). The functional defects of these proteins cause increasing of the stability of Aux/IAA proteins and reduction in auxin response. The domain II in the N-terminal half of Aux/IAA protein is responsible for auxin-dependent ubiquitin-mediated degradation (Leyser, 2002). The mutations in domain II of Aux/IAA proteins increase the protein stability dramatically and result in a loss of auxin response. The transgenic plants expressing the AXR3NT-GUS, the fusion protein of N-terminal of AXR3 and GUS, are useful tool to examine the stability of Aux/IAA proteins. Increasing GUS activity has been reported in the axr1 and tir1-1 mutants containing the HS::AXR3NT-GUS gene (Gray et al., 2001). In our experiments, PCIB counteracted the 2,4-D-induced reduction of GUS activity in the HS::AXR3NT-GUS line, suggesting that PCIB acts through stabilizing the Aux/IAA proteins. Furthermore, PCIB did not affect GUS activity of the axr3–1NT-GUS whose stability is no longer regulated by auxin because of the mutation in domain II. These results together with the fact that PCIB did not inhibit CHX-induced IAA11 gene expression suggest that PCIB acts negatively on auxin-signaling pathway upstream of ubiquitin-mediated degradation of Aux/IAA proteins.
The hypothesized PCIB action is that PCIB binds to the auxin receptor and inhibits the auxin action (MacRae and Bonner, 1953). If the receptor was present on the cell surface and compounds could easily access to the receptor without any regulation, inhibitory effect of PCIB could be observed at the same time when auxin effects are detected. However, the presence of a 1-h lag-period to detect the PCIB action indicates that PCIB does not simply compete with auxin in the binding site on the cell surface. This time lag could be attributed by an explanation that the auxin-binding site inhibited by PCIB may be located inside the cell and lag-period may be due to the time required for PCIB entering into the cells. Although there are some evidences that a functional extracellular auxin-binding protein perceives auxin signal (Steffens et al., 2001), the presence of auxin uptake carriers such as AUX1 and altered physiology of aux1 mutants indicate that auxin signal for root growth is perceived intracellularly. The fact that the lack of AUX1 affected the expression level of Aux/IAA genes and BA::GUS transgene (Abel et al., 1995; Oono et al., 1998) strongly suggests that expression level of auxin-regulated gene is also controlled by intracellular auxin. Many functional auxin-binding sites were identified inside the cells (Jones, 1994; Kim et al., 2001). These binding sites may be the targets of PCIB. Alternative explanation for the lag-period of PCIB action could be due to that PCIB does not interact directly to functional auxin-receptor for early auxin gene expression. However, several biochemical experiments clearly showed that PCIB interferes with auxin binding to auxin-binding proteins (e.g. Ray et al., 1977; Moloney and Pilet, 1981; Prasad and Jones, 1991; Sugaya et al., 2000). Further experiments such as molecular genetic analysis will reveal the precise mechanism of PCIB action.
PCIB Influences Arabidopsis Root Growth
Several Arabidopsis mutants and cosuppression transgenic lines such as axr1, tir1, and CSN5tg, which are defective in auxin signaling because of loss of the components of ubiquitin-related protein degradation pathway, exhibit fewer lateral roots and reduced sensitivity to the gravity (Schwechheimer et al., 2001). Reduced lateral root formation and less sensitivity to the gravity are also reported in other auxin-signaling mutant, ask1-1 and ecr1-1, respectively (Gray et al., 1999; Dharmasiri et al., 2003). The similar characters were also observed in PCIB-treated seedlings, supporting our idea that PCIB impairs the cellular auxin response and thereby inhibits the initiation of lateral roots and gravity sensing. In contrast, the elongation of primary roots was inhibited by both auxin and PCIB, and these two compounds did not counteract each other. Burström (1950) also reported that 10 μm PCIB reduced the rate of wheat root elongation. Growth of primary roots is consequences of coordinated cell division, cell differentiation, and cell elongation of many different types of cells, all of which are regulated by auxin. Exogeneously applied PCIB impairs auxin response but probably would not equally affect to each auxin response in various cells, resulting in disruption of normal cellular organization and inhibition of root growth. It is interesting that the auxin-signaling mutants axr1-3, axr2-1, and tir1-1, but not the auxin influx or efflux mutants aux1s or eir1-1, showed PCIB resistance. This implies that PCIB targets auxin signal pathway to inhibit primary root growth. Evans et al. (1994) demonstrated that axr1 mutants are defective in both promotion and inhibition of primary root growth by low and high concentrations of auxin, respectively. Thus, it might be reasonable that inhibition of auxin response by PCIB is also ineffective in these auxin-signaling mutants. Although further characterization is required, we are speculating that PCIB-mediated inhibition of primary root growth is due to the inhibition of cellular auxin response by PCIB.
