INTRODUCTION
Von Willebrand disease (VWD) is the most common inherited bleeding disorder,1 first discovered under the observations of Erik von Willebrand. In 1924, a 5-year-old girl was admitted to a hospital in Finland with mucocutaneous bleeding. Upon further investigation of both her and many of her relatives, von Willebrand reported differences between known hemophilia and the new “hereditary pseudo-hemophilia.”1 In the early 1970s, the medical community began to understand that a new factor (von Willebrand factor, or VWF) was responsible for the hemophilia-like symptoms.2
Although VWD is now recognized, diagnosis and treatment remain complex.3 In a study reviewing women with menorrhagia and known VWD, an average of 16 years elapsed before these women were correctly diagnosed.3 The delay in proper diagnosis can be attributed to physician lack of experience with VWD3 and the lack of a clear and concise diagnostic test to definitively point to VWD. Instead, clinicians are forced to use a variety of tests and surmise the results to make a differential diagnosis. To make matters more difficult, physicians have to consider interlaboratory variation, changes in VWF due to external variables (stress, surgery, exercise, anxiety, crying) and internal variables (systemic inflammation, pregnancy, menstruation, and use of oral contraception).4 After diagnosis, the severity of VWD presents clinicians with treatment challenges that are described herein.
The evolution of treatment of VWD parallels the course of scientific discovery about the disorder itself. Most products on the market to treat VWD in the United States were first indicated for treatment of hemophilia. Humate-P® is a VWF/Factor VIII (FVIII) concentrate, approved in the United States in 1986 first for hemophilia A, then in 1999 for VWD.* Because of its primary ratio of 2.4:1 (VWF:FVIII), Humate-P® is used primarily for treatment of VWD in patients with spontaneous and trauma-induced bleeding episodes, as well as in the prevention of excessive bleeding after surgery.5 A stringent, carefully monitored manufacturing process for Humate-P® has produced a strong safety record, with more than 500 million units infused without any documented reports of viral transmission.5
The introduction of new National Heart, Lung, and Blood Institute (NHLBI) guidelines have renewed awareness of VWD. This Product Profiler reviews the disorder and the evidence base for use of Humate-P® in the treatment of VWD.
Discovery of VWD
VWD is the most common genetically inherited coagulopathy. VWD occurs with a prevalence estimated at 66 to 100 cases per million symptomatic individuals, or 1% to 2% of the general population.1,2 VWD is caused by an anomaly in a blood protein called von Willebrand factor. This glycoprotein (GP) promotes adhesion and aggregation of platelets at wound sites, thereby inducing platelet plug formation. When VWF is defective or deficient, as in VWD, patients may experience easy bruising; frequent or prolonged nosebleeds; heavy or prolonged menstrual bleeding; or prolonged bleeding episodes following even minor injuries, childbirth, surgery, or dental work.1
Recognition of bleeding disorders has an ancient history reaching back to texts of the second century. It was not until the aforementioned Erik von Willebrand, an internist in Helsinki, described a genetic disorder with characteristics different from those of hemophilia. Unlike hemophilia, this disorder affected both sexes with predominantly mucosal bleeding, later determined to be related to loss of platelet adhesion and dependent on a plasma GP other than antihemophilic factor (FVIII). This GP was ultimately identified as VWF and the condition named VWD.6
