Abstract
The Wnt planar cell polarity (Wnt/PCP) pathway signals through small Rho-like GTPases to regulate the cytoskeleton. The core PCP proteins have been mapped to the Wnt/PCP pathway genetically, but the molecular mechanism of their action remains unknown. Here, we investigate the function of the mammalian PCP protein Vang-like protein 2 (Vangl2). RNAi knockdown of Vangl2 impaired cell-cell adhesion and cytoskeletal integrity in the epithelial cell lines HEK293T and MDCK. Similar effects were observed when Vangl2 was overexpressed in HEK293T, MDCK or C17.2 cells. The effects of Vangl2 overexpression could be blocked by knockdown of the small GTPase Rac1 or by dominant-negative Rac1. In itself, knockdown of Rac1 impaired cytoskeletal integrity and reduced cell-cell adhesion. We found that Vangl2 bound and re-distributed Rac1 within the cells but did not alter Rac1 activity. Moreover, both transgenic mouse embryos overexpressing Vangl2 in neural stem cells and loop-tail Vangl2 loss-of-function embryos displayed impaired adherens junctions, a cytoskeletal unit essential for neural tube rigidity and neural tube closure. In vivo, Rac1 was re-distributed within the cells in a similar way to that observed by us in vitro. We propose that Vangl2 affects cell adhesion and the cytoskeleton by recruiting Rac1 and targeting its activity in the cell to adherens junctions.
Keywords: Cell adhesion, Neurulation, PCP, Wnt
Introduction
Cell adhesion is an essential process for morphogenesis of higher organisms. It is necessary for the formation of epithelial sheets and is tightly regulated during delamination. An imbalance in this process can lead to cancer (Lee et al., 2006). Adherens junctions are specialized adhesive structures that connect cadherins with the actin microfibers via β-catenin and α-catenin, thereby connecting the cytoskeleton of neighboring cells. In the neural tube, adherens junctions are present as ring-like structures surrounding the apical end of the cells, which face the lumen of the neural tube. During nervous system development, defects in the integrity of adherens junctions lead to defects in cranial neural tube closure (Ybot-Gonzalez and Copp, 1999).
Interestingly, defects in neural tube closure have been associated with genetic alterations in the Wnt planar cell polarity (Wnt/PCP) signaling cascade (Kibar et al., 2001; Murdoch et al., 2001a; Murdoch et al., 2001b), strictly determined by the core PCP proteins. Wnt/PCP involves activation of the small Rho-like GTPases RhoA and Rac1 (Fanto et al., 2000; Habas et al., 2003; Kim and Han, 2005; Strutt et al., 1997), leading to JNK activation (Fanto et al., 2000; Paricio et al., 1999), modulation of the cytoskeleton (Wong and Adler, 1993), and/or altered gene transcription (Fanto et al., 2000; Schambony and Wedlich, 2007).
Homologs of Drosophila PCP signaling genes are found in vertebrates, and have been mapped to the Wnt/PCP pathway genetically, although the molecular mechanism of their action remains unknown. These include Vangl2 (strabismus in lower vertebrates), Prickle and Celsr1 (flamingo). They were first characterized as regulators of convergent extension, a process during gastrulation and neurulation where lateral cells migrate and intercalate at the midline, thus narrowing and elongating the embryo. Both reduced and increased levels of PCP proteins disturb convergent extension, indicating a dose-dependent effect (Carreira-Barbosa et al., 2003; Darken et al., 2002; Goto and Keller, 2002; Takeuchi et al., 2003; Veeman et al., 2003). In addition to their role in convergent extension, Vangl2, Celsr1 and Scrb1 also regulate PCP in the mammalian inner ear (Montcouquiol et al., 2006). Vangl2 is expressed throughout the neural tube. At least part of the role of Strabismus in zebrafish convergent extension is to regulate cell polarity in the neural tube, but it has also been suggested that it facilitates convergent extension movements by regulating cell adhesion (Ciruna et al., 2006).
We have previously reported that adherens junctions are disrupted in transgenic mouse embryos overexpressing Wnt7a in the developing neural tube (Shariatmadari et al., 2005). The expression of Vangl2 mRNA was increased in mouse embryos overexpressing Wnt7a, which led us to speculate that the Wnt7a-induced effect is at least partly mediated by Vangl2 and Wnt/PCP signaling. Here, using gain-of-function and loss-of-function approaches in vivo and in vitro, we report that Vangl2 indeed affects the cytoskeleton and cell-cell adhesion. Moreover, we demonstrate that Vangl2 achieves these effects in concert with the small GTPase Rac1 (which binds Vangl2) and relocates to the site of active cytoskeletal remodeling close to the membrane in a Vangl2-dependent way.
Results
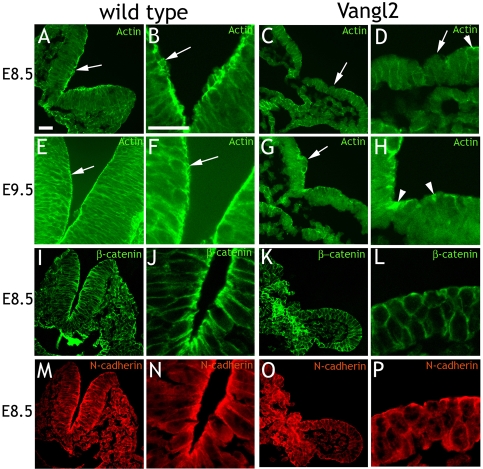
Vangl2 affects cell shape and actin distribution in HEK293T and MDCK cells
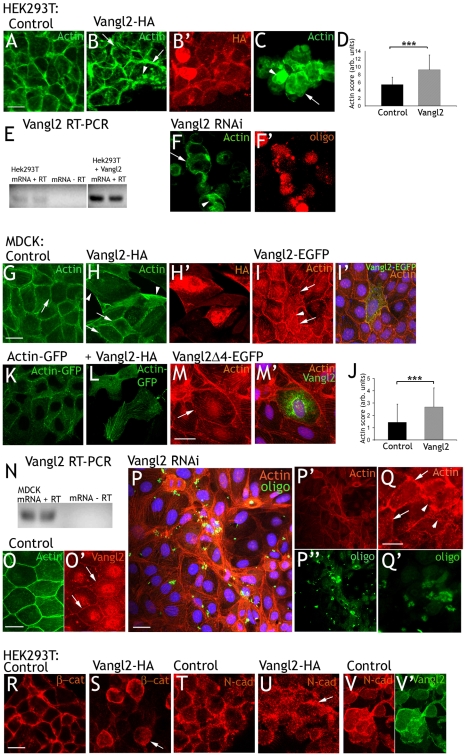
In order to study the effect of Vangl2 on cell shape and actin localization in vitro, 80% confluent HEK293T cells were transfected with control plasmid (DsRed) or Vangl2-HA expression plasmid. Transfection rates were high, and only experiments with a transfection rate of more than 80% were analyzed further. Control transfected cells grew in monolayers, where the cells consistently organized themselves in a honeycomb fashion. Labeling F-actin with FITC-phalloidin revealed distinct and continuous cortical (circumferential) actin (Fig. 1A). Vangl2-HA reduced the areas of the monolayers, and cells appeared to be more loosely attached to each other. Cortical actin was more discontinuous than in control cells, and intracellular actin deposits were observed (Fig. 1B). Vangl2-HA was present in, or close to, the cell membrane, but was also present to a varying degree in the cytoplasm (Fig. 1B′). Vangl2-HA also yielded a high proportion of single cells or clusters of few, loosely attached cells. The cortical actin in these cells was sparse and highly discontinuous, and intracellular actin deposits were common (Fig. 1C). The effects of Vangl2 were quantified by three observers. Individual control (n=16) or Vangl2 overexpressing (n=14) cells were blindly rated. This yielded a number that was higher the more the cell diverted from a hypothetical ideal (see Materials and Methods). On average, control cells were rated 3.4, whereas Vangl2-expressing cells received a rating of 7.2 (Fig. 1D). The difference was significant (P=8.78×10−9).
