Abstract
Alternative oxidase (Aox) is a nuclear-encoded mitochondrial protein. In soybean (Glycine max), the three members of the gene family have been shown to be differentially expressed during normal plant development and in response to stresses. To examine the function of the Aox promoters, genomic fragments were obtained for all three soybean genes: Aox1, Aox2a, and Aox2b. The regions of these fragments immediately upstream of the coding regions were used to drive β-glucuronidase (GUS) expression during transient transformation of soybean suspension culture cells and stable transformation of Arabidopsis. The expression patterns of the GUS reporter genes in soybean cells were in agreement with the presence or absence of the various endogenous Aox proteins, determined by immunoblotting. Deletion of different portions of the upstream regions identified sequences responsible for both positive and negative regulation of Aox gene expression in soybean cells. Reporter gene analysis in Arabidopsis plants showed differential tissue expression patterns driven by the three upstream regions, similar to those reported for the endogenous proteins in soybean. The expression profiles of all five members of the Arabidopsis Aox gene family were examined also, to compare with GUS expression driven by the soybean upstream fragments. Even though the promoter activity of the upstream fragments from soybean Aox2a and Aox2b displayed the same tissue specificity in Arabidopsis as they do in soybean, the most prominently expressed endogenous genes in all tissues of Arabidopsis were of the Aox1 type. Thus although regulation of Aox expression generally appears to involve the same signals in different species, different orthologs of Aox may respond variously to these signals. A comparison of upstream sequences between soybean Aox genes and similarly expressed Arabidopsis Aox genes identified common motifs.
The alternative oxidase (Aox) is one of the most studied components of the plant electron transport chain since the first reports of its activity in 1925 (Day et al., 1980; Moore and Siedow, 1991). It is encoded in the nucleus and imported into mitochondria via the general import pathway and is responsible for cyanide-insensitive respiration. Together with the rotenone-insensitive internal and external NAD(P)H dehydrogenases, it provides additional branch points for input and output in the plant mitochondrial electron transport chain.
With the development of an Aox monoclonal antibody (Elthon et al., 1989) and initial cloning of the Aox gene from Sauromatun guttatum (Rhoads and McIntosh, 1991), Aox has been intensively studied. Aox protein and gene expression have been characterized in a diverse range of species under a variety of conditions, and Aox is used as a model for nuclear-encoded mitochondrial proteins (Vanlerberghe and McIntosh, 1994, 1997; Day et al., 1995; McIntosh et al., 1998; Daley et al., 2003). Despite this intense interest, the function of Aox remains speculative, aside from thermogenic plants, where it plays a role in pollination (Seymour, 2001). Among the proposed roles for Aox, the most compelling ones revolve around protection from reactive oxygen species and maintenance of the TCA cycle to allow continued production of carbon skeletons in situations of high ATP (Lambers, 1982; Purvis and Shewfelt, 1993; Wagner and Krab, 1995; Vanlerberghe et al., 1995; Millenaar et al., 1998; Moller, 2001; Yip and Vanlerberghe, 2001; Maxwell et al., 2002).
In all species examined to date, Aox is encoded by a small multigene family that displays tissue, developmental, and environmental regulation (Whelan et al., 1996; Vanlerberghe and McIntosh, 1997; McCabe et al., 1998; Considine et al., 2002). These multigene families can be divided into two subfamilies (Considine et al., 2002), Aox1 and Aox2, of which there are varying copy numbers in different plants. For example, in soybean (Glycine max), there is one Aox1 type, GmAox1, and two Aox2 types, GmAox2a and GmAox2b. Arabidopsis, on the other hand, has four Aox1 types, AtAox1a, AtAox1b, AtAox1c, and AtAox1d, and one Aox2 member.
The members of the Aox family are differentially expressed in plant tissues, with much of this work carried out in soybean. These studies have been aided by the ability to separate and identify the individual proteins by SDS-PAGE and by N-terminal protein sequencing (Finnegan et al., 1997; Tanudji et al., 1999). Detailed tissue, developmental, and induction studies in soybean have shown that the GmAox2 genes are expressed during normal plant growth, with GmAox2b expressed throughout the whole plant and GmAox2a confined to photosynthetic tissues, whereas GmAox1 expression has only been detected in response to various inducers (Finnegan et al., 1997; McCabe et al., 1998; Djajanegara et al., 2002) and at very low levels in infected cells from soybean nodules (Millar et al., 1997).
Multiple signaling pathways may be involved in the induction of Aox. Induction of Aox1 in soybean cell cultures by citrate is sensitive to the protein kinase inhibitor staurosporine, but induction by reactive oxygen species or antimycin A is not (Djajanegara et al., 2002). In tobacco (Nicotiana tabacum) cell cultures, induction of Aox1 expression by antimycin A (and Cys) is blocked by phosphatase inhibitors, whereas induction by citrate is not (Vanlerberghe et al., 2002).
To gain further insight into the mechanism of Aox gene regulation, we have used regions upstream of the soybean Aox genes to drive the expression of the β-glucuronidase (GUS) reporter protein in soybean cells and Arabidopsis plants, and we have characterized regions of the fragments important for promoter activity. The reporter gene expression patterns were then compared with the detailed expression profiles of all five members of the Arabidopsis Aox gene family.
RESULTS
Isolation of Soybean Aox Genomic Clones for Promoter Analysis
To carry out promoter analysis of all Aox genes from soybean, it was necessary to isolate the genomic clones for GmAox2a and 2b, previously called Aox2 and Aox3, respectively (Whelan et al., 1996; Considine et al., 2002). The genomic sequence for GmAox1 has been reported previously (Whelan et al., 1999). Screening of a soybean genomic library produced a clone that contained both GmAox2a and 2b arranged in tandem (Fig. 1). The sequence from both strands was determined, from approximately 2 kb upstream of the GmAox2b translation start site to 200 bp downstream of the GmAox2a stop codon (Gene accession no. AY303971). Comparison with the corresponding cDNA sequences (Finnegan et al., 1997) showed that the intron-exon boundaries for both genes fell between codons and were bordered by the 5′-GU and AG-3′ consensus sequences of higher plants (Liu and Filipowicz, 1996). The intron locations are well conserved in all Aox genes to date except for AtAox2 from Arabidopsis (Rhoads and McIntosh, 1991; Whelan et al., 1999; Saisho et al., 2001). The sequence of the coding regions differed by two nucleotides between the cDNA and genomic regions for GmAox2a, resulting in a single-amino acid difference, and by one nucleotide for GmAox2b, which also resulted in a single-amino acid difference (accession nos. GmAox2a, U87906; GmAox2b, U87907). The amino acids that differ, Val to Leu for GmAox2a and Gly to Asp for GmAox2b, are at positions that are not conserved between Aox proteins from diverse plants. The 5′ end of the GmAox2b cDNA did not match with the genomic fragment, and three independent amplifications were carried out on genomic DNA to determine the correct sequence at this position. This analysis indicated that the genomic sequence obtained here was correct and that the 30 bp at the 5′ end of the cDNA (Finnegan et al., 1997) was an artifact of library construction. In addition, the sequence from one end of a soybean genomic bacterial artificial chromosome clone (accession no. AZ044777), is identical to that determined here for the genomic clone.