Auxin Action Inhibitors Could Be Useful Tools for Molecular Dissection of Auxin Signaling
Application of exogenous auxin transport inhibitors or the mutants having altered response to these inhibitors provide useful information in understanding the molecular mechanism of auxin transport and its role on plant development (Muday and DeLong, 2001). On the other hand, although several classes of compounds such as classical antiauxins, xyloglucan oligosaccharides, anion-channel blockers, and phospholipase A2 inhibitors have been shown to have an inhibitory effect on auxin action (Jönsson, 1961; McDougall and Fry, 1989; Scherer and Arnold, 1997; Thomine et al., 1997), they were less studied at molecular level, and their mode of action was not well understood until lately. Recently, Mauro et al. (2002) reported that oligogalacturonides inhibit the induction of auxin-responsive genes in tobacco. Unlike PCIB, the oligogalacturonides inhibit secondary auxin-responsive genes but not primary auxin-responsive genes, suggesting that oligogalacturonides act on relatively late auxin response. Yokonolide A and YkB 22-membered spiroketal-macrolides were isolated from S. diastatochromogenes as inhibitors of early auxin gene expression by screening against BA::GUS expression (Hayashi et al. 2001). The inhibitory effect of YkB on BA::GUS expression is quite strong compared with PCIB (Fig. 1). Further characterization of YkB suggested that YkB blocks degradation of Aux/IAA proteins (Hayashi et al., 2003). PD098059 and U0126, which are mitogen-activated protein kinase inhibitors, are also reported to inhibit BA::GUS expression (Mockaitis and Howell, 2000). More detailed characterization of these auxin response inhibitors including PCIB by combining the pharmacological approaches and molecular genetics, would provide new insights to elucidate the mechanism of auxin signaling.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All transgenic and mutants lines except the CycB1;1::GUS and pIB-1.6TC lines were derived from Arabidopsis Columbia (Col-0) ecotype. The CycB1;1::GUS and pIB-1.6TC lines were derived from C24 and Wassilewskija ecotypes, respectively (Ferreira et al., 1994; D'Agostino et al., 2000). Transgenic lines containing BA::GUS (BA3), BA::mgfp5-ER (line 23.1.1), and DR5::GUS are described by Oono et al. (1998), Aspuria et al. (2002), and Ulmasov et al. (1997), respectively. HS::GUS, HS::AXR3NT-GUS, and HS::axr3-1NT-GUS are described by Gray et al. (2001). Surface-sterilized seeds were plated on GM (one-half-strength Murashige and Skoog salts, 1% [w/v] Suc, and 0.5 g L–1 MES, pH 5.8, containing 1× B5 vitamins and 0.8% [w/v] Bacto Agar) in Eiken number 2 square plates (Eiken Kizai, Tokyo). For synchronous germination, the seeds were kept in the dark for 2 to 4 d at 4°C and then transferred to the growth room at 23°C under continuous light (20–40 μmol m–2 s–1).
