Abstract
Phytohormones regulate plant responses to a wide range of biotic and abiotic stresses. How a limited number of hormones differentially mediate individual stress responses is not understood. We have used one such response, the compatible interaction of tomato (Lycopersicon esculentum) and Xanthomonas campestris pv vesicatoria (Xcv), to examine the interactions of jasmonic acid (JA), ethylene, and salicylic acid (SA). The role of JA was assessed using an antisense allene oxide cyclase transgenic line and the def1 mutant to suppress Xcv-induced biosynthesis of jasmonates. Xcv growth was limited in these lines as was subsequent disease symptom development. No increase in JA was detected before the onset of terminal necrosis. The lack of a detectable increase in JA may indicate that an oxylipin other than JA regulates basal resistance and symptom proliferation. Alternatively, there may be an increase in sensitivity to JA or related compounds following infection. Hormone measurements showed that the oxylipin signal must precede subsequent increases in ethylene and SA accumulation. Tomato thus actively regulates the Xcv-induced disease response via the sequential action of at least three hormones, promoting expansive cell death of its own tissue. This sequential action of jasmonate, ethylene, and SA in disease symptom development is different from the hormone interactions observed in many other plant-pathogen interactions.
Plants use phytohormones to respond to a wide range of environmental stimuli in a highly specific manner. Yet all of the hormones that mediate these responses are pleiotropic, with involvement in multiple developmental and stress responses. For example, the gaseous hormone ethylene is a critical component of responses to mechanical damage, herbivory, and pathogen attack in addition to normal developmental processes such as fruit ripening and senescence (Abeles et al., 1992). Because many hormones are involved in responses to multiple stresses, each of which leads to a specific downstream response, there must be mechanisms in place to integrate these signals in an orderly manner. Thus, complex networks of hormonal interactions, both agonistic (O'Donnell et al., 1996) and antagonistic (Pieterse and Van Loon, 1999) must be rapidly integrated into a single response appropriate for an external stimulus.
We have investigated a specific plant-pathogen interaction as a model to identify mechanisms of phytohormone interactions. Plant responses to pathogens involve complex signaling pathways regulating numerous cellular reactions that contribute to the overall response. These responses are classified as either resistant or susceptible depending on the speed and extent of the visible reaction and the ability of the host to limit pathogen growth. Inhibited pathogen growth, resistance, is often accompanied by a hypersensitive response (HR), involving localized and restricted necrosis of the cells immediately surrounding the site of infection (Morel and Dangl, 1997). In contrast, the macroscopic symptoms of susceptible responses typically are not spatially limited. A number of common cellular reactions accompany both compatible and incompatible interactions, including changes in gene expression, synthesis of antimicrobial metabolites, and reinforcement of cell walls (Hammond-Kosack and Jones, 1996).
Many host reactions to pathogen infection are influenced by the phytohormones, ethylene, salicylic acid (SA), and the oxylipin jasmonates such as jasmonic acid (JA; Dong, 1998; Penninckx et al., 1998; Schenk et al., 2000). The roles of these hormones are dependent upon the particular host-pathogen interaction (Knoester et al., 1998; Hoffman et al., 1999). On the basis of the interactions that have been studied, a general rule of hormonal action has been proposed in which resistant responses to biotrophs require SA, whereas responses to necrotrophs require JA and ethylene (Feys and Parker, 2000). Roles for ethylene, SA, and JA have also been proposed in regulation of susceptible responses (Bent et al., 1992; Lund et al., 1998; Greenberg et al., 2000; Pilloff et al., 2002). Again, the hormone requirements are dependent upon the particular interaction. In some instances, these hormones are involved in determining the level of host basal resistance (Delaney et al., 1994; Glazebrook et al., 1996). In other cases, their actions are only involved in production of disease symptoms and do not affect growth of the pathogen. Separation of bacterial growth from the macroscopic disease symptoms demonstrates that pathogen-induced disease is an active host response.
As mentioned above, several lines of evidence support a model consisting of parallel SA- and JA/ethylene-mediated defense responses, with extensive negative feedback between them. For example, the JA/ethylene-inducible gene PDF1.2 is more highly expressed in the SA-deficient sid2 mutant in response to infection with a fungal pathogen (Dewdney et al., 2000). Conversely, a JA-related mutant constitutively expresses the SA-regulated PR1 gene (Petersen et al., 2000). Recent global expression profiling studies largely support this view. Glazebrook et al. (2003) showed that JA and ethylene cooperatively interact whereas SA acts in opposition to them. However, expression of at least some genes is induced by either JA or SA, indicating that these hormones are capable of cooperative interactions as well (Schenk et al., 2000; Glazebrook et al., 2003). It must be noted that, due to the abundance of well-defined mutants, most such studies have been conducted in Arabidopsis. Therefore, it will be important to determine whether this complex signaling network is generally applicable to different combinations of plant and pathogen interactions.