Figure 1.
Structure of the soybean genomic region containing GmAox2a and GmAox2b. A map of the 8.844-kb genomic region containing the genes for Aox2a and Aox2b, showing the tandem arrangement and exon structure for the two genes. Exons are shown as boxes. The direction of transcription is from left to right. Aox2b is shown in light gray, and Aox2a is shown in black. The EMBL3 bacteriophage right arm of the characterized clone is indicated.
Characterization of Promoter Activity in Soybean Cell Cultures
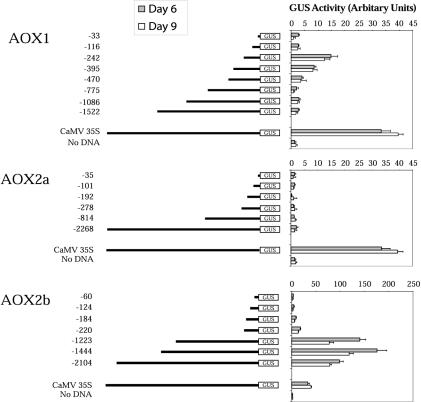
Soybean suspension culture cells were used initially to determine the promoter activity of the available GmAox1, GmAox2a, and GmAox2b upstream regions. The regions 1.5 kb upstream of the translational start site of GmAox1 and 2.1 and 2.2 kb upstream from the translational start site of GmAox2a and 2b were fused independently to the GUS reporter gene in pCAMBIA1301 (accession no. AF234297). The GUS constructs were then used to transiently transform soybean cells using a biolistic gun (Iida et al., 1990; Godon et al., 1993; Schledzewski and Mendel, 1994). As a control for variation in transformation rate, the cauliflower mosaic virus (CaMV) 35S promoter was placed in front of the luciferase (Luc) gene. The Aox promoter constructs and the control plasmid were mixed before shooting, and GUS activity was normalized to Luc activity for each transformation. Additionally, the CaMV 35S promoter was fused to GUS to allow the relative strength of the soybean promoters to be compared with that of this well-characterized promoter system.
Before undertaking these transient transformations, we determined which Aox proteins were present in the cultured soybean cells throughout a 9-d growth period. Previously, it has been shown that the proteins encoded by the three soybean genes have distinct mobilities on SDS-PAGE gels, and thus an Aox protein detected by immunoblotting can be assigned to a gene (Finnegan et al., 1997; McCabe et al., 1998; Tanudji et al., 1999). On the basis of immunoblot analysis, GmAox2b is the most abundant Aox protein (Fig. 2A) throughout the growth cycle, peaking at d 6 to 7. GmAox1 was only detected between d 4 and 7, peaking at d 6. GmAox2a protein was never detected in these cultures, based on the fact that no protein band was evident with the same apparent molecular mass to that observed for GmAox2a in soybean cotyledons (Fig. 2A, lane C). To determine whether the transformation procedure had any effect on the amounts of the endogenous Aox protein present in soybean suspension cells, d-6 and -9 cells were subjected to biolistic transformation and immunoblot analysis, which was carried out 48 h later (at which time the maximum activity of GUS and Luc was present using the CaMV 35S promoter). This procedure had no noticeable effect on expression of the various Aox proteins, except that the low level of GmAox1 detected at d 6 before cells were transformed (Fig. 2B, lane 1) was not present 48 h later (Fig. 2B, lane 2). This was not unexpected because in the normal developmental profile GmAox1 protein was not present in d-8 cells (Fig. 2A, lane 8). On the basis of these results, we proceeded to use d-6 and -9 cells for promoter analysis.
Figure 2.
Immunoblot analysis of Aox proteins using an anti-Aox antibody. Numbers on the left represent apparent molecular masses in kilodaltons, where the 36-kD band represents GmAox2b, the 34-kD band represents GmAox2a, and the 32-kD band represents GmAox1. A, The profile of Aox proteins present in soybean suspension cells after subculture. Lane numbers indicate the age of the culture in days after subculture. Lane C, contains mitochondria purified from 7-d-old soybean cotyledons. B, Analysis of Aox protein after biolistic transformation. Lane 1 contains d-6 cells before transformation, and lane 2 contains the same cells 48 h after biolistic transformation. Lane 3 contains d-9 cells before transformation, and lane 4 contains the same cells 48 h after biolistic transformation. Lane C contains mitochondria from 7-d-old soybean cotyledons.
Transformations using upstream regions of the three soybean genes showed that the fragments from GmAox1 and 2b, but not that from GmAox2a, had promoter activity in cells (Fig. 3). This is in agreement with the immunoblot analysis where endogenous GmAox2a protein was never observed (Fig. 2A). With the two active promoters, GmAox2b promoter strength was almost 10 times that of GmAox1 at maximal activity of both promoters (–242 construct for GmAox1 versus –1,444 construct for GmAox2b; Fig. 3). In addition, GUS activity driven by the GmAox1 upstream fragment did not display a significant difference when d-6 and -9 cells were transformed. With the GmAox2b upstream fragment, transforming d-9 cells resulted in promoter activity that was consistently 80% the strength obtained compared with transforming d-6 cells. GUS expression under the control of the CaMV 35S promoter was always slightly higher in older cells, indicating that the lower activity observed when transforming d-9 cells with the GmAox2b fragment was specific for the promoter activity of this fragment.
Figure 3.
Analysis of GUS activity driven by 5′-truncated forms of the GmAox1, GmAox2a, and GmAox2b upstream fragments after transient transformation of soybean cells. Day six (gray) and d 9 (white) cells were used. A schematic representation of each construct is shown to the left along with the length of each upstream fragment in base pairs. The results from measurement of GUS activity using a fluorimetric assay for each construct are shown. The activities of the CaMV 35S promoter and cells exposed to uncoated gold particles are also shown. A minimum of five independent transformations were carried out for each construct, and each transformation was normalized to the level of the cotransformed Luc activity. Error bars indicate se values.
The GmAox1 1.5 kb upstream fragment showed no significant promoter activity above background until the sequences upstream of –470 with respect to the translational start were deleted (Fig. 3). A further 75-bp deletion to position –395 bp, doubled the promoter activity, and deletion of another 153 bp, to –242 bp, further increased activity again to the maximum observed. Removal of another 126 bp, to position –116, abolished activity to background levels. These results indicate that there are negative elements between –1,522 and –470 and between –470 and –242 that control the expression of GmAox1. Because no promoter activity was observed with the complete –1,522 upstream region of GmAox1 in d-6 cells but Aox1 protein was present (Fig. 2), it appears that additional positive elements exist either further upstream or in the 3′ end of this gene.
Analysis of the GmAox2b promoter also suggested the presence of repressor elements. Deleting 670 bp from the upstream end of the longest fragment created a construct with –1,444 bp upstream of the translational start, which consistently displayed 1.75 times the promoter activity of the full-length fragment. Removal of approximately 1 kb between –1,223 bp and –220 bp resulted in a large loss of GUS activity, indicating that positive elements driving expression were present in this region. Noticeably, the expression driven by the –1,223 fragment was up to 10-fold greater than that driven by the GmAox1 upstream fragments and 1.5- to 3-fold stronger than that of the CaMV 35S promoters.
