Abstract
A prominent feature of striated muscle is the regular lateral alignment of adjacent sarcomeres. An important intermyofibrillar linking protein is the intermediate filament protein desmin, and based on biochemical and structural studies in primary cultures of myocytes it has been proposed that desmin interacts with the sarcomeric protein nebulin. Here we tested whether nebulin is part of a novel biomechanical linker complex, by using a recently developed nebulin knockout (KO) mouse model and measuring Z-disk displacement in adjacent myofibrils of both extensor digitorum longus (EDL) and soleus muscle. Z-disk displacement increased as sarcomere length (SL) was increased and the increase was significantly larger in KO fibers than in wild-type (WT) fibers; results in 3-day-old and 10-day-old mice were similar. Immunoelectron microscopy revealed reduced levels of desmin in intermyofibrillar spaces adjacent to Z-disks in KO fibers compared with WT fibers. We also performed siRNA knockdown of nebulin and expressed modules within the Z-disk portion of nebulin (M160-M170) in quail myotubes and found that this prevented the mature Z-disk localization of desmin filaments. Combined, these data suggest a model in which desmin attaches to the Z-disk through an interaction with nebulin. Finally, because nebulin has been proposed to play a role in specifying Z-disk width, we also measured Z-disk width in nebulin KO mice. Results show that most Z-disks of KO mice were modestly increased in width (~80 nm in soleus and ~40 nm in EDL fibers) whereas a small subset had severely increased widths (up to ~1 μm) and resembled nemaline rod bodies. In summary, structural studies on a nebulin KO mouse show that in the absence of nebulin, Z-disks are significantly wider and that myofibrils are misaligned. Thus the functional roles of nebulin extend beyond thin filament length regulation and include roles in maintaining physiological Z-disk widths and myofibrillar connectivity.
Keywords: Muscle, Cytoskeleton, Contractility, Sarcomeric structure, Nemaline myopathy
Introduction
The sarcomere is the basic contractile unit of striated muscle, and is composed of highly ordered thin (actin) filaments and thick (myosin) filaments (Squire, 1997). The cytoskeleton surrounding this elegant contractile molecular machinery is required as a scaffold to ensure efficiency of contraction. Longitudinally, sarcomeres are connected by Z-disk lattices that anchor thin filaments and transmit force along the myofibril. The strikingly regular lateral alignment of Z-disks between adjacent myofibrils ensures that during contraction myofibrils change length in unison, thus preventing damage to membrane systems that span between myofibrils and that are responsible for activating and relaxing the sarcomeres (T-tubules, sarcoplasmic reticulum). The mechanisms and proteins responsible for maintaining lateral myofibrillar alignment are incompletely understood. An important intermyofibrillar linking protein is the intermediate filament protein desmin, but the mechanisms that regulate its attachment to the myofibril itself have not been established (Capetanaki et al., 2007; Costa et al., 2004; Wang and Ramirez-Mitchell, 1983). Here we tested whether nebulin is part of the mechanical linkage by using a recently developed nebulin knockout (KO) mouse model (Witt et al., 2006). In the absence of nebulin, Z-disks assemble (Bang et al., 2006; Witt et al., 2006) and, thus, the nebulin KO makes it possible to test the role of nebulin in Z-disk alignment. Although misalignment has been noted in previous work on a nebulin KO (Bang et al., 2006), it has not been studied in detail. These studies were motivated by in vitro studies that suggested that the Z-disk portion of nebulin interacts with desmin (Bang et al., 2002) and more recently, nebulin modules M160-164 were reported to be involved in this interaction (Conover et al., 2009).
Nebulin is a giant filamentous protein that plays a role in controlling the thin filament length in skeletal muscle (Bang et al., 2006; Castillo et al., 2009; Littlefield and Fowler, 2008; McElhinny et al., 2003; Witt et al., 2006). The N-terminal region of nebulin is located near the pointed end of the thin filament, and the C-terminal region (~50 kDa) is anchored in the Z-disk and contains multiple phosphorylation motifs involved in signaling events (McElhinny et al., 2003). Research into the functions of nebulin has been stimulated by the identification of mutations in the nebulin gene (including in its Z-disk region) that cause nemaline myopathy (NM) in humans. NM is a member of a class of muscle disorders that phenotypically have in common a severe muscle weakness and structural abnormalities of Z-disks (Gurgel-Giannetti et al., 2001; Pelin et al., 1999).
Yeast two-hybrid screens have shown that the C-terminus of nebulin interacts with desmin (Bang et al., 2002), and that the nebulin modules M160-164 are involved in this interaction (Conover et al., 2009). The functional significance of this requires further study. In the present study we investigated whether nebulin-desmin interaction is important for myofibrillar connectivity, by measuring longitudinal Z-disk displacement between adjacent myofibrils in muscle fibers from wild-type and nebulin knockout mice. We also performed immunoelectron microscopy with anti-desmin antibodies, studied the expression level of desmin, and expressed the C-terminal nebulin repeats M160-M170 in myotubes and studied the effect on desmin localization. Finally, because nebulin has been suggested to play a role in specifying Z-disk width (Millevoi et al., 1998), a role that is likely to be independent of the role of nebulin in laterally linking Z-disks, we also measured Z-disk width and determined the expression level of α-actinin, a major Z-disk actin-linking protein (Sjoblom et al., 2008). Our findings suggest that nebulin plays a role in specifying Z-disk width and in lateral registration of Z-disks in adjacent myofibrils.
