Abstract
Brassinosteroids (BRs) play important roles throughout plant growth and development. Despite the importance of clarifying the mechanism of BR-related growth regulation in cereal crops, BR-related cereal mutants have been identified only in rice (Oryza sativa). We previously found that semidwarf barley (Hordeum vulgare) accessions carrying the “uzu” gene, called “uzu” barley in Japan, are non-responding for brassinolide (BL). We then performed chemical and molecular analyses to clarify the mechanisms of uzu dwarfism using isogenic line pairs of uzu gene. The response of the uzu line to BL was significantly lower than that of its corresponding normal line. Measurement of BRs showed that the uzu line accumulates BRs, similar to known BR-insensitive mutants. The marker synteny of rice and barley chromosomes suggests that the uzu gene may be homologous to rice D61, a rice homolog of Arabidopsis BR-insensitive 1 (BRI1), encoding a BR-receptor protein. A barley homolog of BRI1, HvBRI1, was isolated by using degenerate primers. A comparison of HvBRI1 sequences in uzu and normal barley varieties showed that the uzu phenotype is correlated with a single nucleotide substitution. This substitution results in an amino acid change at a highly conserved residue in the kinase domain of the BR-receptor protein. These results may indicate that uzu dwarfism is caused by the missense mutation in HvBRI1. The uzu gene is being introduced into all hull-less barley cultivars in Japan as an effective dwarf gene for practical use, and this is the first report about an agronomically important mutation related to BRs.
Brassinolide (BL) is a firstly identified plant steroid hormone, isolated from rape (Brassica napus) pollen (Grove et al., 1979). Diverse plant species have been found to contain BL and a variety of structural analogs, called brassinosteroids (BRs). With their characteristic physiological effect on plant growth and development, BRs should be included as essential plant hormones, along with GAs, auxins, cytokinins (CKs), abscisic acid (ABA), and ethylene. The effect of BRs on germination, elongation growth, flowering, and sex expressions of plants have been reported, and various application techniques have been tested in the greenhouse and in the field (Yokota, 1999). BR applications have often increased grain and vegetable yields. Plants treated with BRs also acquired resistance to or tolerance against such stresses as cold, drought, salt, disease, and herbicide. In field tests, however, BR effects were unstable and not replicable. The biological activity of these BRs disappeared rapidly due to deactivation and was influenced by environmental conditions (Kamuro and Takatsuto, 1999).
In addition to studies on agricultural applications of BRs, BR physiology has also been studied (Yokota, 1997; Altmann, 1999; Bishop and Yokota, 2001). After many BR-deficient and -insensitive mutants were identified in Arabidopsis, BR biosynthesis and signaling have been rapidly clarified. BR biosynthesis mutants such as deetiolated 2 (det2; Chory et al., 1991) and constitutive photomorphogenesis and dwarfism (cpd; Szekeres et al., 1996) were identified by screening mutants that show de-etiolation in the dark (impaired skotomorphogenesis), and their dwarf phenotypes were recovered as the wild type by BR treatment. In BR signaling, Arabidopsis brassinosteroid-insensitive 1 (bri1) was firstly identified as a BR-insensitive mutant and does not show root-growth inhibition by BL (Clouse et al., 1996). bri1 showed a dwarf phenotype similar to a BR-biosynthesis mutant, but the dwarf phenotype of bri1 could not be recovered as a wild type by BR treatment. BRI1 encodes a Leu-rich repeat (LRR) protein kinase (Li and Chory, 1997), and it acts as a BR receptor (He et al., 2000; Wang et al., 2001). A second brassinosteroid-insensitive 2 (bin2) mutant has been identified (Li et al., 2001b) in addition to bri1. BIN2 encodes a GSK3/SHAGGY-like kinase, and acts as a negative regulator to control BR signaling in plants (Li and Nam, 2002). Further genetic screening has identified additional components involved in BR signaling: BRI1-Associated Receptor Kinase 1 (BAK1; Li et al., 2002), bri1 Suppressor 1 (BRS1; Li et al., 2001a), bri1-EMS-Suppressor 1 (BES1; Yin et al., 2002b), and Brassinazole-Resistant 1 (BZR1; Wang et al., 2002). BR-insensitive mutants bin3 and bin5 were recently shown to have defects in the Arabidopsis homologs of archaebacterial topoisomerase VI subunit A and SPO11 (Yin et al., 2002a). Their physiological roles in relation to BR signaling have yet to be clarified.
In addition to the Arabidopsis mutants, tomato (Lycopersicon esculentum) dwarf (Bishop et al., 1999), dumpy, and curl-3 (cu3; Koka et al., 2000; Montoya et al., 2002) and garden pea (Pisum sativum) lka and lkb (Nomura et al., 1997) have been identified as BR-related mutants in dicots. In monocots, however, only two BR-related mutants have been identified from rice (Oryza sativa). One is a BR-insensitive mutant, d61, defective in the rice homolog of Arabidopsis BRI1 (Yamamuro et al., 2000). The other is a recently identified BR-deficient mutant, brassinosteroid-dependent 1 (brd1), defective in the BR-6-oxidase gene (Hong et al., 2002; Mori et al., 2002). No other BR-related mutant has been identified in monocots. To clarify the physiological roles of BRs in monocots, especially in agronomically important cereals, BR-related mutants must be identified.
In a previous report on the screening of dwarf mutants using the leaf unrolling test, a sensitive technique for examining the BL response of barley (Hordeum vulgare), we found that barley accessions carrying a single recessive gene uzu, called “uzu” barley in Japan, do not respond to exogenously applied BL. The uzu dwarf may have originated in Japan and its semidwarf character leading to lodging resistance is very suitable for high yields, so the uzu gene is currently being introduced into all hull-less barley cultivated in Japan. The mechanism of uzu dwarfism has yet to be clarified, however.
Before BRs have gained wide acceptance as a plant hormone, most physiological studies of dwarf plants focused on the role of classical plant hormones, such as GA and auxin. The effect of these hormones on dwarf plants has been examined in many species. In the pea, stunted phenotypes of lka and lkb mutants were not fully recovered by GA and auxin treatment. Further study showed that lka is a BR-insensitive mutant and lkb a BR-deficient mutant (Nomura et al., 1997). The semidwarf phenotype of uzu has long been studied in relation to GA and auxin (Suge, 1972; Kuraishi, 1974; Inouhe et al., 1982; Honda et al., 1996), but no clear results have shown that uzu has a defect in GA/auxin-related signaling or biosynthesis. Reduced indole 3-acetic acid (IAA) content has been detected only in coleoptiles of uzu grown in the dark, but the effects of the uzu gene have been detected in all organs, including leaves, ears, and seeds, as well as coleoptiles. This result does not, however, explain all uzu gene effects. Regarding the BR insensitivity of uzu, uzu dwarfism must further be studied in relation to BRs.
We studied uzu in detail by physiologically determining uzu responsiveness to BRs and other plant hormones. We also performed the chemical analysis of endogenous BR levels and the molecular cloning of an uzu candidate gene to clarify dwarfism by this agronomical important dwarf gene.