Treatment with Plant Growth Regulators and Root Growth Analysis
For short-term treatments (0–6 h) with plant growth regulators, the vertically grown seedlings were transferred to GM liquid incubation media (GM without vitamins and agar) containing plant growth regulators and incubated in the dark. For long-term treatment (3–5 d) to examine root growth, 5-d-old seedlings grown on GM were transferred on fresh GM containing plant growth regulators and continued to grow in the same condition described above. The number of emerging lateral roots was counted by eye, and the length of primary root was recorded before and after 5-d incubation by digital camera. For gravitropic response, the position of the root tip was marked on the plate before and after treatment, and root images were recorded by digital camera. Primary root length and the angle of the root tip were measured by the NIH image software. Plant growth regulators were dissolved in dimethyl sulfoxide to make 1,000× stock solution. The same concentration of dimethyl sulfoxide was added in the control treatments. IAA, PCIB, and TIBA were purchased from Sigma-Aldrich (St. Louis); 1-NOA was from Aldrich Chemical Co. (Milwaukee); NPA and 2,4,6-T were from Tokyo Kasei Kogyo (Tokyo); DCA was from Avocado Research Chemicals Ltd (Lancashire, UK); 3-IBA and TFIBA were kindly provided by Dr. Masato Katayama (National Institute of Advanced Industrial Science and Technology, Nagoya, Japan); and other plant growth regulators were from Wako Pure Chemical Industries, Ltd. (Osaka).
Gene Expression Analysis
For GUS histochemical analysis, seedlings treated with auxin and other plant growth regulators were transferred to GUS-staining buffer (100 mm sodium phosphate, pH 7.0, 10 mm EDTA, 0.5 mm K4Fe[CN]6, 0.5 mm K3Fe[CN]6, and 0.1% [v/v] Triton X-100) containing 1 mm X-gluc and incubated at 37°C for 15 to 22 h unless indicated. Quantitative GUS fluorometric assay was performed as described by Stomp (1992). Protein concentration was determined with a protein assay reagent (Bio-Rad Laboratories, Hercules, CA) and bovine serum albumin (Sigma B-4287) as standard.
For RNA hybridization analysis, total RNA was isolated from shoots and roots by the procedure of Theologis et al. (1985), size-fractionated with a 1% (w/v) agarose gel containing formaldehyde, and transferred onto a Nytran (Schleicher & Schuell, Keene, NH) membrane. Hybridization was performed by using 32P-labeled probes according to the manual provided by Schleicher & Schuell. The blots were then exposed to x-ray film. DNA fragments for the probes were prepared by digesting plasmids containing cDNA of IAA1 and IAA11 (Abel et al., 1995) with EcoRI/HindIII and EcoRI/NcoI, respectively, and BA-mgfp5-ER/pPZP121 (Aspuria et al., 2002) with EcoRI/SacI. The DNA fragment for 17S rRNA is described by Zarembinski and Theologis (1993). Digital images of autoradiograms were generated by scanning x-ray films and quantitative analysis of radioactivity of each band was performed with NIH image software.
For real-time RT-PCR, total RNA was isolated from 7-d-old seedlings using RNeasy Plant Mini Kit (Qiagen, Tokyo) with on-column DNase digestion according to the provided handbook. The real-time RT-PCR was performed on the LightCycler Instrument (Roche Diagnostics, Mannheim) with the LightCycler-RNA Master SYBR Green I Kit (Roche Diagnostics) according to the manufacturer's protocol using the oligonucleotide primer sets (5′-GGTCTTACGTTGAGCCTTGG and 5′-GTGGCTGAAGCCTTAGCTTG for IAA11, 5′-CTGATAGCGCGTGACAAAAA and 5′-GGCACAGCACATCAAAGAGA for GUS, and 5′-CTTGCTTTCACCCTTGGTGT and 5′-TCCCTCGAATCCAGAGATTG for EF1αA4 gene). Each reaction (20 μL) was performed with 50 or 100 ng of total RNA, 0.3 μm each of forward and reverse primers, and 3.25 mm Mn(OAc)2. The reactions were incubated at 61°C for 20 m for reverse transcription, followed by denaturation at 95°C for 30 s and PCR with 45 cycles of 1 s at 95°C, 5 s at 55°C, and 13 s at 72°C. The specificity of the PCR amplification was checked with a melting curve analysis program and agarose gel electrophoresis of PCR products. Relative amount of specific mRNA was estimated using the standard cDNA preparation of known size and molecular concentration and normalized to the EF1αA4 mRNA level.