Results
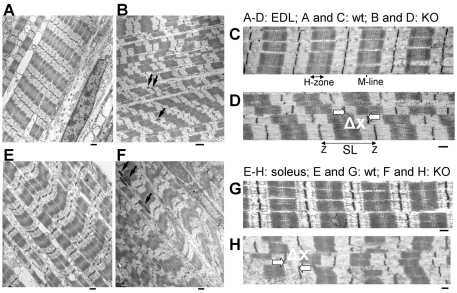
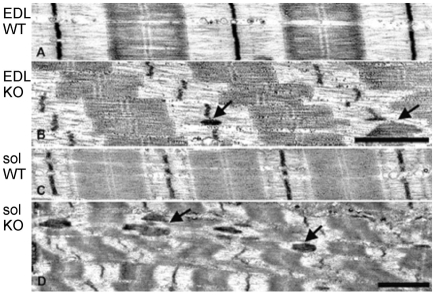
The role of nebulin in myofibrillar connectivity was studied using a nebulin KO mouse model (for details, see Witt et al., 2006). We focused on EDL and soleus muscles because they contain predominately fast and a large number of slow twitch fibers (Danieli-Betto et al., 2005), respectively, allowing us to determine whether there are fiber-type-specific changes in myofibrillar connectivity in the nebulin KO mouse. The ultrastructural sarcomeric organization of WT and nebulin KO muscle is shown in Fig. 1. WT myofibrils of both EDL (Fig. 1A,C) and soleus (Fig. 1E,G) muscle had well-aligned sarcomeres (minimal Z-disk displacement in adjacent myofibrils), uniform Z-disk width and an A-band with a visible H-zone (light zone devoid of thin filaments) in the center of the sarcomere. By contrast, a number of abnormalities were observed in the KO fibers. As previously reported for the tibialis cranialis muscle (Witt et al., 2006), sarcomeres of both EDL (Fig. 1B,D) and soleus KO fibers (Fig. 1F,H) did not have H-zones and their Z-disk widths were sometimes irregular with the presence of nemaline rod bodies (rod-shaped inclusion body consisting of Z-disk material, see also below). A difference that we noted, especially in stretched fibers, was that Z-disks of adjacent myofibrils were not well in register but instead were longitudinally displaced. Thus, in addition to the absence of H-zones, which is due to variable thin filament lengths in nebulin KO mice (Witt et al., 2006), Z-disk abnormalities were present as well, including Z-disk misalignment.
Fig. 1.
Sarcomeric structure in EDL and soleus skinned skeletal muscle fibers of WT and nebulin KO mice. (A-D) EDL muscle; (E-H) soleus muscle. WT fibers have a regular structure with well-aligned sarcomeres. In KO fibers, sarcomeres are misaligned and the Z-disk structure is non-uniform, with nemaline bodies (arrows). (D,H) Measurement of longitudinal Z-disk displacement (ΔX, defined as longitudinal displacement of nearby Z-disk in adjacent myofibrils) in skeletal muscles. Note large Z-disk displacement in both EDL and soleus KO fibers. Scale bars: 0.5 μm (A,B,E,F); 2 μm (C,D,G,H). (Each result is from a different mouse. Fibers were stretched 80%.)
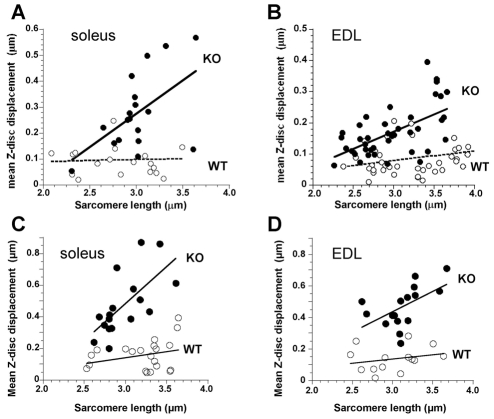
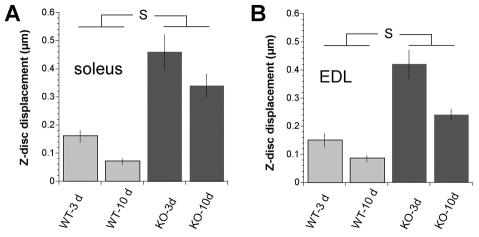
Z-disk misalignment is further highlighted in the sample micrographs shown in Fig. 1D,H. This figure also shows how longitudinal Z-disk displacement (ΔX) of adjacent myofibrils was quantified. We stretched EDL and soleus muscle fibers from nebulin KO and WT mice to different sarcomere lengths and measured ΔX. Results are shown in Fig. 2A,B. We used linear regression analysis to determine the relationship between Z-disk displacement and sarcomere length (SL) and compared the slopes by analysis of covariance. Findings in WT fibers indicate that Z-disk displacement is relatively small at SLs of 2.0-2.5 μm, and that displacement increases slightly as sarcomeres are stretched, with similar findings in soleus and EDL fibers. However, slopes were not significantly different from zero. The increase with SL was significantly steeper in KO fibers than in WT fibers [EDL slopes: 0.03 and 0.11 (P=0.002); soleus slopes 0.01 and 0.26 (P=0.003)]. For soleus and EDL KO fibers the slopes were not significantly different from each other, indicating that no major differences exist between myofibrillar connectivity of these muscle types. To address the concern that differences between WT and KO muscles might be due to muscle degeneration, we also studied muscle from WT and KO mice that were only 3 days old. Supplementary material Fig. S1 shows typical electron micrographs, and analyzed results are shown in Fig. 2C,D. Results are similar to those of the 10-day old mice: displacement increased significantly more steeply with SL (P=0.005 in soleus and P=0.01 in EDL) in KO fibers than in WT fibers. We determined (Fig. 3) the mean Z-disk displacement at SLs >2.9 μm (to make our findings comparable to those obtained on desmin KO fibers [see Discussion by Shah et al. (Shah et al. 2002)]. In 10-day-old WT mice the mean displacement of EDL fibers was 0.08±0.05 μm (n=25) and of soleus fibers, 0.07±0.03 μm (n=8). For age-matched KO fibers the values were 0.21±0.09 μm (n=23) and 0.31±0.03 μm (n=14) for EDL and soleus fibers, respectively. The values obtained in 3-day-old mice are also shown in Fig. 3 (gray bars). The mean displacements were again significantly larger in the KO muscles. The effect of postnatal day and genotype was assessed by ANOVA with Tukey's HSD post-hoc comparisons. There were statistically significant differences between all KO and WT group but not within any of the WT and KO groups.
Fig. 2.
Z-disk displacement in relation to SL of EDL and soleus muscle from nebulin KO and WT muscles. (A,C) EDL muscle; (B,D) soleus muscle. 10-day-old mice were used for A and B and 3-day-old mice for C and D. Results from KO fibers (black circles) are significantly different from WT fibers (white circles) (P<0.001). Each value represents the mean of ~50 measurements made on a single electron micrograph; ~20 micrographs were obtained from randomly selected fibers from five different mice.
Fig. 3.