Characterization of Promoter Activity in Arabidopsis
To determine the tissue specificity of the soybean Aox upstream regions, we carried out stable transformation of Arabidopsis and analyzed the expression of GUS using histochemical staining. Although soybean and Arabidopsis diverged more than 100 million years ago, Arabidopsis has been extensively used in the analysis of promoter regions from a wide variety of plant species (Ermolaeva et al., 2003). The tissue specificity of many soybean gene promoters has been explored in Arabidopsis including that of the auxin-induced GH3 and SAUR 15A; the vegetative storage protein vspB; the late embryogenesis abundant protein GmPM9; the β-subunit of β-conglycinin; the heat shock proteins Gmhsp17.3-B, Gmhsp17.5-E, and Gmhsp17.6-L; a major latex protein homolog Msg; and the nodule-related ENOD40(2) (Kilby et al., 1995; Prandl et al., 1995; Ulmasov et al., 1997; Xu et al., 1997; Mirabella et al., 1999; Stromvik et al., 1999; Lee et al., 2000; Crone et al., 2001; Awazuhara et al., 2002; Berger et al., 2002). We investigated whether the tissue specificity observed at the mRNA and protein levels in soybean (Finnegan et al., 1997; McCabe et al., 1998) was maintained in Arabidopsis.
Histochemical analysis of transgenic plants where GUS expression was driven by the entire upstream fragments outlined in Figure 3, yielded a whole-plant perspective of the promoter activity of the Aox fragments. The GmAox1 upstream fragment drove expression throughout the plant and was strongest in vascular tissue, especially that of the root (Fig. 4, i and ii). The vascular activity of this fragment seemed to increase in leaves with increasing age (Fig. 4, iii–v). The GmAox2a promoter only drove expression in aerial parts of the plant, and again this appeared strongest in vascular tissue (Fig. 4, vi). Message and protein derived from this gene in soybean are also found only in the shoot (Finnegan et al., 1997; McCabe et al., 1998). The strongest expression was confined to the basal area of the cotyledons and increased with cotyledon age (Fig. 4, vii–ix). The GmAox2b promoter supported expression throughout the plant, and expression increased throughout cotyledon and leaf development. Strong expression was also observed in the hydathodes of the leaves (Fig. 3, x–xv). In soybean, Aox2b (formerly called Aox3) protein is also found throughout the plant, and expression increases during cotyledon development (Finnegan et al., 1997; McCabe et al., 1998).
Figure 4.
Histochemical localization of GUS activity during vegetative growth in Arabidopsis transformed with the full-length GmAox1, GmAox2a, and GmAox2b upstream fragments fused to the GUS reporter gene. i through v, GmAox1-GUS. i, Whole 26-d-old plant. ii, Mount of 22-d-old Arabidopsis root. iii through v, Sixteen-, 22-, and 26-d-old leaves, respectively. vi through ix, GmAox2a-GUS. vi, Whole 26-d-old plant. vii through ix, Ten-, 22-, and 26-d-old cotyledons. x through xv, GmAox2b-GUS. x, Whole 26-d-old plant. xi through xiii, Ten-, 22-, and 26-d-old cotyledons. xiv and xv, Sixteen- and 26-d-old leaves, respectively.
Deletion analysis of the GmAox2a upstream fragment showed that deletion of approximately 2 kb allowed root expression (Fig. 5, vii–ix). The –278 bp of promoter showed weak GUS expression in Arabidopsis compared with the –2,268 and –814 constructs (Figs. 4, vi, and 5, vii versus viii). An additional deletion to produce the –192 construct resulted in strong activity in root vascular tissue (Fig. 5, ix).
Figure 5.
Histochemical localization of GUS activity in Arabidopsis transformed with the full-length GmAox1, GmAox2a, and GmAox2b upstream regions fused to the GUS reporter gene. i through vi, Reproductive tissue. i through iii, Floral buds expressing GUS under the control of the GmAox1, GmAox2a, and GmAox2b upstream fragments, respectively. ii, Inset, An older flower bud after pollination from a plant with the Aox2a-GUS construct. iv, Section of anthers from a plant containing the GmAox2a-GUS construct. v, Mature flower from a GmAox2b-GUS plant. vi, Mature flower for GmAox1-GUS plant. vii through ix, Deletion analysis of the GmAox2a upstream fragment showing roots with 814-, 278-, and 192-bp fragments of the upstream region.
Each of the three upstream fragments showed differential promoter activity throughout the flower. Although all three fragments showed some activity in sepals (Fig. 5, i–iii), only the GmAox2a fragment supported strong activity in the anthers (Fig. 5 ii). Cross section of the anthers indicated that the expression was not in the microspores but in the tapetal cells that surround the pollen sacs (Fig. 5, iv). This anther expression was developmentally regulated and did not appear in mature flowers. (Fig. 5, ii, inset). In contrast, GmAox2b promoter activity was observed in the sepals at all stages of floral development. In mature flowers, activity was detected in the filaments supporting the anthers, the tip of the style, and the stigma (Fig. 5, v). The GmAox1 upstream fragment drove expression throughout the whole of the carpel and in the filaments (Fig. 5, vi).
Comparison of Soybean and Arabidopsis Aox Promoter Sequences
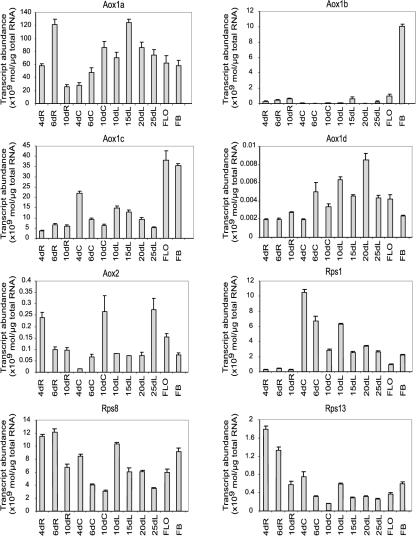
To aid the identification of upstream regions that control the expression of Aox, the soybean Aox upstream sequences were compared with those from Arabidopsis. These are the only dicot plants for which upstream sequences for all Aox genes are available. To ensure that comparisons were made between promoters driving the expression of genes with similar expression profiles, the expression patterns of the five Arabidopsis Aox genes was first analyzed in various tissues. Additionally, we analyzed the expression of three ribosomal genes: Rps1, a nuclear-encoded plastid ribosomal protein; Rps13, a nuclear-encoded mitochondrial ribosomal protein; and Rps8, a cytosolic ribosomal protein (Adams et al., 2002). The expression profile of the mitochondrial Rps13 was different from that of all of the Aox genes, indicating that the patterns of Aox mRNA expression were not simply a result of general mitochondrial biogenesis or activity.