Acknowledgments
We thank Drs. Tom Guilfoyle for the DR5::GUS line; Ottoline Leyser and Stefan Kepinski for the HS::AXR3NT-GUS, HS::axr3-1NTGUS, and HS::GUS lines; Joe Kieber for the pIB-1.6TC line; Cropdesign (Gent, Belgium) for CycB1;1::GUS; Masato Katayama for kind gift of TFIBA and 3-IBA, and the Arabidopsis Biological Resource Center for providing mutants seeds. We also thank Drs. Seiji Tsurumi and Ayako Sakamoto for critical reading of the manuscript. We are indebted to Dr. Hiroshi Watanabe and members of the Department of Ion-beam-applied Biology at the Japan Atomic Energy Research Institute for valuable help and discussions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027847.
A.R. was supported by the Japanese Society for the Promotion of Science Postdoctoral Fellowship for Foreign Researchers, and E.T.A. was supported by the Science and Technology Agency of Japan Nuclear Researchers Exchange Program.
References
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251: 533–549 [DOI] [PubMed] [Google Scholar]
- Abel S, Theologis A (1996) Early genes and auxin action. Plant Physiol 111: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspuria ET, Ooura C, Chen GQ, Uchimiya H, Oono Y (2002) GFP accumulation controlled by an auxin responsive-promoter as a nondestructive assay to monitor early auxin response. Plant Cell Rep 21: 29–34 [Google Scholar]
- Ballas N, Wong L-M, Theologis A (1993) Identification of the auxin-responsive element, AuxRE, in the primary indoleacetic acid-inducible gene, PS-IAA4/5, of pea (Pisum sativum). J Mol Biol 233: 580–596 [DOI] [PubMed] [Google Scholar]
- Benfey PN (2002) Auxin action: slogging out of the swamp. Curr Biol 12: R389–R390 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Burström H (1950) Studies on growth and metabolism of roots: IV. Positive and negative auxin effects on cell elongation. Physiol Plant 3: 277–292 [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell Physiol 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL Jr, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- D'Agostino IB, Deruère J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124: 1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ (1995) Plant Hormones. Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Delbarre A, Muller P, Imhoff V, Morgat JL, Barbier-Brygoo H (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198: 532–541 [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M (2002) AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis cullin AtCUL1 is required for auxin response. Plant Cell 14: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S, Dharmasiri N, Hellmann H, Estelle M (2003) The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis. EMBO J 22: 1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Ishikawa H, Estelle M (1994) Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin-response mutants. Planta 194: 215–222 [Google Scholar]
- Ferreira PCG, Hemerly AS, de Almeida Engler J, Van Montagu M, Engler G, Inzé D (1994) Developmental expression of the Arabidopsis cycline gene cyc1At. Plant Cell 6: 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C, Haard NF (1973) Initiation of ripening in Bartlett pear with an antiauxin α(p-chlorophenoxy)isobutyric acid. Plant Physiol 52: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fujita H, Syono K (1996) Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol 37: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ (1998) Aux/IAA proteins and auxin signal transduction. Trend Plant Sci 3: 205–207 [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Jones AM, Ogino K, Yamazoe A, Oono Y, Inoguchi M, Kondo H, Nozaki H (2003) Yokonolide B, a novel inhibitor of auxin action, blocks degradation of Aux/IAA factors. J Biol Chem 278: 23861–23867 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ogino K, Oono Y, Uchimiya H, Nozaki H (2001) Yokonolide A, a new inhibitor of auxin signal transduction, from Streptomyces diastatochromogenes B59. J Antibiotic 54: 573–581 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff V, Muller P, Guern J, Delbarre A (2000) Inhibitors of the carrier-mediated influx of auxin in suspension-cultured tobacco cells. Planta 210: 580–588 [DOI] [PubMed] [Google Scholar]