Fig. 1.
Vangl2 affects cell shape and actin distribution. (A) Control transfected HEK293T. Circumferential actin is continuous. (B,C) Cells overexpressing Vangl2-HA grow in sheets (B) with an irregular buildup, or in unstructured clusters (C). Circumferential actin is unevenly distributed (arrows), and intracellular actin deposits are observed (arrowheads). (B′) Vangl2-HA is located to the cell cortex and the cell cytoplasm. (D) On average, control cells received an acting rating of 3.4, Vangl2-expressing cells 7.2 Error bars represent s.d. ***P=8.78×10−9. (E) Reverse transcriptase (RT) PCR shows that untransfected HEK293T cell express Vangl2. Vangl2-overexpressing cells yield bands of the same size (dividing lanes have been omitted). (F) Vangl2 RNAi knockdown. Cortical actin is diffuse (arrow) and intracellular actin deposits (arrowhead) are abundant. (F′) Transfected cells are identified by a fluorescent oligo. (G) Control transfected MDCK cells grow in a regular pattern. (H) MDCK cells transfected with Vangl2-HA are irregular in shape and often larger than the surrounding untransfected cells. Actin is irregularly distributed in the cellular cortex and the cytoplasm (arrowheads). Note the difference in appearance to the two untransfected cells (arrows). Vangl2-HA distribution is shown in H′. (I) Vangl2-EGFP affects cells similarly as Vangl2-HA. Transfected cells are indicated by arrows. Arrowhead points at actin deposits in the cytoplasm. (I′) Merged panel shows actin in red, Vangl2-EGFP in green and DAPI in blue. (J) Blind rating of the actin distribution in control (score 1.4) and Vangl2-HA transfected cells (score 2.7). Error bars represent s.d. ***P=2.4×10−5. (K) MDCK cells transfected with actin-GFP. Note that all labeled cells are transfected. (L) Vangl2-HA affects cell shape and actin distribution. (M) Vangl2Δ4-EGFP has no apparent effect on MDCK cells. Arrow points at cortical actin in a transfected cell. (M′) Merged panel show Vangl2-Δ4-EGFP distribution. (N) RT PCR indicates the presence of endogenous Vangl2 in MDCK cells. (O,O′) Overlap between endogenous Vangl2 (arrows in O′) and circumferential actin. (P) Vangl2 RNAi knockdown affects cell size and actin distribution (only actin in P′, transfection indicated by oligo in P″). (Q,Q′) Vangl2 RNAi knockdown cells (arrowheads) stands out from adjacent untransfected cells (arrows). (R,T) β-catenin and N-cadherin display a similar distribution as actin in the cell cortex of control HEK293T cells. (S,U) Vangl2-HA expressing cells display a more diffuse, cytoplasmic distribution. (V,V′) N-cadherin (V) and endogenous Vangl2 (V′) overlap in the cell cortex of untreated HEK293T cells. Scale bars: 10 μm. Actin, FITC-phalloidin- or TRITC-phalloidin-labeled actin; HA, HA-tag; GFP, GFP fluorescence.
The HEK293T epithelial cell line derives from embryonic kidney, which has been shown to express Vangl2 (Katoh, 2002). RT-PCR confirmed that HEK293T cells express Vangl2 (Fig. 1E). To investigate the loss of Vangl2 function in HEK293T cells, we performed RNAi knockdown directed against human Vangl2 (transfection was confirmed with a fluorescent oligo). Vangl2 knockdown caused extensive cell loss during the washes, indicating reduced cell-substrate and cell-cell adherence. The remaining cells were more loosely attached to each other than were control cells, cortical actin was diffuse or discontinuous, and intracellular actin deposits were abundant (Fig. 1F). In sum, Vangl2 loss-of-function had similar, but more accentuated, effects on cytoskeletal actin, cell morphology and adherence to those of Vangl2 gain-of-function.
We then went on to study MDCK cells, which are more suitable for studies of cell polarity. MDCK cells were transfected with control (DsRed), the Vangl2-HA or the Vangl2-EGFP expression plasmids. HA or EGFP positivity was used as the marker of transfection efficiency. This varied substantially, but only clearly transfected cells were analyzed further. The effects were evaluated 24 or 48 hours post-transfection. Control MDCK cells grew in large monolayers, where the cells often attached to each other in a honeycomb-like fashion. Labeling F-actin with FITC- or TRITC-phalloidin revealed distinct and continuous cortical (circumferential) actin (Fig. 1G). Vangl2-HA or Vangl2-EGFP transfected cells were irregular in shape, often larger than average, and more elongated (Fig. 1H,H′,I,I′). They were frequently distinct both from control transfected and adjacent, untransfected cells. Note the difference in Fig. 1H (untransfected cells marked with arrows) and Fig. 1I (EGFP shown in Fig. 1I′). Actin was irregularly distributed in the cell cortex and cytoplasm (arrowheads in Fig. 1H,I). Three observers blindly rated how intermittent Vangl2-EGFP overexpressing or control (EGFP) cells differed from adjacent, untransfected cells (high rating = high difference; see Materials and Methods). On average, control transfected cells received a rating of 1.4 and Vangl2-transfected cells 2.7 (Fig. 1J; P=2.4×10−5).
We also co-transfected cells with GFP-actin and control (DsRed) or HA-Vangl2 plasmids. GFP fluorescence served a double role as a marker of transfected cells, while visualizing actin. Vangl2-HA effects on GFP-actin were comparable to those on endogenous actin (compare control transfected cells in Fig. 1K with Vangl2-HA-transfected cells in Fig. 1L).
To test whether the PDZ (post synaptic density protein, Drosophila disc large tumor suppressor and zonula occludens-1 protein)-binding domain was necessary for the ability of Vangl2 to affect the actin cytoskeleton, we transfected MDCK cells with Vangl2Δ4-EGFP, a construct lacking the four amino acids in the PDZ-binding domain (Kallay et al., 2006). Cells expressing Vangl2Δ4-EGFP did not display any aberrant phenotype, indicating that interaction with other proteins via the PDZ-binding domain is essential for Vangl2 to cause the phenotype (Fig. 1M,M′).
Like HEK293T, the MDCK cell line was derived from embryonic kidney, which expresses Vangl2 (Katoh, 2002). Indeed, MDCK cells also expressed endogenous Vangl2 as shown by RT-PCR (Fig. 1N). An antibody against Vangl2 yielded weak immunolabeling in the cell membranes (Fig. 1O′, arrows), which overlapped with circumferential actin (Fig. 1O). To investigate loss of Vangl2 function in MDCK cells, we performed RNAi knockdown. Co-transfection with a FITC-labeled oligo was utilized to identify transfected cells. Vangl2 knockdown cells were smaller in size than untransfected (including adjacent) cells (Fig. 1P,P′,P″,Q,Q′). The control cells ordered cortical actin, and intracellular stress fibers were replaced by a diffuse and seemingly unorganized actin distribution. Observe the disorganized appearance of a cluster of cells transfected with Vangl2 RNAi (Fig. 1P,P′,P″), and how transfected cells differ from their untransfected neighbors (Fig. 1Q,Q′). In areas with many knockdown cells, cell-cell adherence seemed to be compromised (Fig. 1P,P′,P″).