The expression pattern of the various Arabidopsis Aox genes differed considerably from each other. AtAox1a was the most highly expressed isoform, being expressed in all tissues and showing a noticeable increase in cotyledons from d 4 to 10 (Fig. 6). For AtAox1b, significant expression was only detected in flower buds, at about 20% of the level observed for AtAox1a (Fig. 6). AtAox1c was the second most dominant isoform and was expressed in most tissues examined, albeit at significantly lower levels than AtAox1a. Its expression decreased in cotyledons from d 4 to 10, in a pattern opposite to that observed for AtAox1a. A similar pattern was also seen in leaves, where AtAox1c expression decreased over d 10 to 25. AtAox1c expression was highest in flowers and floral buds (Fig. 6). Expression of AtAox1d was barely detectable in any tissues, even in floral tissues, in contrast to the other genes (Fig. 6). AtAox2 expression was also very low in the tissues examined, being about 100-fold less than AtAox1a, -b, and -c (Fig. 6).
Figure 6.
Expression of the Arabidopsis Aox gene family. Transcript levels were measured for each of the Aox genes from Arabidopsis in different tissues and at different developmental stages using real time PCR, using three independent cDNA preparations. The expression of three ribosomal protein genes, RspI, Rsp8, and Rsp13, is shown for comparison. The tissues examined were roots from 4-, 6-, and 10-d-old plants (4dR, 6dR, and 10dR); cotyledons from 4-, 6-, and 10-d-old plants (4dC, 6dC, and 10dC); the first rosette leaf from 10-, 15-, 20-, and 25-d-old plants (10 dL, 15 dL, 20 dL, and 25 dL); mature flowers (FLO); and young floral buds (FB). The transcript amounts are presented as absolute transcript abundance in moles per microgram of total RNA.
It is interesting that the expression of orthologous Aox genes in soybean and Arabidopsis (GmAox1 versus AtAox1a, -b, -c, and -d; GmAox2a and -2b versus AtAox2) display quite different expression patterns in planta, suggesting that their functional roles have diverged since these two plant lineages separated during evolution (Ermolaeva et al., 2003). For example, the promoter activity of the soybean GmAox2a and -2b upstream regions resembles the expression of Arabidopsis AtAox1a, -1b, and -1c, in that all were expressed in aerial tissues. The sequences of the upstream regions from these genes were compared in an attempt to identify motifs responsible for their common tissue expression. Four motifs that have been previously characterized in other promoter regions were identified: one 6-bp negative element, the S1F box, and three positive elements, a 5-bp ASF-1 motif, a 6-bp I box, and a 9-bp SEF 1 motif. Four other shared motifs not previously described were identified from the sequence comparisons of the soybean and Arabidopsis Aox promoters (Table I). The function of these elements needs to be tested, and this will provide a focus for further functional dissection of these Aox promoters.
Table I.
Sequence motifs common to regions upstream of soybean GmAox2a and 2b and Arabidopsis AtAox1a, -b, and -c
The numbers in parentheses indicate the number of occurrences in the particular upstream region. Motifs identified using motif sampler are given a probability score, where >0.8 is considered significant. Letters other than A, C, G, and T represent degenerate bases, A-T = W, C-G = S, and C-T = Y.
| Motif | Position | Located | Name and Function / Probability Score | Reference |
|---|---|---|---|---|
| Motifs previously characterized in other promoter regions | ||||
| ATGGTA | GmAox1: -1,104; GmAox2a: -675 and -1,110; GmAox2b: -1,227, -1,230, and -660 | AtAox1a (1) and AtAox1b (2), and GmAox1 (2), GmAox2a (2), and GmAox2b (3) | S1F box; cis-acting negative element in non-photosynthetic tissue | Zhou et al. (1992); Lagrange et al. (1997) |
| TGACG | GmAox2b: -800 and -1,025 | AtAox1a (2), AtAox1b (2), and GmAox2b (2) | ASF-1 motif; TGA1a-binding site, high root expression | Benfey et al. (1990); Jupin and Chua (1996) |
| GATAAG | GmAox2a: -130; GmAox2b: -1,000 | AtAox1c (2), GmAox2a (1), and GmAox2b (1) | I Box; cis-acting element involved in light responsiveness | Giuliano et al. (1988); Donald and Cashmore (1990) |
| ATATTTAww | GmAox2a: -890; GmAox2b: -146, -150, -410, and -500 | AtAox1b (1), GmAox2a (1), and GmAox2b(4) | SEF 1 motif; SEF1-binding site, may be involved in young tissue development | Allen et al. (1989); Lessard et al. (1991) |
| Motifs not previously characterized in other promoter regions | ||||
| ATAAAyCTwGCTC | GmAox2b: -70 | AtAox1a (2) and GmAox2b (1) | 0.99 | NA |
| wwwGATAwC | GmAox2b: -290, -310, and -370 | AtAox1a (2) and GmAox2b (3) | 0.87 | NA |
| CGGTswwGA | GmAox2a: -200 and -428 | AtAox1c (2) and GmAox2a (2) | 0.94 | NA |
| wGAAGAwG | GmAox2a: -90 and -320 | AtAox1c (4) and GmAox2a (2) | 0.84 | NA |
DISCUSSION
We report here the genomic sequence encompassing the soybean GmAox2a and -2b genes. Together with the previously sequenced soybean GmAox1 gene, this allowed an initial characterization of the Aox promoter regions for the whole family. Differences between the previously published cDNA sequences and the genomic sequences were found that resulted in changes in one amino acid residue each in GmAox2a and -2b. These amino acids are at positions that are not conserved between plant Aox proteins and therefore appear to represent allelic variation only.
Soybean Aox2a and -2b represent a tandem duplication that, by sequence comparison with soybean bacterial artificial chromosome ends, maps to linkage group A2, near the RFLP marker A110 (accession no. AZ044777; Soybase, available at http://soybase.org). Arabidopsis Aox2 is located in a region represented by clone MSJ1, which is within 0.15 Mb of clone MBK5. This clone represents part of a region of known synteny between Arabidopsis chromosome 5 and soybean linkage group A2 (Grant et al., 2000). Therefore, Arabidopsis Aox2 is likely to be a true ortholog of the original soybean Aox2 locus (Fitch, 2000). The duplication event that gave rise to the tandem arrangement of soybean GmAox2a and GmAox2b may have occurred after the separation of the soybean and Arabidopsis lineages (Ermolaeva et al., 2003) or alternatively, Arabidopsis may have lost the second Aox2 gene. The genomic structure for Aox2 genes from other dicot plants will be necessary to distinguish between these two possibilities (Considine et al., 2002).
The genomic regions immediately upstream of all three of the soybean Aox coding sequences were examined for promoter activity in both soybean cells and Arabidopsis plants. All three upstream fragments had promoter activity and were able to drive expression of the GUS reporter gene in Arabidopsis. The GmAox1 and Aox2b upstream regions functioned in soybean cells in a manner consistent with the expression of the endogenous GmAox proteins. By making deletions to the upstream regions, functional regions could be assigned.