RESULTS
Response of Leaf Segments to Plant Hormones
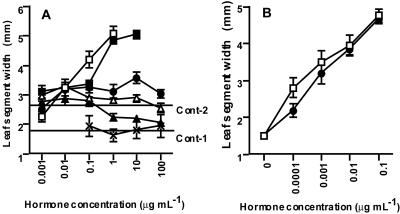
We used the leaf unrolling test to determine the responsiveness of the first leaf segment of darkgrown barley to BL. To determine whether other hormones affect the unrolling response of barley cv Bowman, we tested GA3, IAA, ABA, jasmonate, and trans-zeatin (t-Z) using this method. Except for t-Z, which showed the same effects as BL, no other plant hormones showed unrolling (Fig. 1A). We studied the unrolling of castasterone (CS), the putative direct biosynthetic precursor of BL, finding it to be similar to that of BL (Fig. 1B). BL activity was not enhanced by the addition of IAA (data not shown).
Figure 1.
Effect of plant hormones on the unrolling response of the barley cv Bowman leaf segment. A, Effects of BL (white square), t-Z (black square), s-ABA (black triangle), GA3 (black circle), jasmonate (white triangle), and IAA (cross). Cont-1 indicates the level of control (no treatment) in the IAA-only experiment. Cont-2 indicates the level of control in other hormone treatments. Ten leaf segments each were examined and averaged. Bars indicate se. B, Effects of BL (white square) and CS (black circle). Ten leaf segments each were examined and averaged. Bars indicate se.
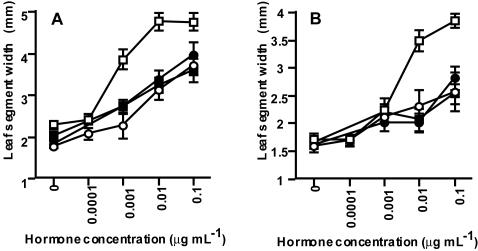
As reported previously (Honda et al., 2003), we examined the leaf unrolling response of 53 isogenic barley cv Bowman dwarf lines and found that the response of two lines, both having the uzu gene, was lower than that of the normal line. To further determine the response of the uzu line, we studied the unrolling response of uzu and normal lines to BL and t-Z using two isogenic line pairs of the uzu gene. One is the barley cv Bowman line containing the uzu gene (GSHO 1963, uzu line) and its corresponding normal line (cv Bowman), and the other is the Akashinriki line excluding the uzu gene (normal line) and its corresponding uzu line (cv Akashinriki). The unrolling response of both uzu lines to 0.1 μg mL–1 of BL solution was similar to that of normal lines to 0.001 μg mL–1 of BL solution (Fig. 2), indicating that the response of uzu is less than normal. The unrolling response of uzu lines to t-Z was similar to that of the normal lines. These results show clearly that the uzu line does not respond specifically to BL.
Figure 2.
Effect of BL and t-Z on the unrolling response of normal and uzu lines. A, Responses of normal and uzu isogenic barley cv Bowman lines. B, Responses of normal and uzu isogenic Akashinriki lines. Leaf segments were treated with BL for normal (white square) and uzu (white circle) lines or with t-Z for normal (black square) and uzu (black circle) lines. Ten leaf segments each were examined and averaged. Bars indicate se.
Response of the Intact Plant to BL
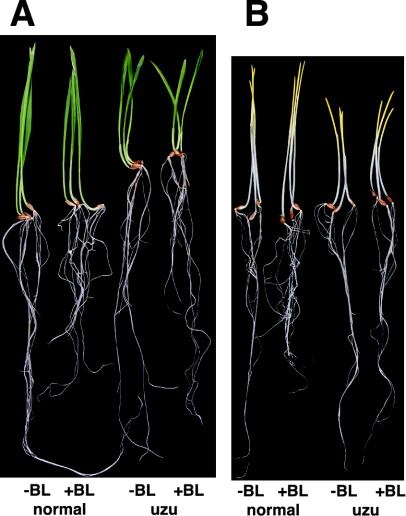
We studied the effect of BR treatment on the intact barley plant using normal and uzu lines, both of which were grown with and without BL under either continuous light or continuous darkness. The effect of BL treatment on the growth of aerial parts was not clear for either the uzu or normal Akashinriki lines, even in continuous light and continuous darkness (Fig. 3, A and B). The root growth of the normal line was clearly inhibited by 0.1 μg mL–1 BL, but that of the uzu line was unaffected. In an examination using another isogenic barley cv Bowman pair, the responses of seedling and root were similar to those for barley cv Akashinriki (data not shown).
Figure 3.
Effect of BL on the growth of normal and uzu isogenic Akashinriki lines. A, Pairs of light-grown seedlings. B, Pairs of darkgrown seedlings. Normal (left) and uzu (right) isogenic lines were grown in the absence (–BL) or presence (+BL) of 0.1 μg mL–1 BL.
Endogenous BRs
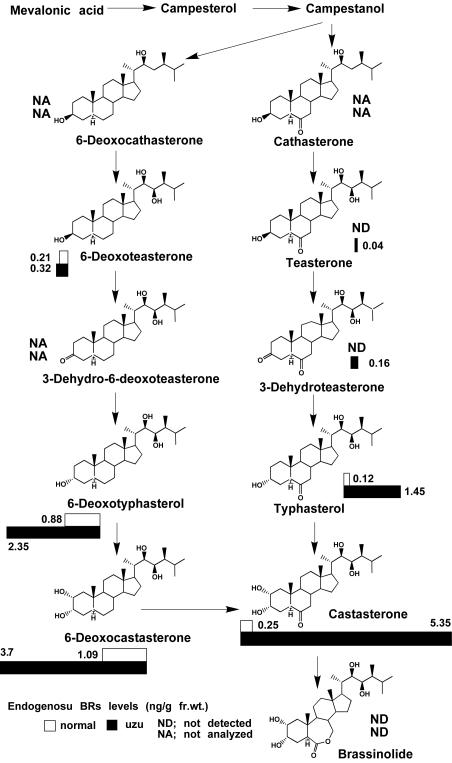
To clarify uzu dwarfism, we studied endogenous BR levels of normal and uzu Akashinriki lines using 7-d-old seedlings grown in continuous light. Endogenous BRs have not been reported in barley. Using full-scan gas chromatography/mass spectroscopy (GC/MS), we identified 6-deoxoteasterone (6-deoxoTE), typhasterol (TY), 6-deoxocastasterone (6-deoxoCS), and CS in both normal and uzu lines, but identified teasterone (TE) and 3-dihydroteasterone only in the uzu line. The occurrence of 6-deoxotyphasterol (6-deoxoTY) in both uzu and normal lines was suggested but not confirmed due to impurities. We conducted GC/selected ion monitoring (SIM) analysis monitoring molecular ions and principal ions, m/z 536 (M+), 446, 431 for 2H6-6-deoxoTY-bismethaneboronate-trimethylsilyl ether, and 550 (M+), 440, 425 for 6-deoxoTY-bismethaneboronate-trimethylsilyl ether and demonstrated the occurrence of 6-deoxoTY in both lines. We also studied the occurrence of cathasterone, 6-deoxocathasterone, and 3-dehydroteasterone, but results were inconclusive because 2H6-labeled BRs, added as internal standards, could not be recovered. Although ions from 2H6-labeled BL added as an internal standard were detected, those from endogenous BL were not detected in either line, probably because BL levels were too low to be detected or were entirely absent. This suggests that the widely accepted biosynthetic pathways of BRs (Fujioka and Sakurai, 1997) also operate in barley (Fig. 4). GC/SIM quantification showed that the CS content of the uzu line (5.35 ng g–1 fresh weight) was about 20-fold higher than that of the normal line (0.25 ng g–1 fresh weight). TY in the uzu line (1.45 ng g–1 fresh weight) was about 12-fold higher than that of the normal line (0.12 ng g–1 fresh weight). 6-deoxoCS (3.7 ng g–1 fresh weight) and 6-deoxoTY (2.35 ng g–1 fresh weight) in the uzu line were about 3-fold higher than those of the normal line (6-deoxoCS, 1.09 ng g–1 fresh weight, 6-deoxoTY, 0.88 ng g–1 fresh weight), whereas 6-deoxoTE levels in the uzu line (0.32 ng g–1 fresh weight) and normal line (0.21 ng g–1 fresh weight) did not clearly differ (Fig. 4). These results show that BRs, closer to CS on the putative biosynthetic pathway, accumulated more clearly in the uzu line. This accumulation pattern of BRs is consistent with other BR-insensitive mutants (Nomura et al., 1997; Noguchi et al., 1999; Yamamuro et al., 2000; Choe et al., 2002; Montoya et al., 2002). These physiological results clearly show the insensitivity of the uzu line to BRs.