JA is one of a group of related oxylipins that have been shown to be involved in pathogen responses (Pieterse et al., 1998; Vijayan et al., 1998; Howe and Schilmiller, 2002). JA is a cyclopentanone that is synthesized from linolenic acid (for review, see Wasternack and Hause, 2002). Following successive action of lipoxygenase, allene oxide synthase, and allene oxide cyclase (AOC), the cyclopentenone 12-oxophytodienoic acid (OPDA) is produced. OPDA is then converted to JA via OPDA reductase and a series of β-oxidation steps. Growing evidence indicates that the cyclopentenones including OPDA are biologically active in pathogen responses (Blechert et al., 1995; Stintzi et al., 2001). There are also indications that responses are mediated by the specific set of oxylipins present, the oxylipin signature. Thus, what are commonly referred to as JA effects in the literature are likely the consequence of multiple biologically active octadecanoid-derived molecules commonly referred to as jasmonates (Wasternack and Hause, 2002).
Hosts that permit substantial pathogen growth but do not exhibit typical disease symptoms are said to be tolerant. We have previously demonstrated tolerance to Xanthomonas campestris pv. vesicatoria (Xcv) in tomato (Lycopersicon esculentum) lines insensitive to ethylene (Never ripe) or inhibited in ethylene synthesis (ACC deaminase [ACD]; Lund et al., 1998). Tolerance was also observed in a tomato NahG line that fails to accumulate SA after Xcv infection (O'Donnell et al., 2001). In tomato, there are two stages of disease symptom development. There is a primary response consisting of localized lesions that is unaltered in the mutants followed by a secondary phase of chlorosis and necrosis that spreads out from the primary infection sites. It is the latter phase that is dependent upon both ethylene and SA and is abolished in the mutants. Xcv-induced disease in tomato, therefore, requires the cooperative action of ethylene and SA. Given the agonistic and antagonistic interactions that can occur among JA, SA, and ethylene, we were also interested in examining the potential role of jasmonates in this defense response. Here, we demonstrate that the susceptible response is dependent upon the cooperative and sequential actions of three hormones, jasmonate, ethylene, and SA.
RESULTS
A Role for Jasmonates in the Host-Dependent Regulation of Xcv-Induced Disease
Jasmonates have been shown to have a major role in host defense against certain pathogens, especially in Arabidopsis (Penninckx et al., 1998; Vijayan et al., 1998; Van Wees et al., 2000). Because the roles of phytohormones in host defense can be dissimilar in different plants, we were particularly interested in examining the role of jasmonates in a susceptible tomato response. We used def1, a mutant with a defective octadecanoid synthesis pathway (Howe et al., 1996), and a line expressing an antisense construct of the JA biosynthetic enzyme, AS-AOC (Stenzel et al., 2003), to determine the role of jasmonates in the tomato:Xcv compatible response. The precise nature of the def1 mutation has not been determined, although it does not accumulate jasmonates in response to wounding. The AS-AOC line is blocked in synthesis of JA at the step before synthesis of OPDA. Disease symptom development was assessed in the two lines and compared with their isogenic wild-type parents following infection with the virulent Xcv strain 93-1. Figure 1 shows disease symptoms resulting from Xcv infection in the four lines 10 d post inoculation. The def1 exhibited an attenuated susceptible response with no visible secondary disease symptoms. The AS-AOC line also showed little of the chlorosis or secondary necrosis that was evident in the wild-type lines. This reduction in symptom development observed in def1 and AS-AOC was confirmed by ion leakage, a quantitative measurement of cell death (Fig. 1B). Because these lines were previously shown to have reduced levels of stress-induced JA (Howe et al., 1996; Stenzel et al., 2003), this result indicates that jasmonates influence host-regulated production of disease symptoms following Xcv infection.
Figure 1.
Reduced disease in Xcv infected AS-AOC and def1. Six-week-old tomato plants were infected with the virulent Xcv strain 93-1, and the progression of disease symptoms was followed visually. A, Comparisons of disease symptom progression 10 d post inoculation in def1 versus its isogenic parent (Castlemart II [CMII]) and AS-AOC versus its isogenic parent (Lukullus [LK]). B, Xcv-induced cell death was quantified by ion leakage measurements.