Both the GmAox1 and GmAox2b upstream sequences appear to possess negative elements that repress their expression. The GmAox1 promoter showed maximal activity with the fragment that only contained 242 bp immediately upstream of the coding region, whereas no GUS activity was detected with fragments extending more than 470 bp upstream. This suggests that repressor(s) of expression are present between positions –1,522 and –470 relative to the translational start. Because GUS activity continued to increase with subsequent deletions up to –242 bp, it is likely that further negative elements are present between –470 and –242. Further deletions inhibited reporter gene activity, indicating that positive element(s) exist between –242 and –116. Because the complete upstream region did not possess promoter activity, it appears that additional elements exist for this gene. Positive elements greater than 1.5 kb upstream have been previously characterized in soybean (Marsolier et al., 1995; Stromvik et al., 1999; Terce-Laforgue et al., 1999), and it is also possible that 3′ elements are responsible, as observed with a number of other plant genes (Trehin et al., 1997; Kirsch et al., 2001; Moreno-Fonseca and Covarrubias, 2001; Wenz et al., 2001; Chen et al., 2002; He et al., 2002; Martinez-Hernandez et al., 2002).
It is interesting to note that GmAox1 is only detected in soybean tissues upon stress treatments (Djajanegara et al., 2002). The role of negative elements in controlling gene expression in plants in response to stress is well documented. HOS 1, 2, and 5 are negative regulators for cold stress (Lee et al., 1999; Xiong et al., 1999; Viswanathan and Zhu, 2002), and ade1 is a negative regulator of abscisic acid signaling under osmotic stress (Foster and Chua, 1999). Characterization of an oxidative stress-inducible peroxidase promoter from sweet potato (Ipomoea batatas) also indicated the presence of negative elements upstream of the positive elements (Kim et al., 2003), as we found in GmAox1 and to a lesser extent in GmAox2. Repression of stress-inducible genes via negative promoter elements appears to be, therefore, a common mechanism of gene regulation in plants.
The deletion of the distal 660 bp from the 2,104-bp fragment of GmAox2b resulted in 1.75 times greater GUS activity. However, even with the negative elements present, the GUS activity driven by this promoter was twice as high as from the CaMV 35S promoter, which is considered a strong constitutive promoter (Benfey et al., 1990; Medberry and Olszewski, 1993). The strength of the GmAox2b promoter and its relatively constitutive expression pattern suggest an important role of Aox in normal growth and development in soybean. Although the GmAox2a promoter supported very little GUS activity in soybean cells, the presence of negative elements is suggested by the observation that deletions of this promoter facilitated root expression in Arabidopsis. In contrast, GmAox1, whose expression is repressed under normal growth conditions in soybean (Finnegan et al., 1997; McCabe et al., 1998; Djajanegara et al., 2002), was expressed throughout the Arabidopsis plant. Obviously, Arabidopsis only recognizes some of the soybean repressor elements.
GUS activity driven by the GmAox2a upstream region was prominent in the anthers of Arabidopsis, perhaps indicative of a role for Aox in pollen development. Such a role has been suggested previously when an Aox antisense construct under the control of a tapetum-specific promoter resulted in reduced pollen viability in tobacco (Kitashiba et al., 1999). Furthermore, in petunia (Petunia hybrida) and common bean (Phaseolus vulgaris), strong Aox expression has been observed in the tapetum and pollen grains (Conley and Hanson, 1994). The expression of the GmAox2a-GUS in Arabidopsis anthers seems to be developmentally regulated (Fig. 5, ii.). Similarly, the AtAox1b transcript (Fig. 6) is up-regulated in floral buds, whereas only low expression is seen in mature flowers. In bean, Aox expression has also been seen to change during bud development, where it has been proposed to be correlated with carbohydrate metabolism (Johns et al., 1993).
Overall, these results suggest that there is some divergence of gene responsiveness between Arabidopsis and soybean, as illustrated by the differences in the expression of the Aox orthologs in the two plants. Although the soybean Aox2 locus is orthologous with that of Arabidopsis Aox2, the expression patterns of the two GmAox2 genes are more closely related to the Arabidopsis Aox1 genes (especially Aox1a and Aox1c). For example, AtAox1a transcript levels increased with age in cotyledons, whereas the levels of AtAox1c transcripts decreased. A similar pattern has been demonstrated in soybean where the levels of GmAox2a mRNA is initially high and decreases over development while the initially low levels of GmAox2b increase (McCabe et al., 1998). These results indicate that it cannot be assumed that orthologous genes in different plants respond to the same signals, at least with respect to Aox gene expression. Rather it seems that although Aox expression responds to similar tissue and development signals in both Arabidopsis and soybean, different orthologs are involved in this response.
MATERIALS AND METHODS
Plant Material
Suspension cells developed from soybean (Glycine max cv Stevens) leaf tissue (Djajanegara et al., 2002), were grown in 250-mL flasks with media (Gamborg et al., 1968) agitated at 130 rpm under approximately 20 μE m–2 s–1 light at 28°C. The cell stocks were maintained in an exponential growth phase by subculturing (1:6 [v/v] dilution) at 7-d intervals. Arabidopsis ecotype Columbia were grown at 22°C under long-day conditions (16 h of approximately 100 μE m–2 s–1 light and 8 h dark). Arabidopsis plants were transformed by Agrobacterium tumefaciens-mediated transformation of flowers (Clough and Bent, 1998). Seeds of the T1 generation were selected for resistance on medium (Gamborg et al., 1968) containing 20 μg mL–1 hygromycin. At least 20 independent lines were examined for each construct.
For transient expression analysis, soybean suspension cells of the appropriate age were aseptically coated onto filter paper (55-mm diameter, Whatman No. 1, Maidstone, UK), which was then placed onto solid media containing soybean culture media supplemented with 200 mm mannitol and incubated for 2 h at 28°C in the dark. Before transformation, 5 μg each of a GUS reporter construct and the luciferase calibration construct was precipitated onto 5 mg of 1-μm (average diameter) gold particles (Chempur, Karlsruhe, Germany) using 2.5 M CaCl2 (Sigma-Aldrich, St. Louis) and 100 mm spermidine (Sigma-Aldrich). For each bombardment, 0.5 mg of particles was used. Bombardment was carried out under vacuum using helium pressure of 1,400 kPa. After transformation the cells were incubated for 48 h at 28°C in the dark on the media-impregnated filter paper discs.
Genomic Library Screening
A soybean genomic library (BD Biosciences Clontech, Sydney) was screened by standard procedures using 32P-labeled probes synthesized from GmAox2a and Aox2b cDNA fragments (Sambrook et al., 1989). The DNA probes were synthesized by random priming using Klenow DNA polymerase and [α-32P]dCTP, using a commercial kit (High Prime, Roche Diagnostics, Sydney). Unincorporated nucleotides were removed using a spin column (ProbeQuant G50, Amersham Biosciences, Sydney). DNA inserts of the isolated clones were sequenced using a commercial kit (ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Mix, Applied Biosystems, Melbourne) and the ABI PRISM 310 Genetic Analyzer.