Early success with fractionated plasma, which inhibited bleeding in hemophilia, did not correct the hemostatic defect in VWD. In 1956, a blood component termed fraction I-O did appear to improve hemostasis in VWD. It also, however, elevated plasma FVIII for 24 hours after administration and carried a small but important risk of transferring blood-borne pathogens. In 1977, Mannucci described positive experience with a vasopressin analogue, desmopressin acetate, that effectively controlled bleeding in most patients with VWD,7 although subsequent reports revealed potentially lethal side effects that limited its use in mild disease and in specific populations.4,8 In the 1980s, advances in the understanding of VWF structure and function, fractionation techniques, and safety yielded the first pasteurized* VWF/FVIII concentrate specifically for treating VWD. Today, it is the mainstay of treatment for VWD8 and nearly universally effective in controlling VWD bleeding episodes when given either episodically (to control discrete bleeding events such as those related to a trauma or monthly menses) or to prevent excessive bleeding during surgery or dental procedures.8
Disease Overview
Inherited as both an autosomal dominant and autosomal recessive disorder, VWD results in prolonged bleeding time despite normal platelet counts in people who carry the disorder. Bleeding from the mucosa, such as in the nasal/oral, gastrointestinal (GI), or reproductive tracts, is the most common clinical symptom. Both sexes and all racial groups are equally affected by VWD.4
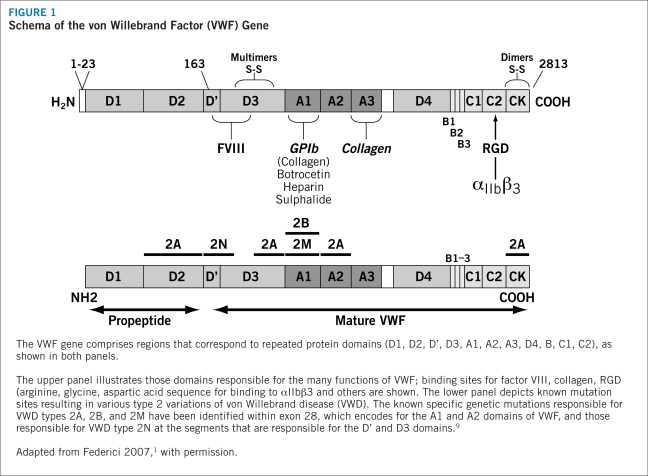
The genetics of VWD present a heterogeneous and diverse expression pattern. The VWF gene is located on chromosome 12 and yields a protein with several regions corresponding to four types of protein domains that comprise the functions of VWF (Figure 1). Genetic mutations within these domains are responsible for various types of VWD (see “classification of VWD,” page 4). An acquired form occurs secondary to other medical disorders, such as autoimmune conditions, some cancers, or cardiovascular disorders,9 but exhibits the same biochemical alterations† of inherited VWD.9–11 This Product Profiler focuses only on hereditary, not acquired, forms of VWD.
FIGURE 1.
Schema of the von Willebrand Factor (VWF) Gene
The wide spectrum of gene mutations in VWD, including large deletions, frameshifts from small insertions or deletions, splice-site mutations, nonsense mutations, and missense mutations,9 are similar to defects found in many other diseases. As a result, it is difficult to identify the presence of the disorder on traditional genetic testing, and in most cases VWD is recognized only during a hemostatic challenge.4 To complicate the assessment of VWD further, circulating levels of VWF appear to vary with blood type, with the lowest levels associated with blood type O.4,12 These levels rise with age, at an approximate rate of 6 U/dL per decade.4 During times of metabolic stress, such as during pregnancy or in patients with malignant disease, VWF levels also may rise.4
The importance of appropriate identification and treatment of VWD is underscored by the release of NHLBI guidelines in 2007. Previously, physicians lacked clear guidance regarding the discrepancies between laboratory methods and the relationships between VWF levels and bleeding. The new guidelines address this need and allow physicians to make more informed decisions about the diagnosis and management of VWD. In addition, the guidelines discuss the improvement of clinical diagnostic tools and optimal treatment options for patients suffering from VWD. Because of the limited number of published studies in this arena, many of the recommendations are based on the opinion of the expert panel.9
Classification of VWD
There are three distinct forms of vWD: type 1, type 2, and type 3 (Table 1). Types 1 and 3 involve a partial or complete deficiency of VWF, respectively. Type 2 involves structural or functional defects of the VWF protein.13 In general, the severity of bleeding worsens from type 1 to 3 disease. It should be understood that severity can differ within each type, and all degrees — mild, moderate, and severe forms — can benefit from correction of the deficient or defective VWF.13
TABLE 1.