- Jones AM (1994) Auxin-binding proteins. Annu Rev Plant Physiol Plant Mol Biol 45: 393–420 [Google Scholar]
- Jönsson A (1961) Chemical structure and growth activity of auxin and antiauxins. In W Ruhland, ed, Encyclopedia of Plant Physiology, Vol 14. Springer, Berlin, pp 959–1006 [Google Scholar]
- Katayama M, Kato K, Kimoto H, Fujii S (1995) (S)-(+)-4,4,4-trifuluoro-3-(indole-3)butyric acid, a novel fluorinated plant growth regulator. Experientica 51: 721–724 [Google Scholar]
- Katekar GF, Geissler AF (1980) Auxin transport inhibitors. Plant Physiol 66: 1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-K, Chang SC, Lee EJ, Chung W-S, Kim Y-S, Hwang S, Lee JS (2000) Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol 123: 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-S, Min J-K, Kim D, Jung J (2001) A soluble auxin-binding protein, ABP57 purification with anti-bovine serum albumin antibody and characterization of its mechanistic role in the auxin effect on plant plasma membrane H+-ATPase. J Biol Chem 276: 10730–10736 [DOI] [PubMed] [Google Scholar]
- Klotz KL, Lagrimini LM (1996) Phytohormone control of the tobacco anionic peroxidase promoter. Plant Mol Biol 31: 565–573 [DOI] [PubMed] [Google Scholar]
- Koshiba T, Ballas N, Wong L-M, Theologis A (1995) Transcriptional regulation of PS-IAA4/5 and PS-IAA6 early gene expression by indoleacetic acid and protein synthesis inhibitors in pea (Pisum sativum). J Mol Biol 253: 396–413 [DOI] [PubMed] [Google Scholar]
- Leopold AC, Klein WH (1952) Maleic hydrazide as an anti-auxin. Physiol Plant 5: 91–99 [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364: 161–164 [DOI] [PubMed] [Google Scholar]
- Leyser O (2002) Molecular genetics of auxin signaling. Annu Rev Plant Biol 53: 377–398 [DOI] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509–530
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae DH, Bonner J (1953) Chemical structure and antiauxin activity. Physiol Plant 6: 485–510 [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Mauro ML, De Lorenzo G, Costantino P, Bellincampi D (2002) Oligogalacturonides inhibit the induction of late but not of early auxin-responsive genes in tobacco. Planta 215: 494–501 [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC (1989) Structure-activity relationships for xyloglucan oligosaccharides with antiauxin activity. Plant Physiol 89: 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon TA, Fernandez-Maculet JC, Yang S-F (1995) Biosynthesis and metabolism of ethylene. In PJ Davies, ed, Plant Hormones. Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 118–139
- Mockaitis K, Howell SH (2000) Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 24: 785–796 [DOI] [PubMed] [Google Scholar]
- Moloney MM, Pilet PE (1981) Auxin binding in roots: a comparison between maize roots and coleoptiles. Planta 153: 447–452 [DOI] [PubMed] [Google Scholar]
- Morris DA (2000) Transmembrane auxin carrier systems-dynamic regulators of polar auxin transport. Plant Growth Regul 32: 161–172 [DOI] [PubMed] [Google Scholar]
- Muday G (2001) Auxins and tropisms. J Plant Growth Regul 20: 226–243 [DOI] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535–542 [DOI] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW (2000) AXR2 encodes a member of Aux/IAA protein family. Plant Physiol 123: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stage of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Tanaka Y, Sakai S (1995) Molecular cloning and analysis of the cDNA for an auxin-regulated calmodulin gene. Plant Cell Physiol 36: 1531–1539 [PubMed] [Google Scholar]