Isolation of Total RNA and cDNA Synthesis
Total RNA was isolated from Arabidopsis tissue using a commercial kit (RNeasy Plant mini, Qiagen, Clifton Hill, Australia). Each batch of total RNA was treated with DNaseI (Roche Diagnostics) and then treated with DNAfree (Ambion, Austin, TX) to remove contaminating DNA and reverse transcribed using Expand Reverse Transcriptase according to the manufacturer's recommendations (Roche Diagnostics). Random primers (Roche Diagnostics) were used in the reverse transcription for the analysis of transcripts in different tissues, and oligo(dT) primer (Roche Diagnostics) was used for the cotyledon transcript analysis. The QIAquick PCR Purification Kit (Qiagen) was used to purify the cDNA before real-time PCR analysis.
Cloning of AtAox
Total RNA was reverse transcribed with the appropriate reverse primer (see below) using Expand Reverse Transcriptase according to the manufacturer's recommendations (Roche Diagnostics). Five microliters of the resulting cDNA was used for PCR with the appropriate forward and reverse primers (30 pmol each) and the Expand High Fidelity PCR system (Roche Diagnostics) according to the manufacturer's instructions. Fragments amplified by PCR were separated on agarose gels and purified (QIAquick Gel Extraction Kit, Qiagen) and ligated into the pCR2.1 vector (Invitrogen, Sydney) according to the manufacturer's instructions.
The following primers were used: Aox1a.F, 5′-GGACCACGTTTGTTCTCGACG-3′; Aox1b.F, 5′-CTGCTGTGACTCACAGCCATC-3′; Aox1c.F, 5′-GCATCAAAGCAACCGACATCC-3′; Aox2.F, 5′-CGTGAGTTCTGTTTCCTCCAC-3′; Aox.R, 5′-CCTCCAACCATTCCWGGWACYG-3′; Aox1d.F, 5′-CCTACAGATCGATTTACCGC-3′; and Aox1d. R, 5′-GGTTTGTATGAATCCCATGG-3′.
Real-Time PCR Analysis of Transcript Levels
Transcript levels were assayed by real time PCR using the iCycler and iQSupermix (Bio-Rad Laboratories, Hercules, CA). Reactions were carried out in a total volume of 25 μL with a final concentration of 0.008% (w/v) bovine serum albumin, 1 μm fluorescein, and 1× SYBR Green, under conditions optimized to minimize primer-dimer formation and maximize amplification efficiency. The iCycler program consisted of four cycles: denaturation, 95°C for 10 min; amplification, 15 cycles at 95°C for 15 s, touchdown annealing from 85°C to 55°C decreasing 2°C per cycle for 30 s, and extension 72°C for 30 s, followed by amplification without touchdown in the annealing phase: 30 cycles at 95°C for 15 s, 50°C for 30 s, and 72°C for 30 s with single data acquisition collected during the extension; melting curve analysis, 95°C for 0 s, 70°C for 60 s, and 95°C for 0 s with a transition rate of 0.1°C s–1 and continuous data acquisition; cooling to 4°C. Samples were analyzed as outlined previously (Considine et al., 2001; Daley et al., 2003). Real time primers: Aox1a. F, 5′-GACGGTCCGTACGGTTTCG-3′; Aox1a. R, 5′-CTTCTGATTCGCGTCCTCTC-3′; Aox1b.F, 5′-CACAGCCATCTTTTGAATCCTAGGG-3′; Aox1b.R, 5′-CATTCAGTTCCATCTTCTTTGG-3′; Aox1c.F, 5′-GCATCAAAGCAAGCGACATCC-3′; Aox1c.R, 5′-TGGCTTCACGCCCCAATAAC-3′; Aox1d. F, 5′-CGACCGGTACTATCATCTTCG-3′; Aox1d.R, 5′-CACTTCCAAGCTGAACCGTC-3′; Aox2.F, 5′-GGCGATTTCAAGATCGGCTC-3′; and Aox2. R, 5′-GTTCCAGGCCAATCCGATC-3′.
Assays for Luc, GUS, and Histochemical Staining and Sectioning
Transiently transformed cells were harvested 48 h after bombardment. Cells were disrupted by grinding in a mortar and pestle under liquid nitrogen. The broken cells were extracted with the lysis buffer and protocol supplied with the Luciferase Assay System kit (Promega, Madison, WI). Luciferase activity assays were carried out according to the manufacturer's instructions, and activity was measured over 10 s using a luminometer (Tr717, Tropix, Bedford, MA). GUS activity was determined using the fluorimetric GUS assay (Jefferson et al., 1987). Samples were taken at 0, 15, 30, and 60 min using 1 m Tris base to stop the reaction. Fluorescence was determined using a spectrofluorophotometer (Shimadzu, RF-5000, Kyoto) with excitation at 365 nm and emission at 455 nm. The normalized GUS activity was determined by dividing the GUS fluorescence value by the luciferase activity value for each sample, thus eliminating variation due to transformation efficiency. A minimum of five replicate bombardments were carried out per construct.
Histochemical localization of GUS was carried out in a solution containing 5 mg mL–1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide as the substrate in a buffer containing 100 mm phosphate buffer (pH 7.0), 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, 10 mm EDTA, and 0.3% (v/v) Triton X-100 and incubating at 37°C (Jefferson et al., 1987). After staining, tissue was incubated in 70% (v/v) ethanol to remove chlorophyll and reduce background. Samples for microscopy were fixed by vacuum infiltration with 2.5% (w/v) glutaraldehyde in 0.05 m phosphate buffer (pH 7.0) and incubated for 3 h. The samples were then dehydrated by washing in 0.05 m phosphate buffer (pH 7.0) followed by a series of 1-h incubations in 100% (v/v) methoxyethanol, 100% (v/v) ethanol, 100% (v/v) 1-propanol, and 100% (v/v) 1-butanol. The samples were finally embedded in glycol methacrylate by infiltrating samples with two changes over 2 d and polymerizing under nitrogen at 60°C overnight. Sections of 4 μm were cut using a microtome (Sorvall JB-4, Newton, CT) and visualized using a light microscope (Zeiss Axioskop 2 plus, Carl Zeiss, Jena, Germany).
Immunoblot Analysis
Extracts from soybean cells used for immunoblot analysis were prepared by first disrupting the cells with a mortar and pestle under liquid nitrogen until a fine powder was produced. This was solubilized in 0.5 m Tris-Cl (pH 7.5), 10 mm EDTA, 1% (v/v) Triton X-100, and 2% (v/v) 2-mercaptoethanol. This was then centrifuged at 20,000g for 5 min, and the supernatant was kept. Soybean cotyledon mitochondria were isolated as described previously (Day et al., 1985). Protein content was determined by the method of Lowry et al. (1951).
Immunoblot analysis was carried out using 40 μg of protein, which was resolved by SDS-PAGE and transferred onto a Hybond-C extra membrane (Amersham Biosciences) using a semidry blotting apparatus (Hoefer Semi-Phor, Amersham Biosciences). Aox proteins were labeled with the AOA monoclonal antibody (Elthon et al., 1989), detected using chemiluminescence (Roche Diagnostics), and visualized using a LAS 1000 (Fuji, Tokyo).