Jasmonates Regulate a Host Pathway That Determines Bacterial Virulence
In the ACD and NahG tomato lines, inhibited in ethylene and SA accumulation respectively, infection with Xcv results in a tolerant response in which disease development is attenuated but there is no measurable effect on bacterial growth (O'Donnell et al., 2001). Host basal resistance to Xcv, the ability to control pathogen growth, is thus independent of ethylene and SA. Rather, the action of both hormones is limited to development of secondary disease symptoms. In contrast, jasmonates do affect basal resistance. Figure 2A shows the relative rates of bacterial growth in each of the lines compared with their wild-type controls. Both def1 and AS-AOC exhibited reduced levels of bacterial growth. The reduction in symptoms observed in these two lines could be related to this difference in bacterial titers, or it may indicate an active role for jasmonates in promoting cell death. Interestingly, when plants were infected with Pseudomonas syringae pv tomato DC3000 (Pst), which produces the phytotoxin coronatine, a toxin with close structural resemblance to OPDA (Weiler et al., 1994), no difference in bacterial growth or disease symptom development was observed among def1, AS-AOC, and their wild-type controls (data not shown).
Figure 2.
Growth of virulent and avirulent Xcv strains on AS-AOC, def1, NahG, and ACD. Bacterial growth was measured in mutant and transgenic lines and compared directly with their isogenic parents. AS-AOC, def1, ACD, and NahG were compared with their isogenic wild-type parents, tomato cvs LK, CMII, UC82B, and MM, respectively. Growth was determined for the virulent 93-1 (A) and the avirulent 87-7 (B) Xcv strains sampled at the indicated times. Infections were performed by dip inoculation in a suspension of 107 cfu mL–1 of 93-1 and 108 cfu mL–1 of 87-7. Each time point represents two discs from separate leaves on each of three plants (± se, n = 6). Values are the average ± se. C, Ion leakage measurements measuring the extent of tissue death associated with lesions formed during the incompatible interaction with avirulent Xcv.
Because loss of jasmonate signaling reduced bacterial growth, we were further interested in evaluating loss of hormone signaling on an R gene-mediated incompatible interaction. Therefore, JA, SA, and ethylene mutants were infected with the avirulent Xcv strain, 87-7 (Bonas et al., 1993). In each case, bacterial growth was unaltered in the mutants relative to their isogenic parents, indicating no significant role for these three hormones in resistance to Xcv, as defined by bacterial growth (Fig. 2B). However, there were significant differences in the lesion sizes associated with the HR in each of the transgenic lines. Hypersensitive cell death was quantified by measuring ion leakage in each line. The AS-AOC and ACD lines exhibited smaller lesions and less cell death than a wild-type control, whereas NahG lesions were larger, developed more rapidly, and exhibited more cell death than the control (Fig. 2C). Thus JA, ethylene, and SA, although not affecting R gene-mediated resistance as defined by bacterial growth, do influence the extent of host cell death.
Jasmonate Action Lies Upstream of Ethylene and SA in the Compatible Interaction
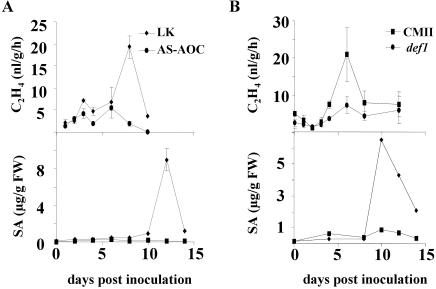
To determine whether and when jasmonate signaling interacts with ethylene and SA in a compatible interaction, the levels of ethylene and SA in Xcv-infected def1, AS-AOC, and their wild-type parents were determined (Fig. 3). AS-AOC and def1 were found to act similarly, with both lines producing less ethylene and less SA than their wild-type controls. These results are consistent with jasmonate action preceding synthesis of both ethylene and SA.
Figure 3.
The effects of altered jasmonates on ethylene and SA accumulation following infection by Xcv. The tomato lines, AS-AOC (A) and def1 (B), along with their isogenic parents (LK and CMII, respectively) were infected with virulent Xcv. The levels of ethylene (top) and free SA (bottom) were determined at the indicated time points. Determinations were performed at least five times, and results from a representative experiment are shown. Each point represents the average ± se of three ethylene measurements.