The mean Z-disk displacement at SLs >2.9 μm. (A) Soleus muscle, (B) EDL muscle. Values are means ± s.d. for 3-day-old and 10-day-old mice. ANOVA with Tukey's HSD post-hoc tests show statistical significance (S) between all KO and WT groups but not within any of the WT and KO groups, with identical results for 3-day-old and 10-day-old mice. No significant differences exist between EDL and soleus fibers. Results are from ~50 measurements made on a single electron micrograph with ~20 micrographs obtained from randomly selected fibers from five different mice.
Thus, in our data, Z-disks in EDL and soleus fibers from nebulin KO mice were, at SLs >2.9 μm, approximately three- to four-fold more displaced than in their WT littermates, and this was true for both age groups. Misalignment was not more severe in the 10-day-old than in 3-day-old mice suggesting that the myofibrillar misalignment is not due to a progressive pathology. Taken together these results demonstrate that nebulin is involved in the lateral registration of both fast and slow skeletal muscle myofibrils.
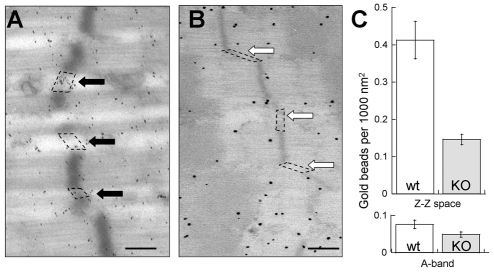
Because it is known that the intermediate filament protein desmin is involved in laterally linking myofibrils (Shah et al., 2004; Shah et al., 2002), we performed immunoelectron microscopy with polyclonal anti-desmin antibodies and gold-conjugated secondary antibodies and studied the localization of desmin in WT and nebulin KO fibers. EDL fibers from WT mice showed an accumulation of gold particles around the Z-disks (Fig. 4A), as well as near the myonuclei (results not shown). In muscle fibers from nebulin KO mice the intermyofibrilar spaces at the level of Z-disks had fewer gold particles (Fig. 4B), whereas near nuclei and costameres enhanced staining was often observed. We quantified the number of gold particles in the Z-disk to Z-disk space (using ImageJ analysis software; Fig. 3C top). The number of gold particle per unit area was significantly reduced in the nebulin KO mice (t-test, P<0.05). Immunogold labeling was also observed throughout the sarcomere, suggesting non-specific labeling. To characterize non-specific labeling, measurements were made in randomly selected areas of the A-band region of the sarcomere. This revealed no difference between WT and KO mice (Fig. 4C, bottom). To determine whether desmin was mislocalized in nebulin KO muscle or, alternatively, had a much reduced expression level, we performed western blot experiments. Analysis revealed an increased desmin expression level in nebulin KO fibers when compared with WT (supplementary material Fig. S1A). Thus, reduced desmin labeling at the Z-disks of KO fibers is not due to a reduced expression level of desmin.
Fig. 4.
Immunoelectron microscopy of EDL muscle. (A) WT muscle had gold particles (indicating the presence of desmin) near the Z-disks (black arrowheads), whereas the nebulin KO (B) muscle was largely devoid of gold particles (white arrowheads) in the Z-disk regions between myofibrils. Bar, 500 nm. (C) Particle counts in the Z-disk to Z-disk space (intermyofibrillar area enclosed by drawing lines from edge to edge of adjacent Z-disks; see examples on micrograph). Results are from three WT and three KO muscle (from three WT and three KO mice) with 15 micrographs per muscle and approx. five Z-disk regions per micrograph. We also counted particles in randomly selected areas in the A-band region of the sarcomere. Significant differences exist between WT and KO fibers for Z-disk to Z-disk measurements only. Note that no differences were found in the size of the gold particles: 14.1±3.6 nm (WT) and 16.8±4.7 nm (KO).
To further study whether nebulin is involved in anchoring desmin to the Z-disk region of the sarcomere we performed cell culture experiments. Our goal was to do this work on cultured mouse fibers (from WT and KO mice) but we were unable to successfully culture these cells and instead performed experiments on quail myoblasts that turned out to be well suited for this work. In order to better understand the assembly of desmin during the differentiation of quail skeletal myotubes in culture, cells were fixed and stained for desmin and α-actinin at various timepoints from 17 hours to 8 days after plating. Based on the staining for the well-characterized Z-disk component, α-actinin (e.g. Rhee et al., 1994; Rudy et al., 2001) and the morphological characteristics of the cells, distinct stages of assembly were identified (supplementary material Fig. S2). At early stages of assembly (~17 hours), both α-actinin and desmin were punctate in appearance. Later, (1-2 days) the dots of α-actinin staining had organized into linear arrays, whereas desmin had formed a peri-sarcomeric mesh-like network that was especially evident around the nuclei. At intermediate stages of assembly (3-4 days) α-actinin was assembled into discrete bands, indicating the maturation of the Z-disk, whereas desmin remained as a network. Finally, in mature stages of assembly (4-8 days) desmin also localized to the Z-disk in distinct striations.
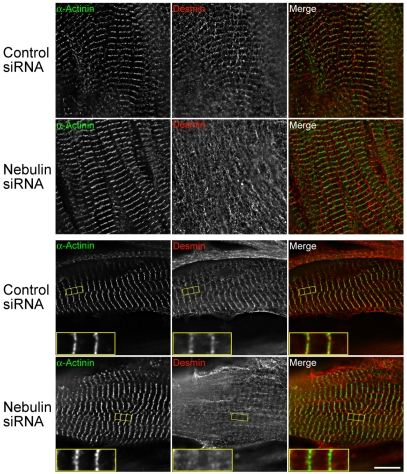
To ascertain the role of nebulin in the assembly of desmin, small interfering RNA (siRNA) was used to reduce the levels of nebulin in quail skeletal myotubes (supplementary material Fig. S3). Quail myotubes were then co-stained with anti-desmin and anti-α-actinin antibodies 4-8 days after siRNA treatment. The reduction of nebulin levels resulted in a marked perturbation of desmin assembly at the Z-disk. The majority of cells lacked any observable desmin Z-disk assembly (Fig. 5, top two rows), whereas in the cells that did have discernible localization of desmin at the Z-disk, the staining was often broader and less well defined in comparison with the controls (Fig. 5, bottom two rows, inset). Note that the lack of desmin staining at the Z-disk is unlikely to be due to a delay in the development of the myotubes, as the phenotype persisted for days following the organization of α-actinin into its mature pattern of assembly.