Promoter Sequence Analysis
Over-represented and previously identified motifs in the Aox promoter sequences were identified using publicly available software. The sequences of the regions upstream of the soybean Aox genes were compared with sequences extending approximately 2 kb upstream of the translational start sites of the Arabidopsis Aox genes identified as having similar patterns of expression using MotifSampler (Thijs et al., 2001). Previously described motifs were detected by comparison of the soybean upstream fragments with the PLACE database (Higo et al., 1999).
Acknowledgments
We appreciate the contribution of undergraduate students Helen Trend and Eric Chan to sequencing the Aox2b/Aox2a region.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.028183.
This work was supported by the Australian Research Council.
References
- Adams KL, Daley DO, Whelan J, Palmer JD (2002) Genes for two mitochondrial ribosomal proteins in flowering plants are derived from their chloroplast or cytosolic counterparts. Plant Cell 14: 931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RD, Bernier F, Lessard PA, Beachy RN (1989) Nuclear factors interact with a soybean beta-conglycinin enhancer. Plant Cell 1: 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awazuhara M, Kim H, Goto DB, Matsui A, Hayashi H, Chino M, Kim S-G, Naito S, Fujiwara T (2002) A 235-bp region from a nutritionally regulated soybean seed-specific gene promoter can confer its sulfur and nitrogen response to a constitutive promoter in aerial tissues of Arabidopsis thaliana. Plant Sci 163: 75–82 [Google Scholar]
- Benfey PN, Ren L, Chua NH (1990) Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J 9: 1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Mitchell-Olds T, Stotz HU (2002) Local and differential control of vegetative storage protein expression in response to herbivore damage in Arabidopsis thaliana. Physiol Plant 114: 85–91 [DOI] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA et al. (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conley CA, Hanson MR (1994) Tissue-specific protein expression in plant mitochondria. Plant Cell 6: 85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Daley DO, Whelan J (2001) The expression of alternative oxidase and uncoupling protein during fruit ripening in mango. Plant Physiol 126: 1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH (2002) Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol 129: 949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone D, Rueda J, Martin KL, Hamilton DA, Mascarenhas JP (2001) The differential expression of a heat shock promoter in floral and reproductive tissues. Plant Cell Environ 24: 869–874 [Google Scholar]
- Daley DO, Considine MJ, Howell KA, Millar AH, Day DA, Whelan J (2003) Respiratory gene expression in soybean cotyledons during postgerminative development. Plant Mol Biol 51: 745–755 [DOI] [PubMed] [Google Scholar]
- Day DA, Arron GP, Laties GG (1980) Nature and control of respiratory pathways in plants: cyanide sensitive and cyanide resistant respiration. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 2. Academic Press, New York, pp 197–241 [Google Scholar]
- Day DA, Neuburger M, Douce R (1985) Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 12: 219–228 [Google Scholar]
- Day DA, Whelan J, Millar AH, Siedow JN, Wiskich JT (1995) Regulation of the alternative oxidase in plants and fungi. Aust J Plant Physiol 22: 497–509 [Google Scholar]
- Djajanegara I, Finnegan PM, Mathieu C, McCabe T, Whelan J, Day DA (2002) Regulation of alternative oxidase gene expression in soybean. Plant Mol Biol 50: 735–742 [DOI] [PubMed] [Google Scholar]
- Donald RG, Cashmore AR (1990) Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J 9: 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol 89: 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolaeva MD, Wu M, Eisen JA, Salzberg SL (2003) The age of the Arabidopsis thaliana genome duplication. Plant Mol Biol 51: 859–866 [DOI] [PubMed] [Google Scholar]
- Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA (1997) Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol 114: 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WM (2000) Homology a personal view on some of the problems. Trends Genet 16: 227–231 [DOI] [PubMed] [Google Scholar]
- Foster R, Chua NH (1999) An Arabidopsis mutant with deregulated ABA gene expression: implications for negative regulator function. Plant J 17: 363–372 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnik PA, Cashmore AR (1988) An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA 85: 7089–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon C, Caboche M, Daniel-Vedele F (1993) Transient plant gene expression: a simple and reproducible method based on flowing particle gun. Biochimie 75: 591–595 [DOI] [PubMed] [Google Scholar]
- Grant D, Cregan P, Shoemaker RC (2000) Genome organization in dicots: genome duplication in Arabidopsis and synteny between soybean and Arabidopsis. Proc Natl Acad Sci USA 97: 4168–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Futterer J, Hohn T (2002) Contribution of downstream promoter elements to transcriptional regulation of the rice tungro bacilliform virus promoter. Nucleic Acids Res 30: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A, Seki M, Kamada M, Yamada Y, Morikawa H (1990) Gene delivery into cultured plant cells by DNA-coated gold particles accelerated by a pneumatic particle gun. Theor Appl Genet 80: 813–816 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns C, Nickels R, McIntosh L, Mackenzie S (1993) The expression of alternative oxidase and alternative respiratory capacity in cytoplasmic male sterile common bean. Sex Plant Reprod 6: 257–265 [Google Scholar]
- Jupin I, Chua NH (1996) Activation of the CaMV as-1 cis-element by salicylic acid: differential DNA-binding of a factor related to TGA1a. EMBO J 15: 5679–5689 [PMC free article] [PubMed] [Google Scholar]
- Kilby NJ, Davies GJ, Snaith MR (1995) FLP recombinase in transgenic plants: constitutive activity in stably transformed tobacco and generation of marked cell clones in Arabidopsis. Plant J 8: 637–652 [DOI] [PubMed] [Google Scholar]
- Kim KY, Kwon Sy, Lee HS, Yunkang H, Bang JW, Kwak SS (2003) A novel oxidative stress-inducible promoter from sweet potato: molecular cloning and characterisation in transgenic plants and cultured cells. Plant Mol Biol 51: 831–838 [DOI] [PubMed] [Google Scholar]
- Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K (2001) A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant J 26: 217–227 [DOI] [PubMed] [Google Scholar]
- Kitashiba H, Kitazawa E, Kishitani S, Toriyama K (1999) Partial male sterility in transgenic tobacco carrying an antisense gene for alternative oxidase under the control of a tapetum-specific promoter. Mol Breed 5: 209–218 [Google Scholar]
- Lagrange T, Gauvin S, Yeo HJ, Mache R (1997) S2F, a leaf-specific transacting factor, binds to a novel cis-acting element and differentially activates the RPL21 gene. Plant Cell 9: 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H (1982) Cyanide resistant respiration: a non-phosphorylating electron transport pathway acting as an energy overflow. Physiol Plant 55: 478–485 [Google Scholar]
- Lee H, Xiong L, Ishitani M, Stevenson B, Zhu JK (1999) Cold-regulated gene expression and freezing tolerance in an Arabidopsis thaliana mutant. Plant J 17: 301–308 [DOI] [PubMed] [Google Scholar]
- Lee P, Hsing Y, Chow T (2000) Promoter activity of a soybean gene encoding a seed maturation protein, GmPM9. Bot Bull Acad Sin 41: 175–182 [Google Scholar]