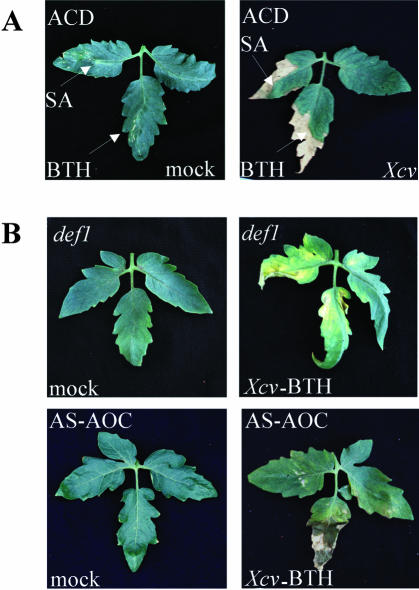
Xcv-infected ACD or Never ripe tomato lines do not accumulate SA, and the addition of exogenous SA to these lines restores disease development in the infected but not mock-inoculated plants (O'Donnell et al., 2001). Blocking ethylene action thus inhibits the ability of the host to promote disease by subsequent accumulation of free SA. If jasmonate action precedes subsequent action of ethylene and SA, then SA application to infected def1 and AS-AOC plants may restore symptom development. When SA was added to def1 and AS-AOC plants, uninfected plants developed disease-like lesions, even at concentrations below 2 mm (data not shown). These concentrations have been previously shown to be nontoxic to uninfected wild-type, NahG, and ACD lines (O'Donnell et al., 2001). Inhibition of JA synthesis, therefore, enhanced sensitivity to exogenous SA. To determine whether stimulation of the SA-signaling pathway would restore disease symptoms, the SA agonist benzo(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester (BTH) was used instead. BTH has been shown to act like SA in terms of symptom restoration in Arabidopsis (Lawton et al., 1996). As can be seen in Figure 4, SA and BTH act similarly in tomato, restoring disease symptoms in the infected but not mock-inoculated ACD leaves. When BTH was applied to infected def1 and AS-AOC, the disease phenotype was restored, consistent with a model in which SA acts downstream of jasmonates and is essential for development of the disease symptoms. Symptom development in tomato, therefore, requires the sequential action of at least three host signals.
Figure 4.
Disease development in leaves treated with the SA agonist BTH or following prior challenge with virulent Xcv. A, Comparison of the effects of SA and BTH addition to the disease-attenuated ACD line. Mock-inoculated (left) and Xcv-infected (right) ACD plants were injected with SA (0.2 mm) and BTH (2.0 mm) 8 d after infection. Photographed 4 d after injection. B, Mock-inoculated (left) and infected (right) plants sprayed with BTH 8 d following infection. Photographs were taken 4 d later.
Early Jasmonate Signaling Is Not Associated with a Measurable Increase in JA
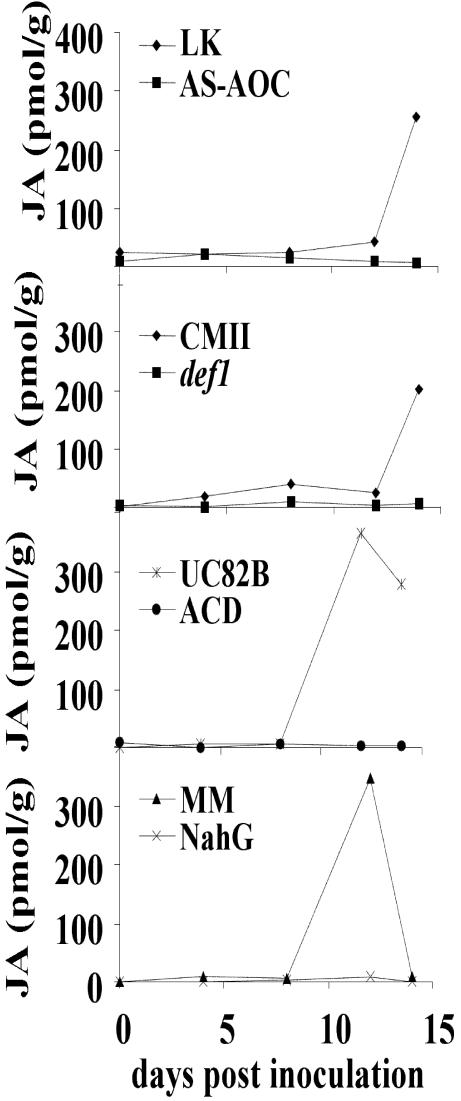
The genetic data above indicate a role for JA signaling at an early stage of the disease process— growth of Xcv in the host. Therefore, we were interested in determining to what level the JA biosynthetic pathway is stimulated and whether altered JA is associated with the change in Xcv virulence observed in def1 and AS-AOC. JA levels were also determined in ACD, NahG, and their respective controls. Figure 5A shows the rate of JA accumulation following Xcv infection in each line. Surprisingly, although def1 and AS-AOC affect the host response at a very early stage, JA accumulation was only detected late in the response in the wild-type controls. No increase in JA was detected in the def1, AS-AOC, ACD, or NahG lines, even at the late time points. JA only accumulated in tissues of the isogenic wild-type parents with extensive secondary disease necrosis containing high levels of free SA. This JA is associated with dying tissue and cannot be responsible for limiting bacterial growth. That JA is a consequence and not the cause of the necrosis was confirmed by feeding experiments in which infected AS-AOC, ACD, and NahG lines were supplied with exogenous JA. Unlike the BTH add-back experiments, the added JA had no effect on symptom development in these lines (data not shown).
Figure 5.
Accumulation of JA following Xcv infection. Levels of JA in AS-AOC, def1, ACD, and NahG compared with their wild-type controls. Plants were infected with virulent Xcv and samples were collected for 14 d.