Fig. 5.
Knockdown of nebulin in primary cultures of quail myotubes perturbs the association of desmin at the Z-disk. Myotubes were double stained with antibodies generated against α-actinin, as a marker of the Z-disk, and desmin, 4-8 days after siRNA treatment. Treatment with nebulin-specific siRNA results in a significant decrease in assembled nebulin (see supplementary material Fig. S3) with a concomitant decrease in assembled Z-disk-associated desmin. In the siRNA-treated cells, desmin staining at the Z-disk was either undetectable (top two rows) or if detectable, was significantly broader and less intense, than in the controls (insets, bottom two rows). The distribution of α-actinin was not disrupted in the identical myofibrils. Scale bar: 10 μm. (Top two rows: example of the most typical cell type in which desmin Z-disk assembly was absent; bottom two rows: example of less typical cell type in which broad and faint Z-disk labeling of desmin was seen.)
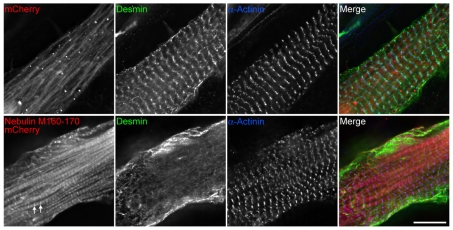
Desmin assembly at the Z-disk was also reduced following the expression of nebulin C-terminal modules M160-170, which contain binding sites for desmin (Bang et al., 2002). Nebulin M160-170 fused to mCherry localized to the Z-disk and along the periphery of the myofibrils, whereas mCherry alone associated with the myofibrils but lacked the distinctive Z-disk distribution (Fig. 6). A significant decrease in the Z-disk localization of desmin was observed in myocytes expressing nebulin M160-170, in comparison to cells expressing mCherry alone (Fig. 6, desmin panels). In three independent experiments we counted the number of cells that were devoid of distinct Z-disk desmin labeling (as in Fig. 6, bottom row). Of all cells transfected with mCherry alone 19.0±1.1% of the cells had diffuse and non-striated desmin labeling; this value was significantly (P<0.001) increased in cultures transfected with nebulin M160-170 fused to mCherry. Thus, both knockdown of nebulin with siRNA and overexpression of M160-M170 did not interfere with the formation of normal striation patterns of α-actinin but did prevent the mature Z-disk localization of desmin.
Fig. 6.
Introduction of a recombinant nebulin fragment (M160-170), which contains the desmin binding site, perturbs the Z-disk distribution of desmin in primary cultures of quail myotubes. Myotubes were double stained for desmin and α-actinin, 6 days after transfection of mCherry alone or nebulin M160-170 fused to mCherry. Scale bar: 10 μm.
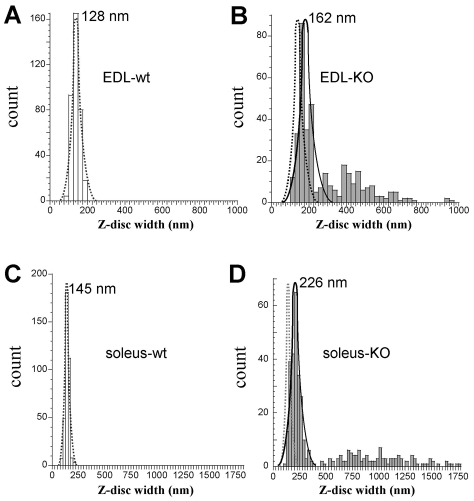
Our studies also provided an opportunity to study the Z-disk structure. Previous work has suggested that nebulin plays a role in specifying Z-disk width (Millevoi et al., 1998) and the Z-disk width is thus expected to be dysregulated in nebulin-deficient muscle. (Note that the role of nebulin in Z-disk width specification is likely to be independent of its role in anchoring desmin.) As noted above, the Z-disk morphology of nebulin KO fibers was also altered, and this phenomenon is further highlighted in Fig. 7. Some Z-disks were unusually wide with an irregular or rounded shape (Fig. 7B,D), similar to the typical structure of nemaline rod bodies (Gurgel-Giannetti et al., 2001). These nemaline rod bodies were found throughout the fibers but had a tendency to be more prominent in areas close to the myonuclei. Consistent with the work of Wallgren-Pettersson and colleagues (Wallgren-Pettersson et al., 1995) we were unable to detect immunogold particles in the IEM experiments described above, indicating that the presence of desmin in rod bodies is unlikely. Previous work by others has shown that the rods contain α-actinin (Wallgren-Pettersson et al., 1995) and we consider it probably that this is the case in the nebulin KO as well, consistent with the increased levels of α-actinin that we found in our western blot experiments (see below). In other cases, the Z-disk exhibited interruptions and a zigzag shape that appeared to be more pronounced at long sarcomere lengths, whereas in severely damaged myofibrils a disintegration of the Z-disk was also observed (which was independent of stretch). Histograms of Z-disk widths are shown in Fig. 8. The average width of the Z-disk in EDL muscle from WT mice was 127±21 nm (range 96-175 nm; Fig. 8A) and in nebulin KO muscle 271±165 nm (range 100-950 nm; Fig. 8B). The average width was significantly larger in soleus muscle from WT mice: 145±20 nm (range 83-200 nm; Fig. 8C), and nebulin KO mice: 465±422 nm (range 100-2075 nm; Fig. 8D). Consistent with wider Z-disks, we found that α-actinin, a major constituent of Z-disks, was upregulated several-fold in KO fibers (supplementary material Fig. S4).
Fig. 7.
Representative electron micrographs of EDL (A,B) and soleus (C,D) muscle from WT (A,C) and nebulin KO 10-day old mice (B,D). In nebulin KO fibers (B and D) wide and irregular Z-disk inclusions that resembled nemaline bodies (arrows) were commonly present. Scale bars: 2 μm (A,B), and 1 μm (C,D). Fibers were stretched 80% in A and D and 60% in B and C.
Fig. 8.
Analysis of Z-disk widths. Histograms of Z-disk widths in EDL (A,B) and soleus (C,D) fibers. Primary peaks were fit by Gaussians. Gaussian peak fit of WT fibers (broken lines) is superimposed on results of KO fibers. Results are from 82 micrographs from five muscles from five different mice. See text for details.