Classification of von Willebrand Disease
| Type 1 | Type 2A | Type 2B | Type 2M | Type 2N | Type 3 | |
|---|---|---|---|---|---|---|
| Quantitative deficiency | ✓ | |||||
| Qualitative deficiency | ✓ | ✓ | ✓ | ✓ | ||
| Complete deficiency | ✓ | |||||
| Inheritance | AD | AD/AR | AD | AD/AR | AR | AR |
| RCo:VWF (levels range) | <30 | <30 | <30 | <30 | 30–200 | Undetectable |
| FVIII levels (range) | 50–200 IU/dL or mildly decreased | 50–200 IU/dL or mildly decreased | 50–200 IU/dL or mildly decreased | 50–200 IU/dL or mildly decreased | Significantly decreased | <10 IU/dL |
AD=autosomal dominant; AR=autosomal recessive
Source: NHLBI 20079
VWF is a relatively large protein made up of a signal peptide (22 amino acids), a large propeptide (741 amino acids), and a mature VWF molecule (2,050 amino acids).2 These different protein regions correspond to four repeating domains that are responsible for the different binding functions of the molecule.2 Monomers of mature VWF are assembled into multimers via disulfide bridges.4 Large complexes are essential for normal platelet adhesion and aggregation under high shear stress conditions, such as those found in arterial capillaries.4
Type 1
Type 1 is the most common form of VWD, accounting for approximately 75% of congenital cases.4 Type 1 disease is marked by a partial quantitative deficiency of functionally normal VWF in platelets or plasma.13 Due to the binding of VWF to FVIII in the plasma, FVIII levels may drop in parallel, warranting replacement of both blood proteins for hematologic normalization.
In type 1 disease, FVIII generally is mildly decreased and VWF levels are low, with no significant decrease in large VWF multimers and only mild bleeding tendency.1,2,13 In many cases, the remaining VWF is able to induce relatively normal platelet adhesion and FVIII binding.9 In cases in which VWF levels range from 30–50 IU/dL, falling just below the normal range (50–200 IU/dL), diagnosis may be difficult; delayed diagnosis can result in unnecessary blood loss, anemia, and, in women, menorrhagia. In cases with VWF levels in the 10–20 IU/dL range (usually related to VWF mutations inhibiting transport of proVWF or promoting rapid VWF clearance from the circulation),15,16 the diagnosis is more apparent.
Type 2
Type 2 composes about 15% to 20% of VWD diagnoses, and is marked by a qualitative defect in the VWF protein. The group is further divided into four subtypes based on pathophysiology:4,17,18
Type 2A VWD is recognized by a reduction in high-molecular-weight VWF multimers in the plasma and a decrease in VWF-dependent platelet adhesion.9 Levels of VWF protein and FVIII may be normal or slightly reduced, but VWF function is markedly decreased as determined by ristocetin cofactor (VWF:RCo) activity assay.19 Impaired coagulation may be related to mutations that inhibit normal assembly or secretion of large multimers or increase susceptibility to proteolytic degradation of VWF, and patients may be predisposed to bleeding due to the deficit of large multimers.9
Type 2B VWD is associated with proteolytic degradation and depletion of large multimers as a result of mutations that increase VWF-platelet binding.9 VWF also exhibits an increased affinity for the platelet GP complex, GP1bα. The overall effect is spontaneous binding of VWF to platelets, leading to platelet agglutination, thrombocytopenia, and rapid VWF clearance.9,20
Type 2M disease is caused by mutation in the GP1b binding domain, which leads to decreased VWF-dependent platelet binding activity despite normal multimer structure.21,22
Type 2N is characterized by a full array of normal VWF multimers but reduced VWF-FVIII binding. As a result, FVIII stability and half-life are reduced, and hemostasis is impaired.9 In most of these cases, FVIII is less than 10% of normal; as such, type 2N VWD may be misdiagnosed as an autosomal recessive form of hemophilia.9,23 Only a FVIII-VWF binding assay can distinguish between the two.