It is possible that there is a rapid and transient increase in JA synthesis early in the compatible response. An increase might also occur specifically in a limited number of cells and therefore be difficult to measure in a large background of non-responding cells. To test whether the failure to measure JA early in the response was due to rapid and transient synthesis, tissues were harvested 4, 8, 12, and 24 h post inoculation, and the JA levels were measured. Again, no increase was detected. To test whether the failure to detect JA was due to localized synthesis, occurring only in those cells immediately surrounding the site of infection, a highly concentrated suspension of Xcv (108 colony forming units [cfu] mL–1) was vacuum infiltrated into the leaves of AS-AOC and its isogenic parent (LK). As a comparison, NahG and its isogenic parent (tomato cv Moneymaker [MM]) were chosen because SA acts at a much later stage of disease development and NahG exhibits tolerance to Xcv in contrast to the resistance observed in AS-AOC. Tissues were harvested, and JA levels were determined. Again, no significant increase in JA was detected before the onset of the secondary disease symptoms. We also quantitated OPDA, a precursor of JA that has been shown to be active in pathogen interactions (Stintzi et al., 2001). In three independent experiments, we observed increases in OPDA of between 2- and 4-fold between 2 and 4 d after infection of wild-type plants. However, in a fourth experiment, no increase in OPDA was observed, despite normal disease symptom development. Thus, we cannot conclude that the increases in OPDA accumulation are biologically significant. Nonetheless, the preponderance of evidence is consistent with a jasmonate signal preceding that of SA. Blocking accumulation of octadecanoids synthesized via the allene oxide synthase/AOC pathway removes a plant-derived virulence factor that is essential for bacterial growth and subsequent disease development.
DISCUSSION
Although much work has been focused on understanding the responses of plants to avirulent pathogens, relatively little is known about how plants mount defense responses to virulent pathogens. The complex response mounted by tomato following infection by virulent Xcv clearly indicates an active defense system involving coordination of multiple hormone-signaling pathways. How these pathways are integrated into a single response has been the focus of this work.
Roles for ethylene and SA in tomato defense against Xcv have been established (O'Donnell et al., 2001). Loss of synthesis or perception for either hormone results in a tolerant response with significantly less tissue damage at the site of infection. Ethylene action precedes and is necessary for subsequent SA action. Accumulation of SA is correlated with and predictive of the extent of expanding necrosis. Here, we have assessed the role of jasmonates in that same compatible tomato-Xcv interaction using an antisense AOC transgenic line and def1. The former line is blocked in production of OPDA and subsequent jasmonates, whereas the latter mutant does not exhibit stress-induced accumulation of jasmonates. Jasmonate action was shown to be essential for full symptom development. Infected AS-AOC and def1 phenotypically resemble infected ACD and NahG lines in that they had greatly reduced symptoms relative to wild-type controls. Inhibiting the jasmonate pathway results in reduced ethylene and SA accumulation, indicating that the plant response initiated by Xcv requires synthesis and cooperative interactions of JA, ethylene, and SA to induce symptom development.
Because the jasmonate-altered lines do not show the Xcv-induced increases in ethylene and SA, the action of jasmonate must precede ethylene (Fig. 6). The SA agonist BTH rescued development of disease symptoms in AS-AOC and def1. Further evidence of an early role for jasmonate is the reduction of bacterial growth in AS-AOC and def1. All of the data are consistent with a sequential mode of action with respect to these three hormones. However, because the response to Xcv occurs over days, it is likely that the response is not limited to these three signaling pathways. Notably, increased JA was not associated with this early response. Although suppression of bacterial growth is manifested before d 4, we were unable to detect JA increases in Xcv-infected wild-type tomato until very late in the response after ethylene synthesis had occurred. This late-appearing JA, associated with necrosis, may be due to loss of membrane integrity during cell death. Further, although JA does accumulate late and is associated with terminal necrosis, exogenous JA application did not restore symptom development in any of the mutant or transgenic lines. Due to lack of availability, we were not able to fully evaluate the effects of OPDA application on symptom restoration.
Figure 6.
Model for hormonal interactions in the primary and secondary responses of tomato leaves to virulent Xcv infection. Reduced synthesis of jasmonates interferes with the primary response, leading to resistance. Following the primary response, ethylene and SA action determine the magnitude of secondary symptom development but do not affect bacterial growth. The full development of disease in an infected leaf thus requires the sequential action of jasmonates, ethylene, and SA.