- Oono Y, Chen QG, Overvoorde PJ, Köhler C, Theologis A (1998) age1 mutants of Arabidopsis exhibited altered auxin-regulated gene expression. Plant Cell 10: 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Uchimiya H (2002) Expression pattern of Aux/IAA genes in the iaa3/shy2-1D mutant of Arabidopsis thaliana (L.). Ann Bot 89: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad P, Jones A (1991) Putative receptor for the plant growth hormone auxin identified and characterized by anti-idiotypic antibodies. Proc Natl Acad Sci USA 88: 5479–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurumi S (2001) Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol 125: 990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130: 1908–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JA, Zenser N, Leyser O, Callis J (2001) Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM, Dohrmann U, Hertel R (1977) Specificity of auxin-binding sites on maize coleoptile membrane as possible receptor sites for auxin action. Plant Physiol 60: 585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev 12: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Scherer GFE, Arnold B (1997) Inhibitors of animal phospholipase A2 enzymes are selective inhibitors of auxin-dependent growth: implications for auxin-induced signal transduction. Planta 202: 462–469 [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng X-W (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292: 1379–1382 [DOI] [PubMed] [Google Scholar]
- Steffens B, Feckler C, Palme K, Christian M, Böttger M, Lüthen H (2001) The auxin signal for protoplast swelling is perceived by extracellular ABP1. Plant J 27: 591–599 [DOI] [PubMed] [Google Scholar]
- Stomp A-M (1992) Using the GUS gene as a reporter of gene expression. In SR Gallagher, ed, GUS Protocols. Academic Press, San Diego, pp 103–113
- Sugaya S, Ohmiya A, Kikuchi M, Hayashi T (2000) Isolation and characterization of a 60 kDa 2,4-D-binding protein from the shoot apices of peach trees (Prunus persica L.): It is a homologue of protein disulfide isomerase. Plant Cell Physiol 41: 503–508 [DOI] [PubMed] [Google Scholar]
- Theologis A, Huynh TV, Davis RW (1985) Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol 183: 53–68 [DOI] [PubMed] [Google Scholar]
- Thimann KV (1977) Hormone action in the whole life of plants. University of Massachusetts Press, Amherst
- Thomine S, Leliévre F, Boufflet M, Guern J, Barbier-Brygoo H (1997) Anion-channel blockers interfere with auxin response in dark-grown Arabidopsis hypocotyls. Plant Physiol 115: 533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle T (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Wang X-J, Hagen G, Guilfoyle TJ (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic S, Gabdoulline RR, Kojic-Prodic B, Wade RC (1998) Classification of auxin plant hormones by interaction property similarity indices. J Comput-Aided Mol Des 12: 63–79 [DOI] [PubMed] [Google Scholar]
- Tsai D-S, Arteca RN (1984) Inhibition of IAA-induced ethylene production in etiolated mung bean hypocotyl segments by 2,3,5-triiodobenzoic acid and 2-(p-chlorophenoxy)-2-mehyl propionic acid. Physiol Plant 62: 448–452 [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overbeek J, Blondeau R, Horne V (1951) Trans-cinnamic acid as an anti-auxin. Am J Bot 38: 589–595 [Google Scholar]
- Xie Q, Frugis G, Colgan D, Chua NH (2000) Arabidopsis NAC1 transduced auxin signal downstream of TIR1 to promote lateral root development. Genes Dev 14: 3024–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W-K, Yang SF (1986) Effect of thidiazuron, a cytokinin-active urea derivative, in cytokinin-dependent ethylene production systems. Plant Physiol 80: 515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A (1993) Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol Biol Cell 4: 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J (2001) Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci USA 98: 11795–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]