Figure 4.
BR levels of normal and uzu isogenic Akashinriki lines and the putative biosynthetic pathway. Levels of BRs (nanograms per gram fresh weight) of the normal line are shown in values above and those of the uzu line in values below.
Synteny of Barley Chromosome 3H and Rice Chromosome 1
Genetic analysis has indicated that the semidwarf plant type of uzu barley is conditioned by a single recessive gene uzu, located on chromosome 3H. The North American Barley Genome Project (http://www.css.orst.edu/barley/nabgmp/nabgmp.htm) provided bin map data on barley, the uzu locus was mapped to Bin6 of chromosome 3H by using the phenotype as a probe, and 39 markers were located on Bin6. A comparative map of barley chromosome 3H and rice chromosome 1 (Smilde et al., 2001) and a high-density rice genetic linkage map (Harushima et al., 1998) are useful for transferring genetic information from rice to barley by using common markers mapped on both barley and rice chromosomes. We then carefully checked markers mapped on both rice chromosome 1 and barley chromosome 3H. One cDNA marker, C1271, located on Bin6 of barley, was mapped on both barley and rice chromosomes. C1271 was located at the same position of C1370 on the high-density map of rice. Yamamuro et al. (2000) reported that C1370 was closely linked to the d61 locus in linkage analysis between RFLP and the rice d61 phenotype. Rice dwarf mutant d61 carries a mutation in the gene (OsBRI1) encoding the rice homolog of Arabidopsis BRI1, a BR-receptor protein, suggesting that the barley BRI1 homolog may be a good candidate for a genetic lesion in uzu.
Cloning of a BRI1 Homolog from Barley
To confirm whether uzu has the mutation(s) in the barley BRI1 gene, we isolated a BRI1 homolog from normal and uzu barley and compared sequences. We started by attempting to amplify fragments of the barley BRI1 homolog by using the PCR with degenerate primers designed from amino acid sequences of Arabidopsis BRI1 and its rice homolog (OsBRI1). BRI1 is a LRR receptor-like kinase, and the sequence shows homology with other proteins containing LRRs and/or a kinase domain. We then carefully chose primer sites to amplify the barley BRI1 gene (Fig. 5), using cDNA derived from normal barley (cv Misato Golden) as a template for PCR. The sequence of the PCR product (875 bp long) was highly similar to Arabidopsis BRI1 and rice OsBRI1 genes. We constructed new gene specific primers and used them for the RACE experiments, which yielded a 3,901-bp fragment. To confirm the sequence of a full-length cDNA, we performed end-to-end PCR using 5′ and 3′ end primers and high-fidelity DNA polymerase. From end-to-end PCR, we cloned a 3,558-bp fragment and then sequenced this fragment. The predicted polypeptide of the fragment was the most similar to OsBRI1 (82.3% identity). A BLAST search showed that the predicted polypeptide showed a high score with rice OsBRI1 (1,625 bits), tomato systemin receptor SR160 (1,131 bits), and Arabidopsis BRI1 (1,087 bits). The recent characterization of the tomato BR receptor (tBRI1) showed that this protein is identical to systemin receptor SR160 (Montoya et al., 2002). The putative polypeptide of barley contained several domains also present in BRI1 homologs, i.e. Arabidopsis BRI1, rice OsBRI1, and tomato tBRI1 (Fig. 5). We then named the gene corresponding to the 3,558-bp fragment “HvBRI1” (GenBank accession no. AB088206).
Figure 5.
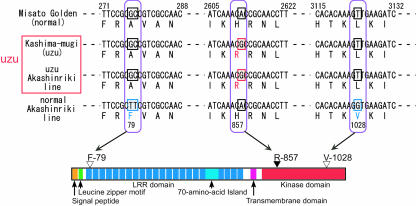
Comparison of BRI sequences. Deduced amino acid sequences of BRI1 homologs from barley (HvBRI1), rice (accession no. AP003453.3), Arabidopsis (accession no. AAC49810), and tomato (accession no. AY179606) were aligned by using ClustalW and BOXSHADE Web sites (http://www.ch.embnet.org/software/ClustalW.html and http://www.ch.embnet.org/software/BOX_form.html). Identical residues are boxed in black; similar residues are shaded in gray. Asterisks indicate positions of degenerate primers. The single line indicates a putative signal peptide. Black circles indicate Leu residues in the Leu zipper motif. White circles indicate the pair of Cys residues. The double line indicates 70-amino acid island in the LRR domain. Dashed line indicates a putative transmembrane domain. I to XI indicate conserved subdomains of eukaryotic protein kinases. The black arrowhead indicates an amino acid change in uzu barley. White arrowhead indicates an amino acid change in the normal Akashinriki line.
The putative HvBRI1 polypeptide contains a signal peptide, a LRR domain including 70-amino acid island, a transmembrane domain, and a kinase domain, all conserved between BRI1 homologs. Two pairs of Cys residues are also present in HvBRI1. HvBRI1 has a Leu zipper motif-like sequence in the region corresponding to other BRI1 homologs, but it is not as typical as Arabidopsis and tomato BRI1 homologs (Fig. 5). The LRR domain consists of 22 tandem copies of LRR, and the copy number of LRR in HvBRI1 is the same as that of the rice BRI1 homolog. Rice and barley BRI1 homologs lack three copies of LRR, compared with those of Arabidopsis, tomato, and pea (the amino acid sequence of pea homolog was demonstrated by Montoya et al. [2002]).