Type 3
Patients with type 3 VWD are essentially or completely devoid of VWF and are moderately deficient in FVIII as well, due to its shortened half-life in the absence of VWF. These patients thus have impaired thrombin generation and fibrin formation, and exhibit a severe hemophilia-like bleeding disorder.24 This disorder is rare, representing less than 5% of patients with VWD,18 and can be life-threatening. It is associated with decreased stability and rapid clearance of FVIII, with levels reportedly falling as low as 5% or less of normal.26
Diagnostic and Laboratory Criteria
A positive bleeding history since childhood may be suggestive of VWD.2 In particular, bleeding is prominent and/or easy bruising is seen in one or more of the five following indicators:2,4,27
Frequent or prolonged nosebleeds
Heavy menstrual bleeding
Prolonged bleeding (>5 minutes) or recurrent bleeding during or following childbirth or surgery
Prolonged/excessive bleeding or mucous membrane bleeding during dental work
Family history of an autosomal dominant or recessive inherited bleeding disorder or easy bruising with indurations, which may also indicate the presence of VWD
Multiple and specialized laboratory evaluations, generally repeated several times over a 1- to 3-month period to compensate for clinical and laboratory variability, are required for a definitive diagnosis of VWD.28 Unfortunately, no single laboratory test firmly establishes a diagnosis of VWD. Instead, an initial hemostatic evaluation is conducted to determine whether there is a coagulation factor deficiency, thrombocytopenia, or other coagulopathy causing the bleeding.9 Typically, laboratory tests include platelet count, complete blood count, partial thromboplastin time (PTT), prothrombin time (PT), and either fibrinogen measure or thrombin time.9
In patients with abnormal results, such as a prolonged PTT that corrects using a 1:1 mixing study, further testing should include evaluation for VWF:Ag, VWF:RCo, and FVIII.9 VWF:Ag measures the amount of protein present in plasma.9 A VWF:RCo assay exposes a plasma specimen to ristocetin, an antibiotic that causes platelet agglutination, indicating reduced VWF activity. This activity is measured via platelet aggregation and is proportional to the reduced levels of VWF in the plasma in subjects with VWD. Decreased concentration of VWF:Ag or decreased activity in VWF:RCo testing is suggestive of type 1 VWD.9 Testing for FVIII helps to determine the ability of VWF to serve as a carrier protein to maintain normal levels of FVIII.9
Additional tests, such as radioimmunoassay or enzymelinked immunoabsorbent assays, can also be used to indicate the amount of VWF antigen in plasma but do not necessarily indicate any structural or functional defects, and thus do not rule out type 2 VWD.9 Structural defects instead must be assessed by means of gel electrophoresis multimer analysis; low- and high-resolution gel electrophoresis techniques are used to distinguish multimeric structure of VWF in plasma and platelets in order to differentiate the variants of VWD.9 A deficiency in FVIII coagulant activity also may be suggestive of VWD due to the protective binding of VWF to FVIII under normal physiologic conditions, and a FVIII binding assay can help to distinguish between disease subtypes.
Closure time (CT) and VWF binding to collagen (VWF: CB) assays also may assist in screening for VWD. CT is a rapid and simple technique for determining VWF-dependent platelet function at high shear stress, and is a good alternative to bleeding time (BT) measures in children or in patients with contraindications. Its limitations are that it appears normal in individuals with type 2N VWD, and it cannot be modified in type 3 patients after administration of a VWF/FVIII concentrate.29 The VWF:CB assay or VWF:CB to VWF:Ag ratio can be used to distinguish types 1 and 2 disease, and should be performed in association with the VWF:RCo test.30,31 Neither assay, however, has been standardized, and therefore is not diagnostic alone and must be performed with other VWD testing.
Several rapid tests for a bleeding disorder, such as activated PTT and BT, may be suggestive of severe type 3 disease, but are not necessarily prolonged in patients with mild disease and therefore are not useful as a global screen for VWD.9 Furthermore, because of the heterogeneity of the disorder and the impact of external influences, repeat testing often is required. For instance, a normal result on a first BT or PT may not rule out VWD; prolongation of response may not be evident until a second or subsequent assessment.