Discussion
Linkage of myofibrils allows for lateral force transmission and limits the degree to which adjacent myofibrils translocate relative to each other during active contraction or passive stretch, thereby preventing damage to intermyofibrillar membrane systems. An important protein involved in linking adjacent Z-disks is the intermediate filament protein desmin which forms a network of filaments that surrounds myofibrils at the level of the Z-disk (Capetanaki et al., 2007; Costa et al., 2004; Lazarides and Granger, 1978; Wang and Ramirez-Mitchell, 1983). The subunit proteins of desmin filaments are elongated coiled-coils with extensive intermolecular ionic and hydrophobic interactions between individual subunits, giving rise to filaments with high tensile strength as well as plasticity (Costa et al., 2004). That desmin tethers adjacent Z-disks is supported by work on a desmin KO mouse in which Z-disk misalignment was shown to occur in stretched muscle (Shah et al., 2002). Previous in vitro work suggested that desmin binds to the sarcomeric protein nebulin (Bang et al., 2002), and in the present study using a nebulin KO mouse model we examined this possibility. We found that myofibrils translocate in muscle devoid of nebulin to a much higher degree than in WT muscle (for definition of translocation, ΔX, see Fig. 1D,H; for results, see Fig. 2). Our work also shows that desmin is present in muscle devoid of nebulin but that it is reduced in the intermyofibrillar spaces that surround the Z-disks (Fig. 4C, top). Consistent with this, we found that both knockdown of nebulin with siRNA and overexpression of M160-M170 did not interfere with the formation of normal striation patterns but did prevent desmin localization at the mature Z-disk (Figs 5 and 6). It is unclear why two distinct phenotypes were observed; that is, lack of detectable Z-disk staining and broader and less defined Z-disk staining. We predict that the broad Z-disk staining might reflect displaced Z-disk desmin. Combined, our knockdown and overexpression studies suggest a model in which desmin attaches to the Z-disk through an interaction with nebulin. This model is consistent with a previous yeast two-hybrid study that found that nebulin modules M163-M183 interact with a 19-kDa central coiled-coil domain (known as coil 1B) of desmin (Bang et al., 2002) and a more recent study in which M160-M164 was found to interact with desmin (Conover et al., 2009). In the absence of nebulin, desmin is unable to bind to these sites on the myofibril and as a result myofibrils differentially translocate when passively stretched. In our work we studied both soleus and EDL muscles, examples of predominately slow- and fast-fiber-containing muscles (Danieli-Betto et al., 2005; Girgenrath et al., 2005), respectively. Essentially the same findings were obtained (Fig. 2) suggesting that the model (desmin binding to nebulin) applies equally to different muscle types. In summary our work on a nebulin KO mouse suggests that myofibrils are laterally linked at the level of the Z-disk by relatively stiff desmin filaments that bind to nebulin.
It is currently unknown whether in addition to Z-disks other linkage sites exist between adjacent myofibrils that are sufficiently stiff to prevent translocation of myofibrils. A comparison of results obtained with the nebulin KO and desmin KO models might shed light on this issue. If the Z-disk is the only site of importance, largely identical Z-disk displacements are expected in the two models. Shah et al. (Shah et al., 2002) reported that in desmin KO fibers with SLs >2.90 μm there was a Z-disk displacement of 0.49±0.10 μm, larger that the values that we found in nebulin KO fibers [~0.2 μm (EDL) and ~0.3 μm (soleus)]. However, Z-disk displacement of WT fibers in the Shah et al. study was, at 0.25 μm, also much larger than in our WT fibers (~0.08 μm). This might indicate that forces were much higher in the Shah et al. study than in ours (we stretched the tendons very slowly and in small increment to keep passive force relatively low). It has been shown in single molecule studies (Kreplak et al., 2008) that at high force, desmin filaments can be elongated several-fold, presumably because the α-helical coiled-coil dimer can be converted into β-sheet-type structures upon stretching. The finding that Z-disk displacement in WT fibers is approximately constant at ~0.08 μm (Fig. 2) suggests that in our study forces were insufficient to cause filament elongation. Because the increase in Z-disk displacement above that of WT fibers is similar in our study to that of Shah et al. (Shah et al., 2002) we consider it likely that the desmin-nebulin linkage site is most important for myofibrillar connectivity. Thus although it is possible that other desmin-Z-disk linkages exist that do not involve nebulin [as suggested by the remaining Z-disk labeling of desmin in nebulin KO mice (see Bang et al., 2006)] the desmin-nebulin linkage is likely to be very important for establishing myofibrillar connectivity. Our similar findings in soleus and EDL muscles, representatives of slow and fast muscle types, respectively, suggest that nebulin-based myofibrillar connectivity is important in different muscle types, irrespective of their activity patterns. The importance of this linkage is also suggested by the upregulation of desmin, which might be viewed as a response to correct compromised myofibrillar connectivity. Finally, it is possible that in addition to desmin and nebulin other proteins also play a role in linking myofibrils. A good candidate is plectin, an ~500 kDa linking protein with isoforms 1d and 1f binding to desmin and Z-disks (Konieczny et al., 2008). It is unknown to which Z-disk protein(s) plectin binds and it is of importance to determine whether plectin is arranged in series or in parallel with the desmin-nebulin linkage.
We found that both soleus and EDL muscles of nebulin KO mice contain electron-dense Z-disk-like bodies that are similar to nemaline rod bodies that are a hallmark of nemaline myopathy in humans (Wallgren-Pettersson et al., 2004). Furthermore, histograms of Z-disk width (Fig. 6) show that the majority of all Z-disks in both WT and nebulin KO sarcomeres can be fit with a Gaussian curve that is shifted to higher values in KO than in WT fibers (by an average of ~80 nm in soleus and ~40 nm in EDL fibers). We conclude that absence of nebulin affects most Z-disks by widening them by a modest amount and that it affects a small subset of sarcomeres more severely (nemaline rod bodies). Previously titin has been suggested to play a role in Z-disk assembly (Gautel et al., 1996) and our present work indicates that nebulin also contributes to establishing and/or maintaining the Z-disk structure. The Z-disk region of titin contains a family of differentially expressed repeats, the titin Z-repeats (Gautel et al., 1996). These Z-repeats are a family of α-actinin-binding motifs, which are differentially expressed in a tissue- and developmental-stage-specific fashion (Gautel et al., 1996). As previously pointed out, it is unlikely that the differential expression of the titin Z-repeats alone can determine the Z-disk width, because too few isoforms exist to account for the wide range of different Z-disk widths (Millevoi et al., 1998). Our present work supports a model in which titin and nebulin together specify Z-disk width, with titin constructing the central region of the Z-disk, including the number and positions where α-actinin cross-links the thin and the titin filaments. Nebulin determines the ending of the Z-disk structure and its transition to the I-band, i.e. nebulin functions as a Z-disk terminator.