We then used HEK293T cells to investigate whether Vangl2 affected the distribution of β-catenin and N-cadherin (the cadherin present in HEK293T cells). Control transfected cells displayed a distinct band of cortical β-catenin (Fig. 1R). In Vangl2-transfected cells, this was more disperse and discontinuous (Fig. 1S). N-cadherin was present in a punctuate band along the cell membrane of control transfected cells (Fig. 1T). Vangl2 transfection resulted in a more diffuse pattern over the cell membrane and cytoplasm (Fig. 1U). Endogenous Vangl2 overlapped with N-cadherin in untreated cells (Fig. 1V,V′).
We conclude that both Vangl2 gain-of-function and loss-of-function disturb the localization of actin and actin-associated components of adherens junctions, such as β-catenin and N-cadherin, in agreement with previous reports on PCP protein function (Carreira-Barbosa et al., 2003; Darken et al., 2002; Goto and Keller, 2002; Takeuchi et al., 2003; Veeman et al., 2003). Furthermore, the PDZ-binding domain seems to be essential in mediating this effect.
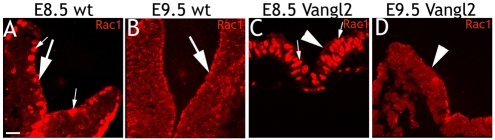
Vangl2 binds and redistributes Rac1
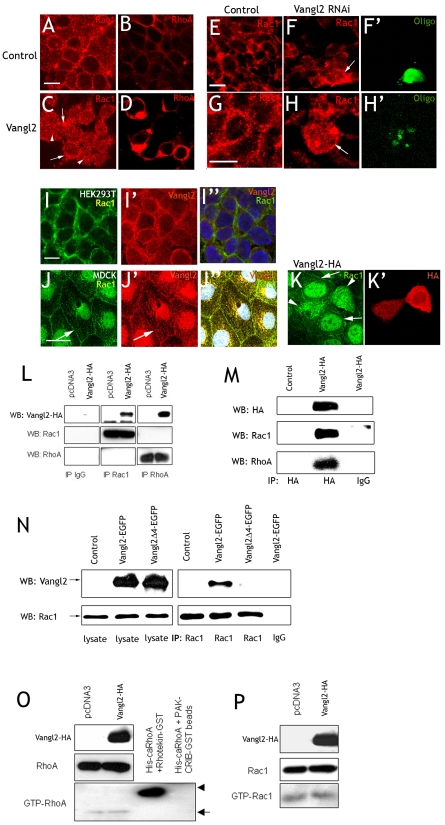
The effectors of PCP signaling, the GTPases RhoA and Rac1, are key regulators of cadherin-mediated cell-cell adhesion. RhoA regulates the formation of stress fibers and the attachment of cells to the substrate (Nobes and Hall, 1999). Rac1 has been implicated in controlling adhesion between epithelial cells (Braga et al., 1997; Jou and Nelson, 1998), and affects adherens junctions (Fischer and Quinlan, 1998; Quinlan, 1999). We therefore went on to investigate the interaction of Vangl2 with RhoA or Rac1. First, HEK293T cells were transfected as before with control (EGFP) or Vangl2-HA. After 24 hours, Rac1 and RhoA immunolabeling in the control cells appeared as distinct puncta focused to the cell membranes, although RhoA labeling was more continuous (Fig. 2A,B). Vangl2 yielded a more diffuse Rac1 distribution, displaying aggregates of Rac1 puncta associated with both the cell membranes and cytoplasm (Fig. 2C). The subcellular distribution of RhoA was similar in Vangl2-expressing cells as in control cells, but the labeling intensity was higher (Fig. 2D). Vangl2 knockdown affected Rac1 distribution similarly to Vangl2 overexpression. Rac1 puncta were distributed throughout the cytoplasm and cell cortex in cells transfected with Vangl2 RNAi (Fig. 2F,H). By contrast, control transfected cells displayed a distinct Rac1 localization in the cell cortex (Fig. 2E,G). Co-labeling for Rac1 (Fig. 2I) and Vangl2 (Fig. 2I′) indicated that both Rac1 and endogenous Vangl2 are present in the cell cortex of untreated HEK293T cells (Fig. 2I″). Control MDCK cells displayed Rac1 labeling in the cell membranes (Fig. 2J,K, arrows) that overlapped with weak, although clearly present, endogenous Vangl2 immunoreactivity (Fig. 2J′,J″). Overexpression of Vangl2-HA resulted in a more diffuse, cytoplasmic distribution of Rac1 (arrowheads in Fig. 2K).
Fig. 2.
Vangl2 binds and redistributes Rac1. (A-I″) HEK293T cells. (A) The cell membranes of control cells display a distinct punctuate Rac1 labeling. (C) Vangl2-transfected cells display strong labeling of Rac1 in the membrane (arrows), and increased cytoplasmic labeling (arrowheads). (B,D) Vangl2 increases RhoA labeling intensity in the cell cortex (B) compared to control cells (D). (E,G,F,H) Rac1 in control and Vangl2 RNAi knockdown cells. (F,H) Vangl2 RNAi knockdown results in a more diffuse cortical Rac1 labeling and increased cytoplasmic labeling. Transfection in F and H is verified with a control oligo (F′,H′). (I-I″) Rac1 (I′) and endogenous Vangl2 (I′) overlap (I″) in the cortex of untreated HEK293T cells. (J-K′) MDCK cells. Rac1 (arrow in J) and Vangl2 (arrow in J′) overlap in the cell membranes of control transfected cells. (J″) Merged picture including DAPI. (K,K′) Cells overexpressing Vangl2-HA (K′, arrowheads in K) display a diffuse, cytoplasmic Rac1 distribution. Adjacent, untransfected cells display distinct Rac1 labeling in the cell membranes (arrows in K). (L-N) Lysates from cells transfected with Vangl2-HA, Vangl2-EGFP or Vangl2Δ4-EGFP were immunoprecipitated as indicated with anti-Rac1, anti-RhoA or anti-HA, and used for western blots with anti-HA, anti-Rac1 or anti-RhoA. (L,M) Distinct bands of the correct size demonstrate that Rac1 and RhoA are part of the same protein complex as Vangl2. (N) Rac1 immunoprecipitates Vangl2-GFP, but not Vangl2Δ4-EGFP. (O,P) Despite the ability of Vangl2 to bind Rac1 and RhoA, pull-down assays shows that the activities of RhoA (O) and Rac1 (P) are not affected by Vangl2. Scale bars: 10 μm.