Comparison of HvBRI1 Sequences in Uzu and Normal Lines
To determine whether uzu has mutation(s) in the HvBRI1 gene, we determined the HvBRI1 sequence in uzu barley (cv Kashima-mugi). The same size fragment was amplified by end-to-end PCR from barley cv Kashima-mugi, then sequenced. A sequence comparison showed that HvBRI1 sequences in barley cvs Misato Golden and Kashima-mugi are the same, except for a single nucleotide substitution (A-2612 to G-2612; Fig. 6). The substitution in barley cv Kashima-mugi results in the change of His (CAC) to Arg (CGC) at residue 857 in subdomain IV of the kinase domain (Fig. 6). His-857 is highly conserved between BRI1 homologs (Fig. 5). No mutant has a change at subdomain IV in currently known BRI1 mutants of Arabidopsis (Li and Chory, 1997; Noguchi et al., 1999; Friedrichsen et al., 2000), tomato (Montoya et al., 2002), and rice (Yamamuro et al., 2000).
Figure 6.
Schema of amino acid changes in HvBRI1. The black arrowhead indicates an amino acid change in uzu barley (cv Kashimamugi and uzu Akashinriki line). White arrowhead indicates an amino acid change in the normal Akashinriki line.
We used the isogenic Akashinriki line pair to determine the effect of the uzu gene on BL sensitivity and BR content and showed that the uzu line was less sensitive to BL and contained higher levels of BRs than the normal line. We then compared HvBRI1 sequences in uzu and normal Akashinriki lines. For sequence comparisons of HvBRI1, we used the sequence in barley cv Misato Golden as the standard. The HvBRI1 sequence in the uzu Akashinriki line showed a single nucleotide substitution from A (barley cv Misato Golden) to G (uzu Akashinriki line) at 2,612 (Fig. 6). The same substitution was detected in barley cv Kashima-mugi, an uzu-type cultivar. HvBRI1 sequences in barley cv Kashima-mugi and the uzu Akashinriki line showed 100% mutual identity. We also determined the genomic DNA sequence of HvBRI1 in the uzu Akashinriki line, and the cDNA and genomic DNA sequence of HvBRI1 showed no difference. A sequence comparison between barley cv Misato Golden and the normal Akashinriki line showed that the normal Akashinriki line has three nucleotide substitutions (G-277 to T-277, C-278 to T-278, and T-3,124 to G-3,124) in the coding region of HvBRI1 (Fig. 6). These substitutions change the amino acid residues at 79 (Ala to Phe) and 1,028 (Leu to Val; Fig. 6). These amino acid residues were not highly conserved between BRI1 homologs as His-857 (Fig. 5). The HvBRI1 sequence in the normal Akashinriki line has a 4-bp (3,457–3,460) and 1-bp (3,487) deletion in the 3′ non-coding region (data not shown). These sequence polymorphisms, detected between the normal Akashinriki line and other cultivars, may be derived from the different parent plants from which these sequences were isolated. However, the His residue at 857 was conserved between barley cv Misato Golden (normal cultivar) and the normal Akashinriki line, as such as the BRI1 homologs of Arabidopsis, tomato, and rice (Figs. 5 and 6). The semidwarf phenotype of uzu barley was therefore correlated with a single nucleotide substitution (A-2,612 to G-2,612), which causes an amino acid change at the highly conserved residue (His-857 to Arg-857) of the kinase domain of a putative BR receptor protein. The mutation on the HvBRI1 gene in uzu barley may cause reduced sensitivity to BRs, resulting in uzu phenotype such as reduced plant height. To confirm these results, linkage analysis is currently in progress between the HvBRI1 gene and uzu phenotype, with results to be published upon completion.
DISCUSSION
As reported previously, BL treatment clearly unrolled the first leaf segments of dark-grown barley, similar to that of wheat (Triticum aestivum). Wada et al. (1985), based on their study of other plant hormone effects in wheat leaf unrolling, reported that CK showed activity similar to that of BL, but GA, ABA, and IAA did not. They also reported that CS showed activity similar to that of BL. Synergism between BL and IAA were reported in physiological processes such as cucumber (Cucumis sativus) hypocotyl growth (Katsumi, 1985), bean (Phaseolus vulgaris) hypocotyl hook opening, adzuki bean (Vigna angularis) epicotyl growth, pea epicotyl growth (Yopp et al., 1981), and rice laminar inclination by intact plants (Fujioka et al., 1998). Wada et al. (1985), however, reported that synergism between IAA and BRs was not observed in the wheat leaf segment. Our barley results correlated well with those for wheat, indicating that leaf unrolling may be physiologically similar for wheat and barley.
Our detailed study using two independent isogenic lines of uzu gene showed that the uzu response to BL was less than normal, whereas the response to t-Z was similar. These results clearly indicate that the uzu line does not respond specifically to BL. Physiological interaction between CK and BRs has been suggested, but not clarified. Chory et al. (1994) showed that the CK-applied Arabidopsis wild strain grown in the dark has a de-etiolated phenotype similar to det2, which is a defective mutant in BR biosynthesis (Li et al., 1996; Fujioka et al., 1997). CK level in the det2 mutant is slightly higher than in the wild type (Chory et al., 1994), whereas that in the uzu line is higher than in the normal line 90 h after imbibition (Honda et al., 1996). The physiological role of BRs and CK and their interaction in leaf unrolling have yet to be clarified, but detailed study of the uzu response to BRs and CK may provide evidence.
Many Arabidopsis dwarf mutants are defective in BR biosynthesis and signaling, such as dim/dwf1 (Takahashi et al., 1995; Klahre et al., 1998; Choe et al., 1999a), det2 (Li et al., 1996, 1997; Fujioka et al., 1997), cpd (Szekeres et al., 1996), dwf4 (Azpiroz et al., 1998; Choe et al., 1998), dwf5 (Choe et al., 2000), and bri1 (Clouse et al., 1996; Li and Chory, 1997; Noguchi et al., 1999). These BR-related mutants show typical characteristics in the dark, such as a short hypocotyl, lack of an apical hook, and cotyledon expansion, regarded as de-etiolated. A recently developed BR-specific biosynthetic inhibitor, brassinazole, mimics these phenotypes in the wild-type Arabidopsis (Asami et al., 2000). BR-related mutants that do not show a de-etiolated phenotype in darkness have also been reported. A dark-grown dwf7 mutant of Arabidopsis, defective in the early step of BR biosynthesis, showed closed cotyledons and hooks similar to those of the normal (Choe et al., 1999b). A sax1 dwarf mutant of Arabidopsis, also defective in an early step of BR biosynthesis, showed an almost wild-type phenotype in darkness, except that the hypocotyl was slightly shorter (Ephritikhine et al., 1999a, 1999b). Nomura et al. (1999) reported that dwarfism of pea lka and lkb mutants was caused by BR insensitivity and deficiency, respectively, and neither mutant showed a de-etiolated phenotype in darkness. A tomato BR-deficient mutant dwarf (Bishop et al., 1999) and BR-insensitive mutant cu3 (Koka et al., 2000) showed a de-etiolated phenotype, whereas BR-deficient dumpy did not show a true de-etiolated phenotype in darkness (Koka et al., 2000). In rice, the mesocotyl and the first leaf sheath of the BR-insensitive mutant d61 (Yamamuro et al., 2000) and BR-deficient mutant brd1 (Hong et al., 2002; Mori et al., 2002) were not elongated in darkness, whereas those of wild type and GA-deficient mutants (d35 and d18) were elongated. Rice BR mutant behavior thus resembles that of de-etiolated mutants in Arabidopsis.