- Lessard PA, Allen RD, Bernier F, Crispino JD, Fujiwara T, Beachy RN (1991) Multiple nuclear factors interact with upstream sequences of differentially regulated beta-conglycinin genes. Plant Mol Biol 16: 397–413 [DOI] [PubMed] [Google Scholar]
- Liu HX, Filipowicz W (1996) Mapping of branchpoint nucleotides in mutant pre-mRNAs expressed in plant cells. Plant J 9: 381–389 [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Marsolier MC, Debrosses G, Hirel B (1995) Identification of several soybean cytosolic glutamine synthetase transcripts highly or specifically expressed in nodules: expression studies using one of the corresponding genes in transgenic Lotus corniculatus. Plant Mol Biol 27: 1–15 [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Lopez-Ochoa L, Arguello-Astorga G, Herrera-Estrella L (2002) Functional properties and regulatory complexity of a minimal RBCS light-responsive unit activated by phytochrome, cryptochrome, and plastid signals. Plant Physiol 128: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Nickels R, McIntosh L (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J 29: 269–279 [DOI] [PubMed] [Google Scholar]
- McCabe TC, Finnegan PM, Harvey Millar A, Day DA, Whelan J (1998) Differential expression of alternative oxidase genes in soybean cotyledons during postgerminative development. Plant Physiol 118: 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L, Eichler T, Gray G, Maxwell D, Nickels R, Wang Y (1998) Biochemical and genetic controls exerted by plant mitochondria. Biochim Biophys Acta 1365: 278–284 [Google Scholar]
- Medberry SL, Olszewski NE (1993) Identification of cis elements involved in Commelina yellow mottle virus promoter activity. Plant J 3: 619–626 [DOI] [PubMed] [Google Scholar]
- Millar AH, Finnegan PM, Whelan J, Drevon JJ, Day DA (1997) Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ 20: 1273–1282 [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H (1998) The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol 118: 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella R, Martirani L, Lamberti A, Iaccarino M, Chiurazzi M (1999) The soybean ENOD40(2) promoter is active in Arabidopsis thaliana and is temporally and spatially regulated. Plant Mol Biol 39: 177–181 [DOI] [PubMed] [Google Scholar]
- Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Moore AL, Siedow JN (1991) The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059: 121–140 [DOI] [PubMed] [Google Scholar]
- Moreno-Fonseca LP, Covarrubias AA (2001) Downstream DNA sequences are required to modulate Pvlea-18 gene expression in response to dehydration. Plant Mol Biol 45: 501–515 [DOI] [PubMed] [Google Scholar]
- Prandl R, Kloske E, Schoffl F (1995) Developmental regulation and tissue-specific differences of heat shock gene expression in transgenic tobacco and Arabidopsis plants. Plant Mol Biol 28: 73–82 [DOI] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL (1993) Does the alternative pathway ameliorate chilling injury in sensitive plant tissue. Physiol Plant 88: 712–718 [DOI] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L (1991) Isolation and characterisation of a cDNA clone encoding an alternative oxidase protein of Sauromatum guttatum (Schott). Proc Natl Acad Sci USA 88: 2122–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho D, Nakazono M, Lee K, Tsutsumi N, Akita S, Hirai A (2001) The gene for alternative oxidase-2 (AOX2) from Arabidopsis thaliana consists of five exons unlike other AOX genes and is transcribed at an early stage during germination. Genes Genet Syst 76: 89–97 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York
- Schledzewski K, Mendel RR (1994) Quantitative transient gene expression: comparison of the promoters for maize polyubiquitin1, rice actin1, maize-derived Emu and CaMV 35S in cells of barley, maize and tobacco. Transgenic Res 3: 249–255 [Google Scholar]
- Seymour RS (2001) Biophysics and physiology of temperature regulation in thermogenic flowers. Biosci Rep 21: 223–236 [DOI] [PubMed] [Google Scholar]
- Stromvik MV, Sundararaman VP, Vodkin LO (1999) A novel promoter from soybean that is active in a complex developmental pattern with and without its proximal 650 base pairs. Plant Mol Biol 41: 217–231 [DOI] [PubMed] [Google Scholar]
- Tanudji M, Djajanegara IN, Daley DO, McCabe TC, Finnegan PM, Day DA, Whelan J (1999) The multiple alternative oxidase proteins of soybean. Aust J Plant Physiol 26: 337–344 [Google Scholar]
- Terce-Laforgue T, Carrayol E, Cren M, Desbrosses G, Hecht V, Hirel B (1999) A strong constitutive positive element is essential for the ammonium-regulated expression of a soybean gene encoding cytosolic glutamine synthetase. Plant Mol Biol 39: 551–564 [DOI] [PubMed] [Google Scholar]
- Thijs G, Lescot M, Marchal K, Rombauts S, De Moor B, Rouze P, Moreau Y (2001) A higher-order background model improves the detection of promoter regulatory elements by Gibbs sampling. Bioinformatics 17: 1113–1122 [DOI] [PubMed] [Google Scholar]
- Trehin C, Ahn IO, Perennes C, Couteau F, Lalanne E, Bergounioux C (1997) Cloning of upstream sequences responsible for cell cycle regulation of the Nicotiana sylvestris CycB1;1 gene. Plant Mol Biol 35: 667–672 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, MacIntosh L (1995) Alternative oxidase activity in tobacco leaf mitochondria: dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol 109: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1994) Mitochondrial electron transport regulation of nuclear gene expression: studies with the alternative oxidase gene of tobacco. Plant Physiol 105: 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48: 703–734 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Robson CA, Yip JY (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 129: 1829–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan C, Zhu JK (2002) Molecular genetic analysis of cold-regulated gene transcription. Philos Trans R Soc Lond B Biol Sci 357: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K (1995) The alternative respiration pathway in plants: role and regulation. Physiol Plant 95: 318–325 [Google Scholar]
- Wenz P, Schwank S, Hoja U, Schuller HJ (2001) A downstream regulatory element located within the coding sequence mediates autoregulated expression of the yeast fatty acid synthase gene FAS2 by the FAS1 gene product. Nucleic Acids Res 29: 4625–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J, Millar AH, Day DA (1996) The alternative oxidase is encoded in a multigene family in soybean. Planta 198: 197–201 [DOI] [PubMed] [Google Scholar]
- Whelan J, Smith MK, Finnegan PM, Day DA (1999) Cloning and sequencing of a genomic clone for the alternative oxidase (accession no. AF083880) from soybean. Plant Physiol 120: 663 [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (1999) HOS5-a negative regulator of osmotic stress-induced gene expression in Arabidopsis thaliana. Plant J 19: 569–578 [DOI] [PubMed] [Google Scholar]
- Xu N, Hagen G, Guilfoyle T (1997) Multiple auxin response modules in the soybean SAUR 15A promoter. Plant Sci 126: 193–201 [Google Scholar]
- Yip JY, Vanlerberghe GC (2001) Mitochondrial alternative oxidase acts to dampen the generation of active oxygen species during a period of rapid respiration induced to support a high rate of nutrient uptake. Physiol Plant 112: 327–333 [DOI] [PubMed] [Google Scholar]
- Zhou DX, Li YF, Rocipon M, Mache R (1992) Sequence-specific interaction between S1F, a spinach nuclear factor, and a negative cis-element conserved in plastid-related genes. J Biol Chem 267: 23515–23519 [PubMed] [Google Scholar]