To test whether Vangl2 can physically interact with Rac1 and/or RhoA we performed immunoprecipitation experiments. Lysates from HEK293T cells transfected with Vangl2-HA plasmid were either precipitated with anti-Rac1 or anti-RhoA and blotted against Vangl2-HA, or precipitated with anti-HA and blotted against anti-Rac1 or anti-RhoA. In both experiments, distinct bands of the expected size were observed (Fig. 2L,M). To test the importance of the PDZ-binding domain, we transfected cells with either Vangl2-GFP or Vangl2Δ4-EGFP, and precipitated with anti-Rac1. Blotting against Vangl2 yielded a band of the correct size [Vangl2 (65 kDa) + GFP (30 kDa) = 95 kDa] in the lane with precipitate from Vangl2-GFP transfected cells, but not in the Vangl2Δ4-GFP lane (Fig. 2N). By contrast, Vangl2 overexpression did not affect the activation state of Rac1 and RhoA, as demonstrated by pull-down assays with GST-PAK1 or GST-rhotekin, effectors that binds to activated Rac1 or RhoA, respectively (Akasaki et al., 1999; Ren and Schwartz, 2000) (Fig. 2O,P). We conclude that Vangl2 can be part of the same protein complex as Rac1 and RhoA, and that the PDZ-binding domain is essential for this, at least in the case of Rac1. Furthermore, Vangl2 affects the subcellular distribution of Rac1.
Rac1 mediates the effects of Vangl2 on the actin cytoskeleton
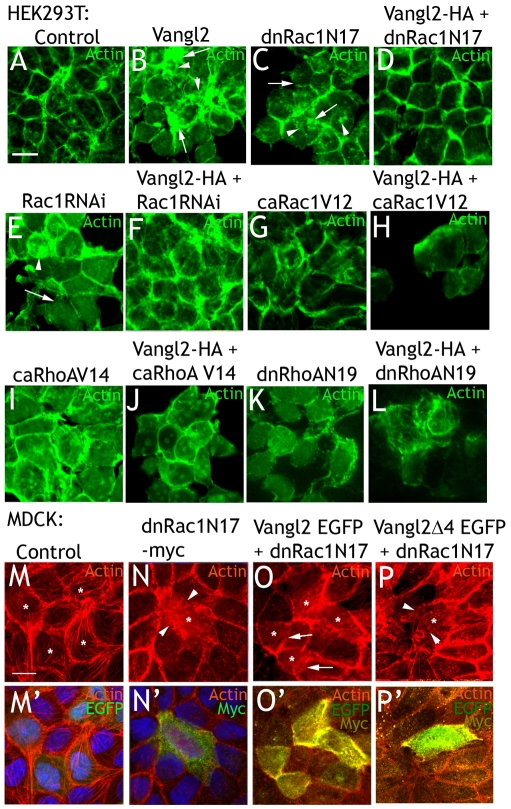
We then investigated whether the effect of Vangl2 on the actin cytoskeleton was mediated by RhoA or Rac1. HEK293T cells were transfected with control plasmid (DsRed) alone, together with Vangl2-HA, or with plasmids expressing dominant-negative Rac1 (dnRac1N17), dominant-negative RhoA (dnRhoAN19), constitutively active Rac1 (caRac1V12), or constitutively active RhoA (caRhoAV14). HA-Vangl2 was also co-transfected with dnRac1N17, dnRhoAN19, caRac1V12 or caRhoAV14. After 24 or 48 hours, the cells were fixed and the actin cytoskeleton was visualized by FITC-phalloidin. Only experiments with a transfection rate of at least 80% were further analyzed.
Cells transfected by dnRac1N17 grew in monolayers of reduced area, clusters or as single cells (Fig. 3C), and resembled Vangl2-expressing cells (Fig. 3B). Cell-cell adherence was apparently reduced compared to control transfected cells (Fig. 3, compare A,C). Cortical actin was discontinuous, and actin deposits were observed within the cells (arrowheads in Fig. 3C). Interestingly, co-transfection of dnRac1N17 and Vangl2-HA resulted in a close-to-normal actin distribution (Fig. 3D). This was not simply due to Vangl2 binding to dnRac1N17 and titrating out its effect, because Vangl2 affected cells treated with Rac1 RNAi similarly. Rac1 RNAi (co-transfected with control vector) had a similar effect as dnRac1N17 transfection (Fig. 3E). Exchanging control vector for Vangl2-HA reversed the effects of transfection with Rac1 RNAi to a large extent (Fig. 3F). Transfection of caRac1V12, caRhoAV14 or dnRhoAN19 affected actin distribution and cell shape (Fig. 3G,I,K). Co-transfection of Vangl2-HA and caRac1V12 accentuated the effects of caRac1V12 alone (Fig. 3H). On the contrary, no obvious interactions were observed when Vangl2-HA was co-transfected with either caRhoAV14 or dnRhoAN19 (Fig. 3J,L).
Fig. 3.
Rac1 mediates the effects of Vangl2 on the actin cytoskeleton. (A) Control transfected HEK293T cells. (B) Vangl2 transfection results in uneven cortical actin (arrows), and cytoplasmic actin deposits (arrowheads). (C) dnRac1N17 impairs cortical actin (arrows) and increases intracellular actin deposits (arrowheads). (D) Co-expression of Vangl2 and dnRac1N17rescues the cytoskeleton from the effects of either agent alone. (E) RNAi knockdown of Rac1 affects the actin cytoskeleton similarly to dnRac1N17. (F) Coexpression of Vangl2 and Rac1 RNAi results in close-to-normal cells. (G) caRac1V12 affects cell shape and actin distribution. (H) Vangl2 enhances the effects of caRac1V12. (I-L) caRhoAV14 (I) and dnRhoAN19 (K) affect cell shape and the cytoskeleton, but no combinatorial effect with Vangl2 is observed (J,L). (M,M′) MDCK cells transfected with EGFP control plasmid (indicated by *) display a similar actin distribution as surrounding, untransfected cells. (N,N′) A cell transfected with dnRAC1N17 (*) display aberrant shape and actin distribution (arrowheads). (O,O′) Cells co-transfected with dnRAC1N17 and Vangl2-EGFP (highly transfected cells indicated by *) are similar in shape and actin distribution as control transfected (M) and surrounding cells. This is particularly obvious in the transfected cells in the lower left (O, indicated by arrows). (P,P′) By contrast, Vangl2Δ4-EGFP is not able to negate the effects of dnRac1N17 in a highly transfected cell (*). Note the extreme paucity of cortical actin (arrowheads in P). (M-P) TRITC-phalloidin panels. (M′-P′) TRITC-phalloidin panels (M-P) merged with EGFP and Myc or DAPI channels. Scale bars: 10 μm.
MDCK cells were then transfected with control plasmid (EGFP) (Fig. 3M,M′), dnRac1N17 (Fig. 2N,N′), or dnRac1N17 in combination with either Vangl2-EGFP (Fig. 3O,O′) or Vangl2Δ4-EGFP (Fig. 3P,P′). After 36 hours, the cells were fixed and the actin cytoskeleton visualized by TRITC-phalloidin. Transfection rates were relatively low, and EGFP fluorescence was used as a transfection indicator. As was the case with HEK293T cells, MDCK cells transfected with dnRac1N17 (Fig. 3N,N′) differed markedly in their actin distribution compared with control transfected cells (Fig. 3M,M′). Cells co-transfected with dnRac1N17 and Vangl2-EGP were significantly more similar to control transfected cells, or to adjacent, untransfected cells (O,O′). Cells co-transfected with dnRac1N17 and Vangl2Δ4-EGFP mostly resembled dnRac1N17-transfected cells (Fig. 3P,P′).
In conclusion, Vangl2 overexpression and Rac1 blockade balance each other. The Vangl2 PDZ-binding domain is essential for this to occur.