There are several potential ways in which jasmonates could mediate the early response to Xcv. First, JA may not be the active jasmonate mediating the response. We observed increased OPDA accumulation following infection, but the increase was inconsistent. It is possible that another unmeasured oxylipin may be mediating this pathogen response. Recent findings of other groups indicate that OPDA is biologically active (for review, see Blee, 2002). For example, Stintzi et al. (2001) showed that OPDA is sufficient to mount defense responses. Rather than JA alone, it has been proposed that the “oxylipin signature” is the overall determinant of a stress response (Kramell et al., 2000; Göbel et al., 2002; Howe and Schilmiller, 2002). Alternatively, sensitivity to jasmonates may be heightened following Xcv infection. Alterations in hormone sensitivity following pathogen infection are not unprecedented (Ciardi et al., 2001). In Arabidopsis, the jasmonate-signaling mutant, coi1, exhibits hyperactivation of SA-mediated defense responses following Pst infection (Kloek et al., 2001). We also observed heightened sensitivity of both the AS-AOC and def1 lines to exogenously applied SA. Thus, it is possible that the JA-related mutants may act by altering sensitivity to other hormones. Future experiments addressing jasmonate sensitivity following pathogen infection are certainly needed. Presently, there are no marker genes suitable for quantitative measurement of jasmonate sensitivity in tomato leaves. Although pin2 is associated with JA synthesis in wounded leaves (Howe et al., 1996), it is not induced following Xcv infection (D. Bies and H. Klee, unpublished data).
The role of jasmonates in resistance has been examined in Arabidopsis, although the story is not entirely clear. Pieterse et al. (1998) reported that the JA-signaling pathway is necessary for induced systemic resistance to Pst and that applied methyl jasmonate and 1-aminocyclopropane-1-carboxylate were capable of inducing resistance. However, Kloek et al. (2001) isolated an allele of the coronatine-insensitive locus (coi1) with enhanced resistance to Pst. This latter result is consistent with our tomato observations. The AS-AOC and def1 lines both exhibit reduced bacterial virulence or elevated resistance, whereas ACD and NahG exhibit a tolerant response in which bacterial growth is not affected. It is possible that the lower ethylene levels as well as reduced symptom development in AS-AOC and def1 may result from reduced bacterial growth, and an indirect relationship/interaction between JA and ethylene that is dependent upon the level of bacterial growth may exist. The observed resistance may be due to suppression of the normal host-susceptible response. Alternatively, the lack of an obvious HR in the def1 and AS-AOC may indicate that reduced bacterial growth results from an altered environment in planta that is less permissive to colonization by Xcv. This concept is supported by results showing that growth of the coronatine-deficient Pst DC3661 is greatly inhibited relative to the coronatine-producing DC3000 strain (Mittal and Davis, 1995). The concept of a loss of function leading to resistance is not unprecedented. The Arabidopsis pmr6 mutant, deficient in a pectate-lyase-like gene, was shown to be more resistant to Erysiphe cichoracearum (Vogel et al., 2002). This resistance was not associated with an HR or altered SA, JA, or ethylene signaling.
Interestingly, while the level of R gene-mediated resistance to avirulent Xcv, as defined by bacterial growth, was not altered in AS-AOC, ACD, or NahG, each line did have altered lesion sizes. Thus jasmonates, ethylene, and SA, although not required for resistance, do regulate the extent of hypersensitive cell death. The AS-AOC and ACD lines produced smaller lesions than controls, whereas NahG displayed an exaggerated HR, which eventually enveloped the entire leaf. Ciardi et al. (2001) showed that increasing ethylene sensitivity leads to larger lesions following infection with avirulent Xcv. The reduced sizes of lesions in ACD and AS-AOC may be a consequence of reduced ethylene synthesis. Whatever the mechanisms, it is interesting that in both the compatible and incompatible tomato responses to Xcv, jasmonates, ethylene, and SA always play a role in the regulation of a cell death response.
The specific relationships among jasmonates, ethylene, and SA are unknown. As illustrated in Figure 6, the jasmonate pathway acts early in the response and affects bacterial growth. Ethylene action is intermediate and is not directly involved in bacterial colonization or the eventual death of the infected tissue but is an essential intermediary between jasmonate and SA. The SA accumulates late in the disease process and is correlated with terminal necrosis. Although jasmonate, ethylene, and SA action are essential to this susceptible response, there are almost certainly other factors yet to be identified. Further, it is highly likely that the timing of these events is critical. The context in which each hormone acts likely influences the outcome of the disease process. Thus, SA induces chlorosis and necrosis only when applied to infected tissue 8 to 12 d after inoculation (O'Donnell et al., 2001). There may be an additional feedback component to this pathway because loss of jasmonate synthesis (as evident in the AS-AOC and def1 lines) significantly increases sensitivity to exogenously applied SA. Thus, alterations in one pathway appear to affect responses to additional hormones.