We compared uzu and normal phenotypes under light and dark conditions. The coleoptile and the first leaf of the uzu line were shorter than those of the normal line under either condition (Fig. 3). The reduced plant height of the dark-grown seedling of uzu may be a de-etiolated phenotype, but no other morphological difference occurred between normal and uzu lines grown in darkness, so uzu barley is not a typical de-etiolated mutant. These results indicate that the BR role in darkness may differ physiologically between plant species; even in Arabidopsis, uncertainty remains about BR-related photomorphogenesis. Symons et al. (2002) found that BR levels in light-grown pea seedlings increased compared with those grown in the dark, suggesting that BRs did not negatively regulate de-etiolation in the pea. BRs in dark- and light-grown seedlings must therefore be measured directly to clarify the BR role in barley.
We found that the BR accumulation pattern in uzu resembled that of other plant hormone-insensitive mutants such as GA-insensitive wheat Rht1 (Appleford and Lenton, 1991) and maize (Zea mays) D8 (Fujioka et al., 1988), BR-insensitive Arabidopsis bri1 (Noguchi et al., 1999) and dwarf12 (Choe et al., 2002), pea lka (Nomura et al., 1999), tomato cu3 (Montoya et al., 2002), and rice d61 (Yamamuro et al., 2000). The most bioactive BR, BL, was not detected in normal and uzu lines, and CS, the putative direct biosynthetic precursor of BL, accumulated dramatically in the uzu line. CS shows activity similar to that of BL in barley (Fig. 1B). CS may act as active BR in barley as predicted in rice where BL is not detected (Yamamuro et al., 2000; Hong et al., 2002). This chemical evidence supports the observation that uzu can be predicted to be a BR-insensitive mutant.
Arabidopsis BR-insensitive 1 (BRI1), encoding a typical plasma membrane-associated LRR receptor-like kinase, is firstly identified as a BR-receptor gene. In this study, a barley BRI1 homolog, HvBRI1, was identified as a candidate for the uzu gene. Sequence comparisons of BRI1 homologs showed that BRI1 homologs of rice and barley are three copies of LRR shorter than those of Arabidopsis, tomato (Fig. 5), and pea (Montoya et al. [2002] demonstrated the amino acid sequence). Furthermore, the Leu zipper motifs of rice and barley BRI1 homologs are not as typical as those of dicots. These differences in the copy number of LRR and the Leu zipper motif might suggest evolutionary diversity of BRI1 between monocots and dicots. Bishop and Koncz (2002) speculated that the LRR region with a putative Leu zipper motif at the N terminus may facilitate the dimerization of BRI1. By using a yeast two-hybrid screen, Arabidopsis BAK1 (BRI1-associated receptor kinase 1) has already been identified as a specific interactor for BRI1 (Li et al., 2002; Nam and Li, 2002). To determine whether barley has the same type of BR-receptor complex, we must identify the BAK1 homolog from barley. Interestingly, the tomato BRI1 homolog is hypothesized to have a dual role in the signaling of BRs and systemin (Montoya et al., 2002; Scheer and Ryan, 2002). Systemin is a peptide hormone that acts as a primary signal of wounding (Pearce et al., 1991). Further analysis is needed to clarify the HvBRI1 function in barley.
Sequence comparisons between normal and uzu barley varieties showed that uzu has a missense mutation in the HvBRI1 gene (A-2,612 to G-2,612), resulting in an amino acid change (His-857 to Arg-857) in subdomain IV of the kinase domain. A comparison of the eukaryotic protein kinase superfamily indicates that subdomain IV contains no invariant residue (Hanks and Hunter, 1995). The single nearly invariant residue is His, corresponding to His-857 of HvBRI1, found as a mutation point for uzu. Single nucleotide substitution causes an amino acid change from His to Arg, both basic amino acid residues. This missense mutation from His-857 to Arg-857 might change the kinase activity of HvBRI1, slightly reducing BR sensitivity in uzu barley. The severe dwarf mutant of tomato, cu3, containing a nonsense mutation in BRI1 homolog, reduces fertility (Koka et al., 2000). A weak allele of cu3, abs1 (cu3-abs), containing a missense mutation in the kinase domain, is more fertile than cu3 (Montoya et al., 2002). If uzu mutation causes mild phenotypic changes, such as decreased plant height without reducing fertility, it may produce high yields due to lodging resistance. Several BR-insensitive and -deficient mutants have been identified from rice, tomato, and pea, but no mutant has been introduced into commercial varieties of these plants, other than uzu.
In a BLAST search, the HvBRI1 sequence showed high identity with several barley expressed sequence tag accessions, isolated from embryos (GenBank accession no. BM37519 and AJ461510), roots (GenBank accession no. AU252398 and BM370202), leaves (GenBank accession no. BE421192), seedling shoots (GenBank accession no. BF622527), and female inflorescence (GenBank accession no. BQ660218). The variety of tissues from which these accessions are isolated suggests that HvBRI1 may be expressed in the entire plant, even though the expression level of HvBRI1 is unknown. For rice, expression of BRI1 homolog is detected in actively dividing and elongating cells, in an organ-specific manner (Yamamuro et al., 2000). Examining the spatial expression pattern of HvBRI1 will be needed to understand the role of BRs in barley. AU252398 is isolated from salt-stressed barley roots by a differential display (Ueda et al., 2002), and salt treatment increases gene expression. BM370202 is isolated from drought-stressed roots and BF622527 from cold-stressed seedlings. BRs are known to enhance resistance to various stresses, such as cold, drought, and salt (Sasse, 1999). Uzu barley is less tolerant of salt than non-uzu barley (Mano et al., 1996). The uzu gene is currently being introduced into all hull-less barley cultivated mainly in southern Japan. Barley varieties cultivated in northern Japan, which must be resistant to snow and cold, have not been given the uzu gene. Evidence from this practical breeding program indicates that uzu barley's lower resistance to stress is due to HvBRI1 gene mutation.