Vangl2 affects cell adhesion and migration in interaction with Rac1
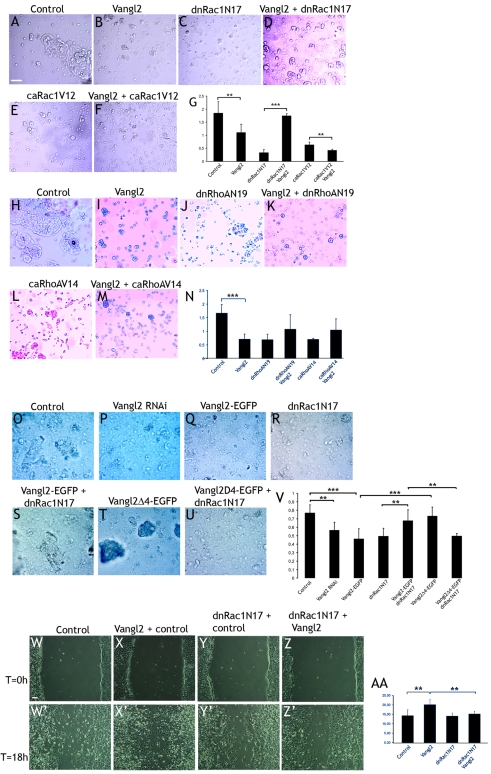
To functionally test and quantify the effects of Vangl2 on cell adherence, MDCK cells were transfected with control (DsRed) plasmid; control plasmid combined with Vangl2-HA, dnRac1N17 or caRac1V12; and Vangl2-HA combined with dnRac1N17 or caRac1V12. The cells were then transferred to hanging drop cultures. After 24-48 hours, aggregation was evaluated and quantified as an aggregation index. The higher the index, the higher the ratio of aggregated to single cells (see Materials and Methods).
Control cells displayed a high level of large and middle-sized aggregates, but single cells were also abundant (Fig. 4A). The aggregation index in Vangl2-expressing cells was 59% of control cells (P=0.027, Fig. 4B,G), indicating that Vangl2 affects cell adherence negatively. dnRac1N17 dramatically reduced the aggregation index (Fig. 4C,G). However, co-transfection of dnRac1N17 and Vangl2 resulted in a fivefold higher aggregation index (523%, P=9×10−6) (Fig. 4G), in essence bringing the aggregation index up to the same level as in control cells (Fig. 4D). caRac1V12 reduced aggregation similarly to dnRac1N17 (Fig. 4E). Co-transfection with Vangl2 enhanced the effect and slightly further reduced the aggregation index (66% of caRac1V12 alone, P=0.022) (Fig. 4F,G).
Fig. 4.
Vangl2 affects cell adhesion and migration in interaction with Rac1: aggregation assays with MDCK cells. (A-D) Control cultures display a high proportion of aggregated cells (A), whereas Vangl2 (B) or dnRac1N17 (C) significantly reduced cell aggregation. (D) Vangl2 combined with dnRac1N17 resulted in a close-to-normal level of cell aggregation. (E,F) caRac1V12 reduced cell aggregation (E), which was further enhanced by Vangl2 (F). (G) Graph with the respective aggregation indexes. (H-M) Vangl2 (I), dnRhoAN19 (J) or caRhoAV14 (L) reduces cell aggregation compared to control transfected cells (H). Addition of Vangl2 does not modify the effects of dnRhoAN19 (K) or caRhoAV14 (M). (N) Quantification of the results. (O,P) Vangl2 RNAi reduces cell aggregation compared to control transfected cells. (Q-S) Vangl2-EGFP (Q) and dnRac1N17 (R) alone reduces aggregation, whereas the combination of Vangl2-EGFP and dnRac1N17 results in close to normal aggregation levels (S). (T) Vangl2Δ4-EGFP results in normal aggregation (95% of control). (U) However, Vangl2Δ4-EGFP is not able to counter the effects of dnRac1N17. (V) Quantification of the results. (W-Z′) Vangl2 increases cell migration in a wound assay. Pictures of the same area were taken at the start of the experiment (T=0, W-Z), and after 18 hours (T=18 hours, W′-Z′). Vangl2-transfected cells (X′) colonize the inflicted wound to a higher extent than the control transfected cells (W′). Dominant-negative Rac1 blocked the effects of Vangl2 in cell migration (Z′), but did not by itself affect cell migration (Y′). (AA) Quantification of the results. Scale bars: 100 μm. Error bars in G, N, V and AA represent s.d. **P=0.03-0.05, ***P<0.01.
In a similar set of experiments with combinations of Vangl2 and dominant negative or constitutive active RhoA, Vangl2 transfection again resulted in a lower level of aggregation (Fig. 4H,I, compare). In this set of experiments, the aggregation index of the Vangl2-treated cells was 42% of control (P=0.0077) (Fig. 4N). Both dnRhoAN19 (Fig. 4J) and caRhoAV14 (Fig. 4L) reduced the aggregation index (Fig. 4N). This was not affected by co-transfection with Vangl2-HA (Fig. 4K,M,N).
In a third set of experiments, we tested the effects of Vangl2 RNAi and Vangl2Δ4-EGFP (Fig. 4O-V). Aggregation in Vangl2-EGFP-treated cells was 60% of control (Fig. 4Q, P=0.003), and in Vangl2-RNAi-treated cells 73% of control (Fig. 4P, P=0.011). Aggregation in Vangl2Δ4-EGFP-treated cells was similar to that in control transfected cells (95% of control, Fig. 4T,V). The difference between Vangl2-EGFP and Vangl2Δ4-EGFP treatment was significant (P=0.007). Co-transfection of Vangl2-EGFP and dnRac1N17 (Fig. 4S) restored normal cell aggregation (88% of control), and thus significantly differed from dnRac1N17-treated cells (P=0.029). However, cells co-transfected with dnRac1N17 and Vangl2Δ4-EGFP (Fig. 4U) displayed a reduced aggregation (65% of control), which was comparable to that of cells transfected with dnRac1N17 or Vangl2-GFP alone. The difference between Vangl2-GFP + dnRac1N17 and Vangl2Δ4-EGFP + dnRac1N17 was significant (P=0.016). In sum, these results suggest that Vangl2 gain-of-function affects cell adherence in a Rac1-dependent manner, and that the PDZ-binding domain is essential for this.
Reduced cell adherence is often paralleled by increased cellular migration. We thus examined whether Vangl2 affects migratory behavior of C17.2 neural stem cells in wound-healing assays. These cells were chosen because they do not adhere to each other as firmly as HEK293 or MDCK cells, which make it easier to scrape away cells without loosening the entire sheet. Cells were transfected with control plasmid (pcDNA3), Vangl2-HA, dnRac1N17 (combined with control plasmid) or with dnRac1N17 and Vangl2-HA. After 27 hours, a scratch wound was introduced in the cell layer with a pipette tip, and the cells were incubated for 18 hours before the results were evaluated and quantified (see Materials and Methods). In control cultures, the test area was invaded by low-to-moderate numbers of cells (Fig. 4W,W′). Vangl2-transfected cell cultures displayed 40% higher number of cells (P=0.0273) in the test area (Fig. 4X,X′). Co-transfection of Vangl2 and dnRac1N17 blocked this effect (P=0.0260, Fig. 4Z,Z′). The observations are quantified in Fig 4AA. We conclude that Vangl2 overexpression can increase cell migration and that this effect can be antagonized by dominant-negative Rac1.