The mechanism by which nebulin terminates the Z-disk might involve interaction between nebulin and Z-disk-localized proteins such as, titin and CapZ. CapZ is a barbed-end actin capping protein that binds near the C-terminus of nebulin (Pappas et al., 2008). In muscle fibers devoid of nebulin [nebulin KO (Witt et al., 2006)] or siRNA knockdown of nebulin in myotubes (Pappas et al., 2008), CapZ does not localize properly, allowing the barbed ends of Z-disks to continue to grow beyond the Z-disk resulting in widened Z-disks. It is unclear why most Z-disks only widen by a modest amount and others develop into nemaline rod bodies. Because similar results were obtained in soleus and EDL, a mainly slow twitch posture muscle and fast twitch intermittently used muscle, respectively, differences in mechanical load might not be the main explanation. Instead we speculate that gradients in protein levels in the muscle fiber play an important role. Clearly further research is required to resolve this question.
In summary, structural studies on a nebulin KO mouse show that in the absence of nebulin, Z-disks are significantly wider and that myofibrils are misaligned. Thus the functional roles of nebulin extend beyond thin filament length regulation and include maintaining physiological Z-disk widths and myofibrillar connectivity. It is interesting to note that the Z-disk functions of nebulin might be performed in cardiac muscle (where nebulin levels are very low or absent) by nebulette, a ~100 kDa nebulin-like protein that shares extensive similarity with the C-terminal region of nebulin (Millevoi et al., 1998). Thus thin filament length regulation might not be the main function of nebulin (because cardiac muscle can do without) but instead the Z-disk functions of nebulin might be most crucial.
Materials and Methods
Muscle specimens
The nebulin KO mouse model (BL6) including its genotyping has been described previously (for details, see Witt et al., 2006). Mice die at a relatively early age as a result of muscle weakness and respiratory failure and, therefore, animals in this study were used at 3 days and 10 days of age. At day 10, the KO mice weighed 2.3±0.4 g (n=8) and the WT mice 7.2±0.8 g (n=6). The values at day 3 were 2.0±0.3 g (KO, n=6) and 2.4±0.3 g (WT, n=5). Mice were killed and the skin from both hindlimbs was rapidly removed before immersing the hindlimbs in relaxing solution (40 mM BES, 10 mM EGTA, 6.56 mM MgCl2, 5.88 mM Na-ATP, 1 mM DTT, 46.35 mM potassium propionate, 15 mM creatine phosphate) containing protease inhibitors [0.005 mM leupeptin, 0.1 mM E-64, 0.2 mM phenylmethylsulphonyl fluoride (PMSF) from a stock in 100% ethanol, pH 7.0] and 1% Triton X-100 (w/v). After skinning overnight, the hindlimbs were washed with relaxing solution; EDL and soleus muscles were quickly removed and one end of their tendons was pinned down with a minuten pin (Fine Science Tools) to a Sylgard elastomer base (Dow Corning, Midland, MI) in a small Petri dish filled with relaxing solution. The muscles were then passively stretched in small increments (in ~30 seconds) to a final length that exceeded the slack length by ~20%, 40%, 60% and 80%, followed by pinning the other end of the muscle tendon to the elastomere dissection base. The muscles were kept in this stretched state for 10 minutes at 4°C, and were then processed for electron microscopy (see below). We typically studied ~20 fibers per muscle. All experiments were conducted and approved by the University of Arizona Institutional Animal Care and Use Committee, following the guidelines ‘Using Animals in Intramural Research’ of the National Institutes of Health.
Transmission electron microscopy
For electron microscopy, stretched EDL and soleus muscle from WT and nebulin KO mice were fixed with 3% paraformaldehyde in PBS for 30 minutes. The muscles were then rinsed for 15 minutes in PBS containing protease inhibitors (Granzier et al., 1997). A secondary fixation was performed in 3% glutaraldehyde containing 2% tannic acid in PBS for 1 hour. After another PBS rinse, the muscles were postfixed in 1% OsO4 in PBS for 30 minutes, and were then dehydrated in an ethanol series of increasing concentrations. Following dehydration, the muscles were first infiltrated with 100% propylene oxide then a mixture of 1:1 propylene oxide:Araldite, and finally embedded in a pure Araldite resin. Ultrathin sections (70 nm) were obtained with a Reichert-Jung ultramicrotome and contrasted with potassium permanganate and lead citrate. During sectioning we ensured that the fiber direction was aligned with the edge of the diamond knife. We typically studied 20 fibers per muscle. Samples were observed using a JEOL 1200 EX or a Philips CM12 electron microscope, operated at 100 kV.
Measurement of Z-disk displacement, sarcomere length (SL) and Z-disk width
A set of digitized electron micrographs from both EDL and soleus muscles were randomly selected for analysis with Adobe Photoshop 8.0. The magnification of the microscope was used for calibration. Displacement of Z-disks (ΔX) in adjacent myofibrils was measured as shown in Fig. 2. Sarcomere length was measured from mid Z-disk to mid Z-disk. The Z-disk width was measured along a single pixel perpendicular line using the density as a gauge for the edge of the Z-disk (density midway between that of the center of the Z-disk and the I-band). Typically ~50 measurements were made per electron micrograph with nine micrographs per experiment (with each experiment representing a different degree of stretch: 0, 20, 40, 60 and 80%). We determined the mean and variance of all displacement values per micrograph, using KaleidaGraph 3.6 version software and plotted results as a function of SL (as in Fig. 2). The Z-disk width values were binned in 25 nm bins and plotted in histograms (as in Fig. 8).