Semidwarf genes originating in East Asia have been used in breeding varieties of rice and wheat with high yields and resistance to lodging and were responsible for the so-called “green revolution” of the 1960s (Athwal, 1971). Rice dwarf gene semidwarf 1 (sd1) encodes an enzyme in the GA-biosynthetic pathway (Sasaki et al., 2002), and wheat dwarf gene Reduced height 1 (Rht-B1 and Rht-D1) encodes a negative regulator of GA signaling (Peng et al., 1999). Uzu barley features such as the dark leaf color, stiff culms, and erect leaves resemble those of green revolution wheat and rice. The uzu line shows a semidwarf plant type whose height is reduced to 80% to 90% of its normal counterpart under normal cultivation in Japan (Fig. 7). Such uzu characteristics are adapted to heavy manuring and dense field planting through a high harvest index and lodging resistance (Takahashi, 1964). Uzu varieties are localized only in southern Japan and on the Korean peninsula. In the 1930s, uzu varieties accounted for over 70% of all barley acreage cultivated in Japan and over 30% of Korean acreage (Takahashi and Yamamoto, 1951). The uzu gene is currently being introduced into all hull-less barley cultivated in Japan. No other major semidwarf gene has been reported for practical use in barley (Lundqvist et al., 1996). Our results suggest that BR insensitivity, caused by a nucleotide substitution in the HvBRI1 gene, and introduced into commercial barley contribute to lodging resistance and a high yield. Although green revolution varieties of wheat and rice have defects in the GA-signaling pathway and/or GA biosynthesis, no practical dwarf gene with the BR signal transduction pathway or BR biosynthesis has been reported. Our report on BR-related mutation in a practical, useful gene thus is highly important in practical agronomics.
Figure 7.

Uzu phenotype. Isogenic Akashinriki line pair was grown in the field. Left, Normal line. Right, uzu line. Bar = 1 m.
CONCLUSIONS
We examined BR sensitivity and content in uzu barley in detail, finding that uzu has the same characteristics as other BR-insensitive mutants. We isolated the barley homolog of the BR-receptor gene, HvBRI1, a candidate for the uzu gene suggested by the marker synteny of rice and barley chromosomes. We compared HvBRI1 sequences in uzu and normal barley and found that the uzu phenotype is correlated with a single nucleotide substitution, resulting in the amino acid change at a highly conserved residue of the BR-receptor protein. Reduced BR sensitivity, due to a missense mutation in the HvBRI1 gene, has been introduced into commercial varieties of barley and can contribute to lodging resistance and high yield. Further investigation of uzu barley will help to improve a better understanding of BR roles in practical agricultural fields.
MATERIALS AND METHODS
Plant Materials and Growth
Two independent isogenic lines of barley (Hordeum vulgare) with the uzu gene were used for a physiological study. One was the Bowman line containing the uzu gene (GSHO 1963) and its corresponding normal line (cv Bowman), established by Franckowiak and Pecio (1992). GSHO1963 has an uzu gene derived from Baitori 11 (old Japanese uzu barley), introduced to barley cv Bowman (two-rowed and hulled type of normal barley) by sixtime backcrossing. These lines were donated to the gene bank at the United States Department of Agriculture. The other was the Akashinriki line excluding the uzu gene (normal line) and its corresponding uzu line (barley cv Akashinriki; six-rowed and hull-less type of uzu barley), established at Okayama University. The normal Akashinriki line was produced by ninetime backcrossing. The parent plant used as a normal type counterpart of barley cv Akashinriki is unknown. The later isogenic Akashinriki pair was used for the chemical analysis of endogenous BRs. To clone the barley BRI1 homolog, we grew normal and uzu lines in the field, harvesting embryos of all lines 21 d after pollination and freezing them in liquid nitrogen. For the pictures of isogenic Akashinriki line pair, we also sewed both plant materials in the field at the end of October 2001, growing them under normal field conditions and taking pictures of them at the end of May 2002.
Leaf Unrolling Test
All experiments were conducted based on the method for wheat (Triticum aestivum; Wada et al., 1985), with simple modifications. Surface-sterilized seeds of barley were placed on wet filter paper and incubated for 1 d in the dark at 25°C. Germinated seeds were transplanted onto a seed growth pouch (Mega International, West St. Paul, MN) containing 50 mL of distilled water and incubated for 6 more d for the normal line and 7 more d for the uzu line. Both were grown in darkness at 25°C. Leaf segments of 1.5 cm long were excised from the region 1.5 to 3.0 cm from the leaf tip under a dim green safelight. Ten of these segments were incubated in 10 mL of distilled water containing an appropriate amount of plant hormones and allowed to grow for 72 h in the dark at 25°C. The unrolling of leaf segments was determined by measuring their width with calipers. In a comparative study of CS and BL response, seeds were sown directly without pre-incubation to avoid the effect of light on transplantation.
Response to BL of the Intact Plant
Surface-sterilized seeds of barley were placed on wet filter paper and incubated for 1 d in the dark at 25°C. Fifteen germinated seeds were transplanted to a seed growth pouch containing 100 mL of distilled water and the appropriate amount of BL and grown for 8 more d under continuous light (approximately 100 μmol m–2 s–1) and in the darkness at 20°C. During examination under continuous light, the side and bottom of the pouch were covered by aluminum foil to avoid exposing the root to light.