Cranial neurulation is impaired in nestin-Vangl2 embryos
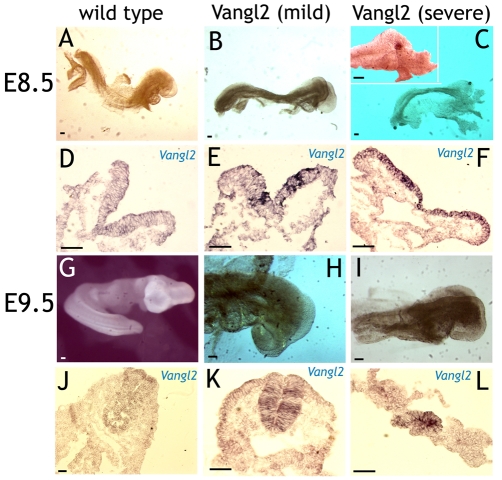
In order to test the effects of Vangl2 overexpression in vivo, a nestin-Vangl2 construct vector that directs the expression of Vangl2 to neural stem cells was generated as previously described (Shariatmadari et al., 2005). Transgenic mouse embryos obtained from pronuclear injections of nestin-Vangl2 were analyzed at embryonic day (E) 8.5 and 9.5. Nestin-Vangl2 embryos were identified by PCR and in situ hybridization with a ribo-probe complementary to Vangl2 mRNA (Fig. 5D-F,J-L). Wild-type E8.5 embryos expressed moderate levels of Vangl2 mRNA in the neural tube, except in the anterior-most and posterior-most sections (Fig. 5D). At E9.5, Vangl2 mRNA expression was found throughout the neural tube (Fig. 5J). Nestin-Vangl2 embryos displayed varying expression levels of Vangl2 mRNA. These ranged from a weak general elevation, combined with patches of markedly elevated expression (Fig. 5E,K), to a highly elevated Vangl2 overexpression throughout the neural tube and/or plate except at the lateral-most edges (Fig. 5F,L).
Fig. 5.
Cranial neurulation is impaired in nestin-Vangl2 embryos. (A-C) E8.5 wild-type and Vangl2-overexpressing embryos. Mildly affected transgenic embryos (B) were slightly smaller than wild-type embryos (A), with somewhat loose bodies and partly splayed out neural folds (E). Severely affected transgenic embryos (C) displayed small, flaccid bodies with the neural folds splayed out (F). (D-F) Transversal sections from the rostral neural tube of embryos A-C showing expression of Vangl2 mRNA. Expression levels correlated well with the severity of the phenotype. (G-I) E9.5 embryos. Cranial neural fold closure was completed in wild-type embryos (G), whereas transgenic embryos displayed an open cranial neural tube (H,I). Transgenic embryos were also smaller in size than their wild-type littermates. (J-L) Vangl2 mRNA expression in embryos G-H. Scale bars: 50 μm.
Mildly affected E8.5 nestin-Vangl2 transgenic embryos were similar or somewhat smaller in size than wild-type littermates (Fig. 5A,B, compare). Their neural folds were more splayed out, and they appeared to have less rigid bodies than wild-type embryos (Fig. 5A,B, compare with 5D,E). Severely affected E8.5 embryos were smaller in size and had less rigid bodies than wild-type embryos (Fig. 5C). Neural tube closure, particularly in the cranial compartment, was impaired, with the neural folds displaying little or no elevation (Fig. 5F). Nestin-Vangl2 embryos investigated at E9.5 were smaller than their wild-type littermates. Wild-type littermates had completed cranial neural tube closure (Fig. 5G), but nestin-Vangl2 embryos still displayed open cranial neural folds (Fig. 5H,I). We conclude that the nestin-Vangl2 embryos display an impaired cranial neurulation, a phenotype similar to that of nestin-Wnt7a transgenic embryos. This is in line with the effects of Wnt7a being partly mediated by Vangl2, as we have suggested previously (Shariatmadari et al., 2005).
Cytoskeletal components of adherens junctions are mis-distributed in nestin-Vangl2 embryos
The distribution of actin microfilaments in transversal sections of neural tubes from E8.5 and E9.5 embryos was investigated using FITC-labeled phalloidin. In wild-type embryos (n=3 each age group), actin microfibers were present as weak circumferential rings in the cortex of each cell (Fig. 6A,E, enlarged in B,F). The apical ends of the neural tube cells, facing the lumen, displayed clear actin enrichment, indicative of adherens junctions. These could be identified as ring-like structures in more oblique cut fields of the sections (arrow in Fig. 6B,F). The neural tube cells of the nestin-Vangl2 embryos (n=3 each age group) displayed more diffuse cortical actin than their wild-type littermates (Fig. 6C,G, enlarged in D,H). Actin enrichment over the adherens junctions was absent or very low. In the cases in which actin enrichment was observed, actin was present in fragmentary patches (Fig. 6D,H, arrowheads). Furthermore, the typical ring-like structures of adherens junctions were not observed.
Fig. 6.
Adherens junctions are aberrant in nestin-Vangl2 embryos. (A-H) Actin distribution over wild-type and nestin-Vangl2 embryos of the indicated ages. Wild-type embryos display clear actin enrichment over the adherens junctions (arrows in A,B,E,F). High magnification reveals ring-shaped adherens junctions (arrows in B,F). Nestin-Vangl2 embryos display stretches without actin enrichment (arrows in C,D,G) and patches of actin (arrowheads in D,H). (I-P) Distribution of β-catenin and N-cadherin correlates with the actin distribution, with clear enrichment over the adherens junctions in wild-type embryos (I,J,M,N), and no enrichment and a patchy distribution in the nestin-Vangl2 embryos (K,L,O,P). B,F,J,N and D,H,L,P are enlarged sections from A,E,I,M and C,G,K,O, respectively. Scale bars: 50 μm.
The distribution of β-catenin and N-cadherin in wild-type embryos (n=2 each age group) overlapped with that of actin, as expected (Fig. 6I,M, enlarged in J,N). In nestin-Vangl2 embryos (n=2 each age group), enrichment over the adherens junctions was absent or very low, similar to that observed for actin, suggesting a functional impairment of the adherens junctions (Fig. 6K,O, enlarged in L,P). This was further supported by observations of the neural tube luminal wall: in wild-type embryos it is smooth, but in nestin-Vangl2 transgenic embryos it is irregular, with groups of cells protruding into the lumen (Fig. 6C,D,G, arrows). Individual cells also appeared more rounded in the transgenic than in the control embryos, suggesting a defect in cell-cell adherence (compare cell shapes in Fig. 6L,P with 6J,N). The embryos thus resembled a previously described telencephalic β-catenin knockout (Junghans et al., 2005).
The cellular distribution of Rac1 is altered in the nestin-Vangl2 embryos
Rac1 immunolabeling was detected over the adherens junctions in neural tubes of wild-type E8.5 (n=4) and E9.5 (n=4) embryos (Fig. 7A,B, arrows). In addition, the E8.5 embryos frequently displayed strong nuclear and/or cytoplasmic Rac1 labeling, mostly in cell soma adjacent to the lumen (Fig. 7A, small arrows). E8.5 nestin-Vangl2 (n=4) embryos displayed prominent nuclear and/or cytoplasmic Rac1 labeling in cell soma scattered over the neural tube. Labeling over the adherens junctions was reduced, displayed paucity or was completely absent (Fig. 7C, arrowhead). E9.5 nestin-Vangl2 embryos (n=4) displayed a reduced level of Rac1 labeling over the adherens junctions (Fig. 7D, arrowhead). Apart from the adherens junctions, wild-type embryos displayed distinct Rac1 labeling at the neural tube cell membranes. In nestin-Vangl2 embryos, labeling of the neural tube cells was more diffuse (compare Fig. 7B and 7D).