Presently, the means by which a complex signaling network is coordinated within a response are poorly understood. Plants respond to both biotic and abiotic stresses by evoking reactions designed to resist and limit damage. The response in each case consists of altered expression of sets of genes appropriate to the stimulus. Some phytohormones such as ethylene are common to many stimuli, yet the outcome is usually unique to the stimulus. For example, ethylene is an integral component of tomato virulent and avirulent responses to Xcv. Yet in the virulent response, ethylene is necessary for subsequent SA, whereas in the avirulent response, ethylene is synthesized earlier and is not followed by SA. Similarly, loss of jasmonate synthesis in def1 reduces the virulence of Xcv and enhances sensitivity to the toxic effects of applied SA. Yet def1 is much more susceptible to herbivory than its wild-type parent (Howe et al., 1996). Thus, “basal resistance” of def1 to Xcv is higher but to insects is severely compromised. It is likely that all of these hormones act in a modular fashion and that action can be agonistic in some circumstances and antagonistic in others. By varying the timing and combinations of their synthesis, appropriate outcomes are achieved. There has been significant investigation of potential points of hormonal interaction as a mechanism for integrating stress responses (O'Donnell et al., 1996; Moller and Chua, 1999; Shinozaki and Yamaguchi-Shinozaki, 2000). The tomato response to virulent Xcv is one in which one hormone response directly influences synthesis of subsequent hormones. Regulation of hormone levels is likely to be a major means of controlling hormonal interactions. Establishing such relationships will require integrated biochemical and genetic approaches.
Finally, it must be noted that the hormonal response described here is different from those of many other plant-pathogen combinations. Whereas in Arabidopsis, pathogen defenses seem to use predominantly SA or JA/ethylene pathways, this tomato response is dependent on sequential action of all three hormones. How a plant responds to different pathogens is likely to depend on the strategies of both partners, e.g. biotrophic versus necrotrophic pathogens or annuals such as Arabidopsis versus perennials such as tomato. The response of Arabidopsis to virulent X. campestris pv. campestris, although dependent on the same hormones, is entirely different with SA action preceding ethylene (O'Donnell et al., 2003). Thus, the concept of model interactions must be taken in this context.
MATERIALS AND METHODS
Plant Growth and Treatments
Tomato (Lycopersicon esculentum) cvs MM, UC82B, CMII, and LK are the parental lines for NahG (Oldroyd and Staskawitz, 1998), ACD (Lund et al., 1998), def1 (Lightner et al., 1993), and AS-AOC (Stenzel et al., 2003), respectively, and were included as controls where appropriate. Plant growth and treatments were performed under ambient temperature and lighting in a greenhouse. Experiments were conducted on 6-week-old plants with five fully expanded leaves. Plants were inoculated with Xcv strain 93-1 (virulent) or 87-7 (avirulent) by submerging in a suspension of bacteria containing MgCl2 (10 mm) and Silwet l-77 (0.0025% [v/v]), for 15 s. Mock inoculations were performed by dipping plants in buffered Silwet only. Each experiment was reproduced at least five times with similar results.
Formulated BTH was ectopically applied by spraying plants 8 d post treatment, in accordance with manufacturer's instructions, or was injected. SA (0.2 mm; Sigma-Aldrich, St. Louis) injection was performed using a syringe and an 18-gauge needle into the abaxial surface of the leaf.
Ion Leakage and Bacterial Growth
Electrolyte leakage and bacterial growth were measured as described previously (Lund et al., 1998).
Ethylene Quantification
Ethylene evolution, from infected and control leaves, was measured by placing excised leaflets in a 5-mL container, capping with a rubber stopper, and incubating for 1 h at room temperature. Ethylene accumulation was then determined from a 1-mL sample of the headspace on a gas chromatograph (model 5890, Hewlett-Packard, Palo Alto, CA), fitted with a flame ionization detector.
SA Determination
SA and SA conjugates were extracted and analyzed as described (Uknes et al., 1993). In brief, 0.5 g of tissue was ground in liquid nitrogen, extracted with 3 mL of 90% (v/v) methanol followed by 2 mL of 100% (v/v) methanol. The combined extracts were then divided in two, dried down, and resuspended in either 2.5 mL of 5% (w/v) trichloroacetic acid, free acid, or phosphate buffer (0.1 m, pH 8.0), total SA content. Conjugated SA was hydrolyzed by acidifying to pH 0.95 with concentrated HCl and boiling for 30 min. Free SA from both fractions was then extracted into ethylacetate: cyclopentane:isopropanol (100:99:1, v/v), twice for free and three times for total, dried down, and resuspended in 20% (v/v) methanol. SA was identified and quantified by reverse phase HPLC on a 5-μm C-18 column (4.6 × 250 mm, Beckman Ultrasphere, Fullerton, CA) and detected using a scanning fluorescence detector (excitation energy 295 nm, emission energy 400 nm; 474, Waters, Milford, MA). Identification and recovery of SA was determined by spiking a noninduced sample with a known amount of an authentic standard.
Extraction and Quantification of Jasmonates
JA and OPDA levels were measured as described by Hause et al. (2000) and Stenzel et al. (2003), respectively.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030379.