Analysis of Endogenous BRs
Surface-sterilized normal and uzu isogenic Akashinriki lines were placed over well-wetted cheesecloth placed on a plastic basket. The basket was placed into a larger container, which contained distilled water, and edges of cheesecloth were dipped in water to ensure seeds had sufficient water. Plants were grown for 2 d in darkness and then for 5 d in continuous light (125 μmol m–2 s–1) at 25°C. Seedlings excluding roots were collected, homogenized in methanol, and filtered. Determination of endogenous BR levels was performed on these extracts corresponding to 136.9 g fresh weight (2,265 plants) for the normal line and 89.4 g fresh weight (2,481 plants) for the uzu line. After 2H6-labeled BRs were added as internal standards, extracts were evaporated, and the residue was adjusted to pH 3 by diluted hydrochloric acid and then extracted by ethyl acetate. Ethyl acetate extracts were washed by a saturated aqueous sodium bicarbonate solution, dried over anhydrous sodium sulfate, and then filtered. Ethyl acetate phases were evaporated to dryness, redissolved, and subsequently partitioned with 80% (v/v) aqueous methanol and n-hexane, and then the 80% (v/v) aqueous methanol phase was evaporated to dryness. The residual solid was dissolved in chloroform and loaded onto a column of silica gel (Wako gel C300, Wako Pure Chemical, Osaka) eluted with chloroform, methanol:chloroform (5:95, 10:90, 20:80, 50:50, v/v), and then methanol. Effluents of the 5:95 and 10:90 fractions were combined, evaporated to dryness, and chromatographed on a column of Sephadex LH-20 (bed volume 400 mL, Pharmacia, Uppsala) with a solvent of methanol:chloroform (4:1, v/v) as the mobile phase, and the effluents of 50 fractions were collected (8 mL each). In purification, commercial available chloroform (Wako Pure Chemical) was used, containing about 1% (v/v) methanol as a stabilizer. Biological activity of fractions was examined using a rice (Oryza sativa) lamina inclination assay (Yokota et al., 1996), and bioactive fractions from 32 to 40 were combined and evaporated to dryness. The residual solid was dissolved in methanol, passed through Bond Elut C18 and DEA (Varian, Palo Alto, CA) cartridges, and evaporated to dryness. The residue was separated by HPLC of Senshu Pak ODS-3251-D (8-mm-i.d. × 250-mm column, Senshu Scientific, Tokyo) and eluted for 20 min by 45% (v/v) aqueous acetonitrile, then by a linear gradient to 100% (v/v) acetonitrile for 20 min, and then for 10 min by 100% (v/v) acetonitrile. The elution was run at a flow rate of 2.5 mL min–1, the column was kept at 40°C, and fractions were collected every minute. After the bioassay, putative BR fractions were converted to bismethaneboronate, bismethaneboronate-trimethylsilyl ether, or trimethylsilyl ether. GC/MS and GC/SIM analyses were carried out by a mass spectrometer (JMS-GCmateII, JEOL, Tokyo) with a gas chromatograph (6890A, Agilent Technology, Palo Alto, CA) fitted with a capillary column (DB-1, 0.25 mm i.d.×15 m, 0.25 μm film thickness, J&W Scientific, Folsom, CA). The carrier gas was helium at a flow rate of 1 mL min–1, the injection port temperature was 260°C, and samples were introduced by splitless injection. The column oven temperature was programmed at 170°C for 1.5 min, before being elevated to 280°C at 37°C min–1, then 300°C at 1.5°C min–1. In GC/MS identification, mass spectra and retention time on GC of putative BRs and 2H6-BRs were compared with those of authentic samples. The content of identified BRs was calculated from peak area ratios between 2H0 and 2H6 molecular ions of each BRs examined by GC/SIM.
Molecular Cloning
Total RNA was isolated from immature embryos of barley (cv Misato Golden) using hexadecyl-trimethylammonium bromide method (Chang et al., 1993). Poly(A+) RNA purified from total RNA (using Dynabeads Olygo (dT)25 resin, Dynal Biotech, Oslo) was subjected to cDNA synthesis (Marathon cDNA amplification kit, BD Biosciences Clontech, Palo Alto, CA). PCR was performed with degenerated primers designed from KCT(K/N) LNWI and AAFEKPL using cDNA as a template, and nested PCR was performed with primers designed from KCT(K/N) LNWI and AMGLLFS (Fig. 5). The nested PCR fragment was cloned into a pGEM-T easy vector (Promega, Madison, WI). DNA sequencing was done using a Big Dye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI PRISM 3100Avant Genetic Analyzer (Applied Biosystems). Sequence analysis was performed using DNASIS pro software (Hitachi Software Engineering Co., Tokyo). RACE reactions were done using a Marathon cDNA amplification kit and Advantage cDNA PCR kit (BD Biosciences Clontech) following manufacturers' instructions, using gene-specific sense (5′-TGGCAGAGCAGTCGGGGAAGATGA-3′) and antisense (5′-TCATCTTCCCCGACTGCTCTGCCA-3′, 5′-TTGAGGGCGGTGAGCGAGGTGAG-3′ and 5′-TCTCCGGCGATCTTGTTGCTGGAC-3′) primers. Amplification products were cloned into a pGEM-T easy vector and sequenced. End-to-end PCR was done to obtain the full-length cDNA of barley cv Misato Golden by using 5′ and 3′ end primers (5′-CGCTTCTCGCATGGTCTCAAGGTAG-3′ and 5′-AGCTTGCGTGGGAACCTCAGAATG-3′) and high-fidelity DNA polymerase (Pyrobest DNA polymerase, Takara Shuzo, Kyoto). The amplified PCR fragment was cloned into a pCR 4 Blunt-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced. For the sequence comparison between uzu and normal lines of barley, end-to-end PCR was done using cDNA derived from the embryo of each line, and the amplified PCR product was cloned and sequenced. We determined DNA sequences from at least five independent clones, thus avoiding potential PCR-induced error.
Acknowledgments
We thank Prof. Nobutaka Takahashi, Prof. Hisakazu Yamane, and Prof. Yasutomo Takeuchi for their helpful comments and ongoing support; Naoyuki Kawada and Megumi Yoshida for providing the barley cv Kashima-mugi seeds; Sachiko Sasaki for technical assistance; members of the field cultivation team at National Institute of Crop Science for cultivation of experimental material; Prof. Takao Yokota, Dr. Takahito Nomura, and Dr. Yasuo Kamuro for their invaluable advice; and Tama Biochemical Co., Ltd., for providing BL and CS standards. We also thank the students at Utsunomiya University who contributed to earlier stages of this work.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026195.
References
- Altmann T (1999) Molecular physiology of brassinosteroids revealed by the analysis of mutants. Planta 208: 1–11 [DOI] [PubMed] [Google Scholar]
- Appleford NEJ, Lenton JR (1991) Gibberellins and leaf expansion in near-isogenic wheat lines containing Rht1 and Rht3 dwarfing alleles. Planta 183: 229–236 [DOI] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S (2000) Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol 123: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal DS (1971) Semidwarf rice and wheat in global food needs. Q Rev Biol 46: 1–34 [DOI] [PubMed] [Google Scholar]
- Azpiroz R, Wu YW, LoCascio JC, Feldmann KA (1998) An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell 10: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. Plant Cell Suppl 14: S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y (1999) The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA 96: 1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T (2001) Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42: 114–120 [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA (1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22 alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S et al. (1999a) The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol 119: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE et al. (1999b) The Arabidopsis dwf7/ste1 mutant is defective in the Δ7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell 11: 207–221 [PMC free article] [PubMed] [Google Scholar]
- Choe S, Schmitz RJ, Fujioka S, Takatsuto S, Lee M-O, Yoshida S, Feldman KA, Tax FE (2002) Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3β-like kinase. Plant Physiol 130: 1506–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Tanaka A, Noguchi T, Fujioka S, Takatsuto S, Ross AS, Tax FE, Yoshida S, Feldman KA (2000) Lesions in the sterol Δ7 reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J 21: 431–443 [DOI] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA (1991) Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3: 445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M (1994) A role for cytokinins in de-etiolation in Arabidopsis. Plant Physiol 104: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111: 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephritikhine G, Fellner M, Vannini C, Lapous D, Barbier-Brygoo H (1999a) The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J 18: 303–314 [DOI] [PubMed] [Google Scholar]