Fig. 7.
Rac1 distribution is altered in nestin-Vangl2 embryos. (A,B) Enrichment of Rac1 over the adherens junctions of E8.5 (A) and E9.5 (B) wild-type embryos (large arrows). Small arrows in A point at cytoplasmic and/or nuclear distribution close to the lumen. (C,D) Rac1 enrichment over the adherens junctions of nestin-Vangl2 embryos is either absent (arrowheads) or highly reduced. Scattered cytoplasmic and/or nuclear Rac1 labeling over the neural tube is observed in E8.5 nestin-Vangl2 embryos (small arrows in C). Scale bar: 50 μm.
Vangl2 loss-of-function in the neural tube is correlated with an impaired cytoskeleton
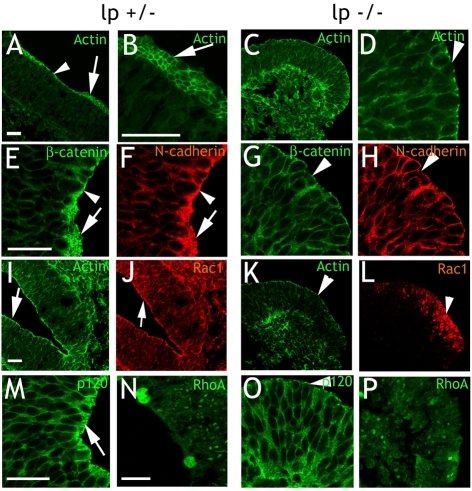
The effects of Vangl2 loss-of-function was studied in loop-tail (lpstudied in loop-tail/studied in loop-tail) mouse embryos, which are homozygous for a Vangl2 loss-of-function mutation (Kibar et al., 2001; Murdoch et al., 2001a). Wild-type mice express Vangl2 throughout the neural tube from E7.5 and onwards (Torban et al., 2007).
First, we compared the distribution of cytoskeletal components in transversal sections of neural tubes from E9.5 wild-type (lp+/+), lp+/− (n=2) and lp−/− (n=2) embryos. FITC-phalloidin labeling of actin was similar in wild-type and lp+/− embryos, and so lp+/− embryos were therefore used as controls. Actin staining in control embryos was as described above. Oblique cut sections with the ring-like adherens junctions apparent were frequently observed (Fig. 8A,B). The most rostral sections of lp−/− neural tubes displayed a normal actin distribution. In sections from hindbrain and caudally we observed lower actin levels over the adherens junctions than in control embryos (Fig. 8C,D,K, compare with 8A,B,I). Phalloidin staining was uneven or punctuate (Fig. 8D). Other cytoskeletal components associated with adherens junctions (β-catenin, N-cadherin, and p120-catenin) displayed a similar distribution as actin, with a clear enrichment in control embryos (Fig. 8E,F,M). In lp−/− embryos, enrichment of these cytoskeletal components over the adherens junctions was less prominent, or absent, in the caudo-lateral parts of the neural tube (Fig. 8G,H,O).
Fig. 8.
The cytoskeleton is impaired in loop-tail embryos. (A,B) Adherens junctions in control (lp+/−) embryos display high actin enrichment (arrowhead in A). In oblique cut sections, the ring-shaped structure of adherens junctions is obvious (arrows in A,B). (C,D) Loop-tail (lp−/−) embryos displays adherens junctions with low levels of unevenly distributed actin (arrowhead in D). Ring-like adherens junctions were not found. (E-H,M,O) In control embryos, double labeling of β-catenin (E) and N-cadherin (F) demonstrate enriched labeling (arrowheads) and ring-like structures (arrows). Loop-tail embryos display weak labeling of β-catenin (G) and N-cadherin (H). Enrichment is frequently absent (arrowheads), at level with the rest of the neural tube. p120-catenin is enriched over the adherens junctions in control embryos (arrow in M), but not in loop-tail embryos (arrowhead in O). (I-L) Control embryos (I,J) display focused Rac1 labeling (J, arrow) that overlap with actin enrichment (I, arrow) over the adherens junctions. Rac1 in loop-tail embryos (L, arrowhead) is not focused to adherens junctions but evenly distributed over the cytoplasm of cells throughout the neural tube. It does not overlap with actin (K, arrowhead). (N,P) RhoA labeling is similar in control (N) and loop-tail (P) embryos. lp, loop-tail. Scale bars: 50 μm.
The loop-tail embryos complete cranial neurulation, but the neural tube remains open from the hindbrain and further caudally. The observed effects on the adherens junctions correlated with this, with no aberrances rostral of the hindbrain. Caudal of this, adherens junctions displayed aberrant distribution of cytoskeletal components. Ring-like adherens junctions were observed in the rostral part of the lp−/− embryos, but not in any of the caudo-lateral sections (Fig. 8C,D,G,H,K,O). Moreover, cells in the lp−/− neural tubes tended to be less orderly arranged, and displayed a more rounded shape than those of controls (particularly evident in Fig. 8G,H).
The distribution of Rac1 was altered in loop-tail embryos
Rac1 immunolabeling was similar in wild-type and lp+/− embryos as expected (Fig. 8J). The distribution of Rac1 was normal in sections of lp−/− embryos rostral of the hindbrain. More caudo-laterally intense Rac1 labeling was observed in the cytoplasm of cells adjacent to the adherens junctions, suggesting that the distinct Rac1 localization was lost (Fig. 8L). Like in nestin-Vangl2 embryos, Rac1 labeling in lp−/− mutants was more dispersed across the neural tube (compare with Fig. 7). Immunolabeling with a second Rac1 antibody yielded a similar diffuse labeling in lp−/− mutants (data not shown). In contrast to Rac1, the distribution of RhoA was unaltered in lp−/− embryos (Fig. 8N,P).
In summary, with the exception of the rostral-most neural tube, distribution of cytoskeletal components and Rac1 was altered over the adherens junctions of E9.5 lp−/− embryos. This complements our gain-of-function data and demonstrates that Vangl2 is required for the adherens junctions formation in vivo.
Discussion
Vangl2 is an essential component of the Wnt/PCP pathway, which acts via activation of the GTPases RhoA and Rac1 (Habas et al., 2003). Here, we used gain-of-function and loss-of-function approaches, both in vitro and in vivo, to demonstrate that Vangl2 affects the cytoskeleton and cell adhesion via Rac1.
Both decreases and increases in Vangl2 levels resulted in aberrant distribution of actin and other cytoskeletal components. Vangl2 is a transmembrane protein and ectopically expressed Vangl2 was located to the cell membrane, but also to the cytoplasmic compartment (Fig. 1B′,H′,I′), where we also observed increased levels of Rac1 granules. Furthermore, Vangl2 knockdown resulted in a diffuse Rac1 labeling over the cytoplasm (Fig. 2F,H). Immunoprecipitation demonstrated that Vangl2 is present in complex with Rac1 and RhoA (Fig. 2L,M,N), and that the PDZ-binding domain of Vangl2 is essential for this (Fig. 2N). However, the overall levels of Rac1 and Rac1 activation were not affected by Vangl2 (Fig. 2O,P). On the basis of these observations, we propose a model in which Vangl2 does not affect Rac1 activity directly, but recruits Rac1 to locally increase its concentration at the membrane or other cellular compartments.