Immunoelectron microscopy (IEM)
Immunogold labeling was performed as previously described (Trombitas and Granzier, 1997). Briefly, skinned muscle fiber bundles from EDL and soleus muscle (n=3) were fixed in 3% paraformaldehyde in PBS, for 30 minutes at 4°C, and washed with PBS, and PBS containing protease inhibitors. Blocking was performed with 1% BSA in PBS containing protease inhibitors for 1 hour at 4°C, followed by incubation with polyclonal anti-desmin antibody (rabbit whole serum, Sigma-Aldrich, D8281) diluted 1:10 in PBS containing protease inhibitors for 48 hours at 4°C. (Note that long labeling durations are commonly used in IEM on skinned muscle.) After rinsing in PBS, the fiber bundles were incubated for 48 hours at 4°C with secondary anti-rabbit Nanogold goat antibody (1.4 nm, Nanoprobes) at a dilution of 1:10 in PBS containing protease inhibitors. The bundles were rinsed with PBS and then fixed in 3% glutaraldehyde containing 0.01% tannic acid in PBS for 30 minutes at 4°C. After rinsing in PBS, aldehydes were quenched with 50 mM glycine in PBS for 15 minutes at 4°C, followed by gold enhancement (Nanoprobes; as per the manufacturer's instructions) for 3-5 minutes depending on fiber bundle thickness. Bundles were washed with PBS and postfixed with 1% OsO4 in PBS for 15 minutes at 4°C. Fiber bundles were washed with distilled water and dehydrated in a graded series of ethanol (50%, 75%, 95%, 100%). Then the bundles were infiltrated with propylene oxide for 15 minutes, then in a mixture of 1:1 propylene oxide:Araldite followed by Araldite alone. Bundles were cut into small fragments and then prepared for mounting, embedding and polymerization for 48 hours at 60°C. Sections were only lightly contrasted in lead citrate, to ensure that gold particles stood out well from the sarcomeric structures, and the sections were examined using either a Jeol 1200EX or a Philips CM12 microscope operated at 100 kV.
Gel electrophoresis and western blotting analysis
Gastrocnemius muscle from 10-day-old WT and nebulin KO mice (n=3) were quick-frozen in liquid nitrogen, pulverized and rapidly solubilized as previously described (Granzier and Irving, 1995). Samples were run on 12% SDS-PAGE gels, and transferred to polyvinylidene difluoride membranes. Blots were probed with anti-desmin polyclonal (Sigma-Aldrich) and sarcomeric anti-α-actinin monoclonal (clone EA-53; Sigma-Aldrich) primary antibodies diluted 1:500. Fluorescently labeled secondary antibodies, Alexa Fluor 680 and Rockland IR Dye 800 conjugate (Molecular Probes) were used as appropriate. Anti-myosin heavy chain (MHC) antibody was used to normalize for protein loading. Protein immunoblots were scanned and quantified by densitometry using the two-color detection Odyssey infrared imaging system (LI-COR Biosciences). MHC was used to normalize for differences in protein loading.
Cell culture, transfections and siRNA treatment
Primary cultures of quail skeletal myotubes were prepared as described (Almenar-Queralt et al., 1999). A nebulin-specific siRNA was previously designed, validated and purchased from Ambion (Austin, Texas; target cDNA sequence: 5′-GTAGCTGACTCTCCAATTA-3′); western blot analysis demonstrated that siRNA treatment reduced endogenous nebulin levels by >90% (Pappas et al., 2008). As a control, a random siRNA was also generated (target cDNA sequence: 5′-CTCGACTAGAGTCTGTCTA-3′). Nebulin modules 160-170, which were previously cloned from mouse heart cDNA (Conover et al., 2002) were inserted into a modified pEGFP-C2 vector in which GFP was replaced with mCherry. Quail myotubes were transfected with 50 nM siRNA of expression plasmid DNA using the lipid-based reagent Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions, 12-24 hours after plating. Four to eight days after transfection, the cells were incubated in relaxing buffer (150 mM KCl, 5 mM MgCl2, 10 mM MOPS, pH 7.4, 1 mM EGTA, 4 mM ATP) for 15 minutes and fixed with 2% paraformaldehyde in relaxing buffer for 15 minutes.
Immunofluorescence microscopy
To observe sarcomeric components, cells were stained as described previously (Pappas et al., 2008). The fixed cells were permeabilized in 0.2% Triton X-100 in PBS, blocked with 2% BSA plus 1% normal donkey serum in PBS, and incubated for 1 hour with primary antibodies diluted in PBS. The primary antibodies consisted of a polyclonal anti-N-terminal nebulin antibody [3.5 μg/ml (McElhinny et al., 2001)], a monoclonal anti-α-actinin antibody (1:15,000; Sigma, A7811) and a polyclonal anti-desmin antibody (1:2; Biomeda, Foster City, CA; 213M). The cells were then washed with PBS for 15 minutes, and incubated with secondary antibodies in PBS for 30 minutes. The secondary antibodies, obtained from Invitrogen and Jackson ImmunoResearch Laboratories, included: Alexa-Fluor-488-conjugated goat anti-mouse and goat anti-rabbit IgG (1:1000), Alexa-Fluor-350-conjugated goat anti-mouse IgG (1:300) and Texas-Red-conjugated donkey anti-rabbit IgG (1:600). Alexa-Fluor-488-conjugated phalloidin was used to stain F-actin (Invitrogen). Coverslips were mounted onto slides with Aqua Poly/Mount (Polysciences, Warrington, PA). The cells were analyzed and images captured using a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA) with a 100× objective (1.3 NA) and a CoolSnap HQ charge-coupled device camera (Photometrics, Tucson, AZ).
Statistical analysis
Data were expressed as mean ± s.d. Unpaired t-tests were used to determine whether Z-disk displacements of EDL and soleus muscles were significantly different, with P<0.05 regarded as statistically significance. The slopes for displacement versus SL data from muscles of wild-type and KO mice were compared by analysis of covariance (ANCOVA) using StatView software (v. 5.01, SAS Institute).