This work was supported by the National Science Foundation (grant no. IBN–0091064 to H.K.), by the Deutsche Forschunggemeinschaft (project C5 of the SFB363 to C.W. and O.M.), and in part by the Florida Agricultural Experiment Station. This is Florida Agricultural Experiment Station journal series number R-09718.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology. Academic Press, San Diego
- Bent A, Innes R, Ecker J, Staskawitz B (1992) Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact 5: 372–378 [DOI] [PubMed] [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH (1995) The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA 92: 4099–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blee E (2002) Impact of phyto-oxylipins in plant defense. Trends Plant Sci 7: 315–321 [DOI] [PubMed] [Google Scholar]
- Bonas U, Conradsstrauch J, Balbo I (1993) Resistance in tomato to Xanthomonas campestris pv vesicatoria is determined by alleles of the pepper-specific avirulence gene AvrBS3. Mol Gen Genet 238: 261–269 [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman D, Jones JB, Klee HJ (2001) Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant-Microbe Interact 14: 487–495 [DOI] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Dewdney J, Reuber TL, Wildermuth MC, Devoto A, Cui J, Stutius LM, Drummond EP, Ausubel FM (2000) Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J 24: 205–218 [DOI] [PubMed] [Google Scholar]
- Dong XN (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1: 316–323 [DOI] [PubMed] [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Metraux JP, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34: 217–228 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel C, Feussner I, Hamberg M, Rosahl S (2002) Oxylipin profiling in pathogen-infected potato leaves. Biochim Biophys Acta 1584: 55–64 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Silverman FP, Liang H (2000) Uncoupling salicylic acid-dependent cell death and defense-related responses from disease resistance in the Arabidopsis mutant acd5. Genetics 156: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Stenzel I, Miersch O, Maucher H, Kramell R, Ziegler J, Wasternack C (2000) Tissue-specific oxylipin signature of tomato flowers: Allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant J 24: 113–126 [DOI] [PubMed] [Google Scholar]
- Hoffman T, Schmidt JS, Zheng XY, Bent AF (1999) Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol 119: 935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (jl5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL (2002) Oxylipin metabolism in response to stress. Curr Opin Plant Biol 5: 230–236 [DOI] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J 26: 509–522 [DOI] [PubMed] [Google Scholar]
- Knoester M, Van Loon LC, Van Den Heuvel J, Hennig J, Bol JF, Linthorst HJM (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Atzorn R, Parthier B, Wasternack C (2000) Octadecanoid-derived alteration of gene expression and the “oxylipin signature” in stressed barley leaves: implications for different signaling pathways. Plant Physiol 123: 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J (1996) Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J 10: 71–82 [DOI] [PubMed] [Google Scholar]
- Lightner J, Pearce G, Ryan CA, Browse J (1993) Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol Gen Genet 241: 595–601 [DOI] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ (1998) Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S, Davis KR (1995) Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv tomato. Mol Plant-Microbe Interact 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Moller SG, Chua NH (1999) Interactions and intersections of plant signaling pathways. J Mol Biol 293: 219–234 [DOI] [PubMed] [Google Scholar]
- Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4: 671–683 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ (2001) Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J 25: 315–323 [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Schmelz E, Moussatche P, Lund S, Jones J, Klee H (2003) Susceptible to intolerance: a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J 33: 245–257 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Staskawitz BJ (1998) Genetically engineered broad-spectrum disease resistance in tomato. Proc Natl Acad Sci USA 95: 10300–10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I, Thomma B, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE et al (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Loon LC (1999) Salicylic acid-independent plant defense pathways. Trends Plant Sci 4: 52–58 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, van Wees S, van Pelt J, Knoester M, Laan R, Gerrits H, Weisbeek P, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilloff RK, Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis gain-of-function mutant Dll1 spontaneously develops lesions mimicking cell death associated with disease. Plant J 30: 61–70 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross talk between two stress signaling pathways. Curr Opin Plant Biol 3: 217–223 [PubMed] [Google Scholar]
- Stenzel I, Hause B, Maucher H, Pitzschke A, Miersch O, Ziegler J, Ryan CA, Wasternack C (2003) Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato: amplification in wound signalling. Plant J 33: 577–589 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S, Winter AM, Delaney T, Vernooij B, Morse A, Friedrich L, Nye G, Potter S, Ward E, Ryals J (1993) Biological induction of systemic acquired resistance in Arabidopsis. Mol Plant-Microbe Interact 6: 692–698 [Google Scholar]
- Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97: 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95: 7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14: 2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2002) Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucleic Acid Res Mol Biol 72: 165–221 [DOI] [PubMed] [Google Scholar]
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F (1994) The Pseudomonas phytotoxin coronatine mimics octadecanoid signaling molecules of higher plants. FEBS Lett 345: 9–13 [DOI] [PubMed] [Google Scholar]