Clinical Manifestations
Mild-to-moderate mucocutaneous bleeding is the most common manifestation of VWD. This is especially evident as easy bruising, prolonged bleeding after injury and during dental work, and prolonged menses and menorrhagia.1 Some cases do not require routine treatment, reflecting the high rate of type 1 VWD relative to other types. This “mild” bleeding can interfere with a rapid and accurate diagnosis for VWD because similar bleeding episodes are observed frequently in the healthy population. In addition, the marker of excess bleeding during menstruation and childbirth in VWD requires careful examination. Menorrhagia may be a sensitive predictor of VWD in women (sensitivity 32–100%), but has limited specificity as similar numbers of healthy women and women with VWD report excessive bleeding during menstruation.32
Excessive and protracted postsurgical bleeding is common in VWD, whereas soft tissue and joint bleeding are observed infrequently and predominantly in persons with type 3 disease or extreme VWF deficiency. Life-threatening bleeding, particularly GI or central nervous system, can occur in persons with type 3 disease and in some individuals with type 2 disease.9 Bleeding severity also may be influenced by concomitant disease or medications, such as worsening among individuals taking aspirin or other nonsteroidal anti-inflammatory drugs.9
Function of VWF
VWF is a complex, multimeric glycoprotein synthesized by endothelial cells and megakaryocytes.1,14,33 Mature VWF proteins are either stored in secretory granules of Weibel-Palade bodies of the endothelial cell or in the alpha-granule of platelets prior to secretion in the event of vessel damage, or circulate as a loosely coiled protein complex.1
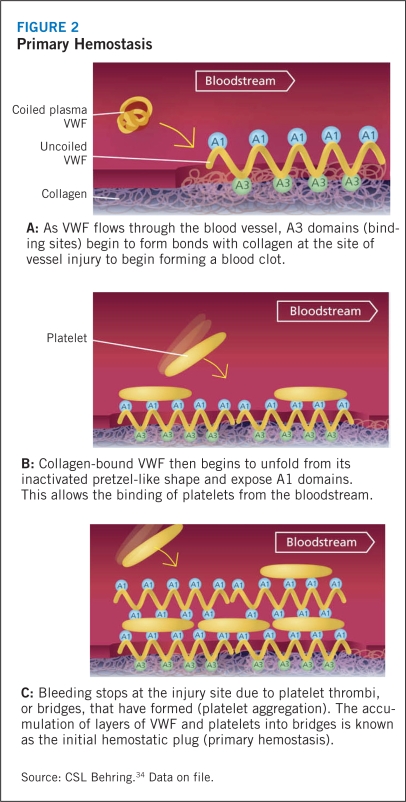
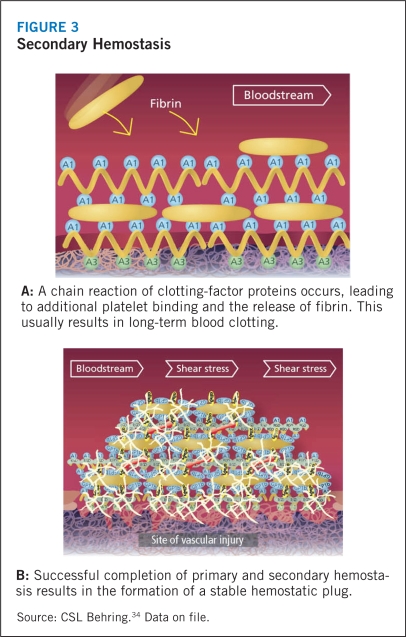
Circulating VWF does not interact with platelets or endothelial cells under normal physiologic conditions. In the event of tissue injury, however, VWF promotes both platelet adhesion and platelet aggregation.1 VWF, particularly the high-molecular-weight multimers, first attach to the subendothelial collagen at the site of injury, where-upon the high shear stress related to rapid blood flow in small vessels and large stenotic arteries activates the subendothelial collagen-bound VWF. This stretches the largest multimers into a filamentous sheet. The GP1bα receptor of circulating platelets adheres to the A1 domain of this reconformed VWF. The platelet adhesion leads to activation and secretion of intracellular proteins in an effort to engulf more platelets in the filament to form a hemostatic plug, a process known as primary hemostasis (Figure 2). Although this soft aggregate plug inhibits bleeding, it is fragile and easily dislodged. In order to secure the plug and complete the thrombus, activation of another platelet receptor protein GPIIb-IIIa35 initiates binding to VWF and fibrinogen, and promotes additional platelet recruitment into the hemostatic plug (Figure 3). During this secondary hemostasis, coagulation factors are activated, fibrin is released, and a stable hemostatic plug is formed.9,4,36
FIGURE 2.
Primary Hemostasis
FIGURE 3.
Secondary Hemostasis
VWF also influences the hemostatic pathway by its role in binding with FVIII. VWF protects FVIII from proteolytic degradation, prolonging its half-life and localizing it when vascular injury occurs. If VWF levels diminish, FVIII experiences a parallel decrease in concentration leading to faulty coagulation.1
Qualitative or quantitative defects in VWF interfere with normal hemostasis via impaired or delayed platelet adhesion and aggregation. As a result, subsequent platelets may not be recruited at full force or in “normal” time frame to complete the platelet plug. Additional coagulation factor reactions may be stalled, leading to the characteristic bleeding complications of VWD.1
Footnotes
Haemate-P® and Humate-P® are the same product. The difference between Haemate-P® and Humate-P® lies in the source of plasma — Humate-P® uses plasma only from U.S. donors. As such, the FDA recognizes the same active ingredients for both, which allows interchangeability of clinical results between the two.
The viral inactivation methods used to manufacture Humate-P® are proven safe and effective in eliminating blood-borne pathogens.
Decreased VWF antigen [VWF:Ag], VWF:RCo, and/or FVIII levels, for instance.