- Ephritikhine G, Pagant S, Fujioka S, Takatsuto S, Lapous D, Caboche M, Kendrick RE, Barbier-Brygoo H (1999b) The sax1 mutation defines a new locus involved in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J 18: 315–320 [DOI] [PubMed] [Google Scholar]
- Franckowiak JD, Pecio A (1992) Coordinator's report. Semidwarf genes: a listing of genetic stocks. Barley Genet Newslett 21: 116–127 [Google Scholar]
- Friedrichsen DM, Joazeiro CAP, Li J, Hunter T, Chory J (2000) Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol 123: 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Li J, Choi Y-H, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J et al. (1997) The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell 9: 1951–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Takatsuto S, Yoshida S (1998) Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry 49: 1841–1848 [Google Scholar]
- Fujioka S, Sakurai A (1997) Biosynthesis and metabolism of brassinosteroids. Physiol Plant 100: 710–715 [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Katsumi M, Phinney BO, Gaskin P, MacMillan J, Takahashi N (1988) The dominant non-gibberellin-responding dwarf mutant (D8) of maize accumulates native gibberellins. Proc Natl Acad Sci USA 85: 9031–9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Carter Cook J (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281: 216–217 [Google Scholar]
- Hanks SK, Hunter T (1995) The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596 [PubMed] [Google Scholar]
- Harushima Y, Yano M, Shomura A, Sato M, Shimano T, Kuboki Y, Yamamoto T, Lin SY, Antonio BA, Parco A et al. (1998) A high-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics 148: 479–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang Z-Y, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- Honda I, Yagoshi T, Saito M, Takeuchi Y, Yanagisawa T, Yamane H, Murofushi N, Takahashi N (1996) Dwarfism of dwarf barley “uzu”. In K Noda, DJ Mares, eds, Pre-Harvest Sprouting in Cereals 1995. Center for Academic Societies Japan, Osaka, Osaka, pp 477–482
- Honda I, Zeniya H, Yoneyama K, Chono M, Kaneko S, Watanabe Y (2003) Uzu mutation in barley (Hordeum vulgare L.) reduces the leaf unrolling response to brassinolide. Biosci Biotechnol Biochem 67: 1194–1197 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y et al. (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32: 495–508 [DOI] [PubMed] [Google Scholar]
- Inouhe M, Sakurai N, Kuraishi S (1982) Growth regulation of dark-grown dwarf barley coleoptile by the endogenous IAA content. Plant Cell Physiol 23: 689–698 [Google Scholar]
- Kamuro Y, Takatsuto S (1999) Practical application of brassinosteroids in agricultural fields. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids. Springer-Verlag, Tokyo, pp 223–241
- Katsumi M (1985) Interaction of a brassinosteroid with IAA and GA3 in the elongation of cucumber hypocotyl sections. Plant Cell Physiol 26: 615–625 [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua N-H (1998) The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD (2000) A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol 122: 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi S (1974) Biogenesis of auxin in the coleoptile of a semi-brachytic barley, uzu. Plant Cell Physiol 15: 295–306 [Google Scholar]
- Li J, Biswas MG, Chao A, Russell DW, Chory J (1997) Conservation of function between mammalian and plant steroid 5α-reductases. Proc Natl Acad Sci USA 94: 3554–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Li J, Lease KA, Tax FE, Walker JC (2001a) BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 98: 5916–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295: 1299–1301 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J (2001b) BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol 127: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Lundqvist U, Franckowiak JD, Konishi T (1996) New and revised descriptions of barley genes. Barley Genet Newslett 26: 22–516 [Google Scholar]
- Mano Y, Nakazumi H, Takeda K (1996) Varietal variation in and effects of some major genes on salt tolerance at the germination stage in barley. Breed Sci 46: 227–233 [Google Scholar]
- Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ (2002) Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 14: 3163–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Nomura T, Ooka H, Ishizaka M, Yokota T, Augimoto K, Okabe K, Kajiwara H, Satoh K, Yamamoto K et al. (2002) Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol 130: 1152–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE (1999) Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121: 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T (1999) Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol 119: 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol 113: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA (1991) Production of multiple plant hormones from a single polyprotein precursor. Science 253: 895–898 [DOI] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F et al. (1999) “Green revolution” genes encode mutant gibberellin response modulators. Nature 400: 256–261 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS et al. (2002) A mutant gibberellin-synthesis gene in rice. Nature 416: 701–702 [DOI] [PubMed] [Google Scholar]
- Sasse J (1999) Physiological actions of brassinosteroids. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroid Plant Hormones. Springer-Verlag Tokyo, pp 137–161
- Scheer JM, Ryan CA (2002) The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc Natl Acad Sci USA 99: 9585–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilde WD, Haluškova J, Sasaki T, Graner A (2001) New evidence for the synteny of rice chromosome 1 and barley chromosome 3H from rice expressed sequence tags. Genome 44: 361–367 [PubMed] [Google Scholar]
- Suge H (1972) Effect of uzu (uz) gene on the level of endogenous gibberellins in barley. Jpn J Genet 47: 423–430 [Google Scholar]
- Symons GM, Schultz L, Kerckhoffs LHJ, Davies NW, Gregory D, Reid JB (2002) Uncoupling brassinosteroid levels and de-etiolation in pea. Physiol Plant 115: 311–319 [DOI] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Takahashi R (1964) Genetic studies on geographical distribution of barley varieties with special reference to uzu or semi-brachytic forms. Ber Ohara Inst Iandw Biol Okayama Univ 12: 217–220 [Google Scholar]
- Takahashi R, Yamamoto J (1951) Studies on the classification and geographical distribution of the Japanese barley varieties III: on the linkage relation and the origin of the “uzu” or semi-brachytic character in barley. Ber Ohara Inst Landw Forsch 9: 399–410 [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua NH (1995) The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Gene Dev 9: 97–107 [DOI] [PubMed] [Google Scholar]
- Ueda A, Shi W, Nakamura T, Takabe T (2002) Analysis of salt-inducible genes in barley roots by differential display. J Plant Res 115: 119–130 [DOI] [PubMed] [Google Scholar]
- Wada K, Kondo H, Marumo S (1985) A simple bioassay for brassinosteroids: a wheat leaf-unrolling test. Agric Biol Chem 49: 2249–2251 [Google Scholar]
- Wang Z-Y, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2: 505–513 [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383 [DOI] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cheong H, Friedrichsen D, Zhao Y, Hu J, Mora-Garcia S, Chory J (2002a) A crucial role for the putative Arabidopsis topoisomerase VI in plant growth and development. Proc Natl Acad Sci USA 99: 10191–10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang Z-Y, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J (2002b) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191 [DOI] [PubMed] [Google Scholar]
- Yokota T (1997) The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 2: 137–143 [Google Scholar]
- Yokota T (1999) The history of brassinosteroids: discovery to isolation of biosynthesis and signal transduction mutants. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids. Springer-Verlag, Tokyo, pp 1–20
- Yokota T, Matsuoka T, Koarai T, Nakayama M (1996) 2-Deoxybrassinolide, a brassinosteroid from Pisum sativum seed. Phytochemistry 42: 509–511 [Google Scholar]
- Yopp JH, Mandava NB, Sasse JM (1981) Brassinolide, a growth-promoting steroidal lactone: I. Activity in selected auxin bioassays. Physiol Plant 53: 445–452 [Google Scholar]