Supplementary Material
Acknowledgments
We are grateful to Germain Wright, Chi Fong, Ellen Taylor, Tiffany Pecor and Gina Zhang for excellent technical assistance. We are grateful to Christian Witt for providing the nebulin KO model, Gloria Conover for generating the M160-164 construct and Marcus DiMarco for generating the primary myocyte cultures. Thanks to Christine Davis (Washington State University, Pullman, WA) and David Elliot (University of Arizona, Tucson, AZ) for kind cooperation at the EM facility core. This work was supported by the European Union (ERARE NEMMYOP to S.L.), and by the US National Institutes of Health (HL083146 to C.C.G. and grants AR-053897 and HL062881 to H.G.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/3/384/DC1
References
- Almenar-Queralt A., Gregorio C. C., Fowler V. M. (1999). Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112, 1111-1123 [DOI] [PubMed] [Google Scholar]
- Bang M. L., Gregorio C., Labeit S. (2002). Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137, 119-127 [DOI] [PubMed] [Google Scholar]
- Bang M. L., Li X., Littlefield R., Bremner S., Thor A., Knowlton K. U., Lieber R. L., Chen J. (2006). Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 173, 905-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y., Bloch R. J., Kouloumenta A., Mavroidis M., Psarras S. (2007). Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 313, 2063-2076 [DOI] [PubMed] [Google Scholar]
- Castillo A., Nowak R., Littlefield K. P., Fowler V. M., Littlefield R. S. (2009). A nebulin ruler does not dictate thin filament lengths. Biophys. J. 96, 1856-1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover G. M., Henderson S. N., Gregorio C. C. (2009). A myopathy-linked desmin mutation perturbs striated muscle actin filament architecture. Mol. Biol. Cell 20, 834-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. L., Escaleira R., Cataldo A., Oliveira F., Mermelstein C. S. (2004). Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Braz. J. Med. Biol. Res. 37, 1819-1830 [DOI] [PubMed] [Google Scholar]
- Danieli-Betto D., Esposito A., Germinario E., Sandona D., Martinello T., Jakubiec-Puka A., Biral D., Betto R. (2005). Deficiency of alpha-sarcoglycan differently affects fast- and slow-twitch skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1328-R1337 [DOI] [PubMed] [Google Scholar]
- Gautel M., Goulding D., Bullard B., Weber K., Furst D. O. (1996). The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J. Cell Sci. 109, 2747-2754 [DOI] [PubMed] [Google Scholar]
- Girgenrath S., Song K., Whittemore L. A. (2005). Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31, 34-40 [DOI] [PubMed] [Google Scholar]
- Granzier H., Kellermayer M., Helmes M., Trombitas K. (1997). Titin elasticity and mechanism of passive force development in rat cardiac myocytes probed by thin-filament extraction. Biophys. J. 73, 2043-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Irving T. C. (1995). Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68, 1027-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel-Giannetti J., Reed U., Bang M. L., Pelin K., Donner K., Marie S. K., Carvalho M., Fireman M. A., Zanoteli E., Oliveira A. S., et al. (2001). Nebulin expression in patients with nemaline myopathy. Neuromuscul. Disord. 11, 154-162 [DOI] [PubMed] [Google Scholar]
- Konieczny P., Fuchs P., Reipert S., Kunz W. S., Zeold A., Fischer I., Paulin D., Schroder R., Wiche G. (2008). Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 181, 667-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreplak L., Herrmann H., Aebi U. (2008). Tensile properties of single desmin intermediate filaments. Biophys. J. 94, 2790-2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Granger B. L. (1978). Fluorescent localization of membrane sites in glycerinated chicken skeletal muscle fibers and the relationship of these sites to the protein composition of the Z disc. Proc. Natl. Acad. Sci. USA 75, 3683-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield R. S., Fowler V. M. (2008). Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin. Cell Dev. Biol. 19, 511-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny A. S., Kolmerer B., Fowler V. M., Labeit S., Gregorio C. C. (2001). The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J. Biol. Chem. 276, 583-592 [DOI] [PubMed] [Google Scholar]
- McElhinny A. S., Kazmierski S. T., Labeit S., Gregorio C. C. (2003). Nebulin: the nebulous, multifunctional giant of striated muscle. Trends Cardiovasc. Med. 13, 195-201 [DOI] [PubMed] [Google Scholar]
- Millevoi S., Trombitas K., Kolmerer B., Kostin S., Schaper J., Pelin K., Granzier H., Labeit S. (1998). Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J. Mol. Biol. 282, 111-123 [DOI] [PubMed] [Google Scholar]
- Pappas C. T., Bhattacharya N., Cooper J. A., Gregorio C. C. (2008). Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol. Biol. Cell 19, 1837-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelin K., Hilpela P., Donner K., Sewry C., Akkari P. A., Wilton S. D., Wattanasirichaigoon D., Bang M. L., Centner T., Hanefeld F., et al. (1999). Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc. Natl. Acad. Sci. USA 96, 2305-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee D., Sanger J. M., Sanger J. W. (1994). The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil. Cytoskeleton 28, 1-24 [DOI] [PubMed] [Google Scholar]
- Rudy D. E., Yatskievych T. A., Antin P. B., Gregorio C. C. (2001). Assembly of thick, thin, and titin filaments in chick precardiac explants. Dev. Dyn. 221, 61-71 [DOI] [PubMed] [Google Scholar]
- Shah S. B., Su F. C., Jordan K., Milner D. J., Friden J., Capetanaki Y., Lieber R. L. (2002). Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle. J. Exp. Biol. 205, 321-325 [DOI] [PubMed] [Google Scholar]
- Shah S. B., Davis J., Weisleder N., Kostavassili I., McCulloch A. D., Ralston E., Capetanaki Y., Lieber R. L. (2004). Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys. J. 86, 2993-3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom B., Salmazo A., Djinovic-Carugo K. (2008). Alpha-actinin structure and regulation. Cell Mol. Life Sci. 65, 2688-2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J. M. (1997). Architecture and function in the muscle sarcomere. Curr. Opin. Struct. Biol. 7, 247-257 [DOI] [PubMed] [Google Scholar]
- Trombitas K., Granzier H. (1997). Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am. J. Physiol. 273, C662-C670 [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C., Jasani B., Newman G. R., Morris G. E., Jones S., Singhrao S., Clarke A., Virtanen I., Holmberg C., Rapola J. (1995). Alpha-actinin in nemaline bodies in congenital nemaline myopathy: immunological confirmation by light and electron microscopy. Neuromuscul. Disord. 5, 93-104 [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C., Pelin K., Nowak K. J., Muntoni F., Romero N. B., Goebel H. H., North K. N., Beggs A. H., Laing N. G. (2004). Genotype-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul. Disord. 14, 461-470 [DOI] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R. (1983). A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J. Cell Biol. 96, 562-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt C. C., Burkart C., Labeit D., McNabb M., Wu Y., Granzier H., Labeit S. (2006). Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 25, 3843-3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.