We suggest that Vangl2, which is located at adherens junctions (Fig. 1O′), is required for the proper recruitment of Rac1 to the adherens junction. Active Rac1 is known to promote actin polymerization at the cell periphery (Hall and Nobes, 2000). The crucial function of Rac1 in the actin cytoskeleton and cell adherence has been demonstrated previously (Jou and Nelson, 1998), and is also confirmed in our experiments with RNAi knockdown of endogenous Rac1 or dominant-negative Rac1. Importantly, previous studies have demonstrated that the actin cytoskeleton is disrupted both by increased and decreased Rac1 signaling (Jou and Nelson, 1998). In line with these findings, we demonstrate that both Vangl2 loss-of-function and gain-of-function result in disruption of the cytoskeleton and decreased cell adherence. Furthermore, our rescue experiments support an interaction between Vangl2 and Rac1. dnRac1N17 is believed to inhibit endogenous Rac1 by competitive inhibition (Coso et al., 1995; Feig and Cooper, 1988), and dnRac1 is thus functionally identical to Rac1 knockdown. Both these conditions disrupt the actin cytoskeleton (Fig. 3C,E,N) and cause adhesion defects (Fig. 4B,I,P,Q). These alterations were rescued by simultaneous Vangl2 overexpression, resulting in an almost normal actin distribution (Fig. 3D,F,O) and cell adherence (Fig. 4D,S). We suggest that Vangl2 overexpression balances the effects of dominant-negative Rac1 or RNAi knockdown by increased recruitment of endogenous Rac1, thus restoring Rac1 levels locally in the cell cortex. This is further supported by our finding that overexpression of Vangl2Δ4-GFP affects neither the actin cytoskeleton (Fig. 1M) nor cell adherence (Fig. 4T). The Vangl2Δ4-GFP mutant lacks the PDZ-binding domain, and immunoprecipitation experiments demonstrate that it is incapable of binding Rac1 (Fig. 2N). In line with this, Vangl2Δ4-GFP fails to rescue the actin cytoskeleton (Fig. 3P) and cell adherence (Fig. 4U) from the effects of dnRac1N17. However, the exact mode of interaction between Vangl2 and Rac1 remains to be elucidated. Other molecules might be involved, for example Scrib, which can bind Vangl2 via its PDZ-binding domain (Kallay et al., 2006).
Many molecules can contribute to membrane localization of Rac1 (Williams, 2003). However, we demonstrate that Vangl2 is present in HEK293T and MDCK cells (Fig. 1E,N), and that Vangl2 knockdown disrupts the actin cytoskeleton (Fig. 1F,P). Furthermore, Vangl2 is known to be expressed in the neural tube cells of wild-type mouse embryos. We suggest that Vangl2 in the neural tube contributes to maintenance of the actin cytoskeleton via Rac1, because Vangl2 loss of function in the lp−/− mutant is correlated with actin filament collapse (Fig. 8C,D) and altered Rac1 distribution (Fig. 8L) in the caudal neural tube. However, the mis-localization of Rac1 could also be secondary to altered cell polarity. More rostral sections of the neural tube in lp−/− mutants displayed normal adherens junctions. This is not surprising because cranial neurulation, which depends on the integrity of adherens junctions (Ybot-Gonzalez and Copp, 1999), proceeds normally in the lp−/− embryos. Instead, the lp−/− embryos display craniorachischisis, a failure to close the neural tube from the hindbrain and caudally. This phenotype is a consequence of an impaired convergent extension (Darken et al., 2002; Goto and Keller, 2002). Interestingly, it has been suggested that Vangl2 influences convergent extension via regulation of cell adhesion (Ciruna et al., 2006).
In conclusion, we demonstrate that Vangl2 affects the cytoskeleton and cell adhesion in epithelial cell lines and in the embryonic neural plate and/or tube. Moreover, we identify the small GTPase Rac1 as a key player in this process. Importantly, both a defective recruitment (Rac1 and/or Vangl2 loss-of-function) and an excessive recruitment (Rac1 and/or Vangl2 gain-of-function) lead to cytoskeletal abnormalities and impaired adhesion. Although further studies are needed to elucidate this mechanism on a structural level, our results indicate that the precise regulation of Vangl2 levels and its interaction with Rac1 is of key importance for the appropriate regulation of cell adhesion and neural tube development.
Materials and Methods
Generation of the Vangl2-HA and nestin-Vangl2 constructs.
A 1566-bp fragment spanning the open reading frame of Vangl2 and flanked by XhoI and HindIII sites was generated by PCR from a cDNA clone containing the Vangl2 coding sequence [I.M.A.G.E. Consortium (LLNL) cDNA CloneID 6509008 (Lennon et al., 1996)] purchased from RZPD (www.rzpd.de; RZPD CloneID IMAGp998J1714075Q3). It was then inserted into the XhoI and/or HindIII site of the pcDNA3-HA expression vector or the NotI site of the human nestin (hnestin) 1852 vector (Lothian and Lendahl, 1997; Shariatmadari et al., 2005). The (human) Vangl2-EGFP and Vangl2D4-EGFP constructs were kind gifts from Lelita T. Braiterman (Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD) (Kallay et al., 2006).
Cell cultures and transfection experiments
HEK293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; GIBCO, cat no. 11971) supplemented with 10% fetal bovine serum (FBS) and antibiotics; MDCK cells in MEM (GIBCO, cat. no. 21090) supplemented with 10% FBS, 2 mM L-glutamine, 1% non-essential amino acids, and antibiotics; and C17.2 cells in DMEM (GIBCO, cat. no. 41966) supplemented with 10% FBS, 5% horse serum, 2 mM L-glutamine and antibiotics (all supplements from GIBCO). HEK293T and MDCK cells were kindly provided by Anita Aperia (Department of Woman and Child Health, Karolinska Institutet), and C17.2 cells by Eric Herlenius (Department of Woman and Child Health, Karolinska Institutet). RNAi hairpins (Stealth siRNA duplex oligoribonucleotides) complementary to human Rac1 and Vangl2 mRNAs were designed by Invitrogen. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Immunocytochemistry
HEK293T, MDCK or C17.2 cells were fixed with 4% paraformaldehyde, permeabilized and blocked in 7% non-fat dry milk and 0.1% Triton X-100 in PBS. Primary antibodies were incubated at 4°C overnight, then rinsed three times in PBS. Secondary antibodies were diluted in PBS. Primary antibodies: anti-β-catenin (BD Biosciences, San Jose, CA) at 1:200 dilution; anti-N-cadherin at 1:100 dilution, anti-Rac1 at 1:200 dilution, anti-RhoA at 1:50 dilution (all from Santa Cruz Biotechnology, Santa Cruz, CA); anti-p120 catenin at 1:100 dilution (BD Biosciences, Franklin Lakes, NJ); anti-Vangl2 (R&D Systems); and anti-HA (Sigma-Aldrich, St Louis, MO) at 1:300 dilution. Endogenous actin was visualized with FITC- or TRITC-phalloidin (Sigma-Aldrich) diluted to 50 μg/ml in PBS.
Actin rating
Actin distribution in HEK293T and MDCK cells was blindly rated by three observers. For HEK293T cells, cortical actin, intracellular actin and general cell shape were given a score of 0-3. A high score indicated a high aberration from a hypothetical normal appearance. The three ratings for each cell were summed, and 1 was added, yielding a cell score of between 1 and 10. The ratings were averaged, and a t-test performed. For MDCK cells, the observers individually rated cortical actin, stress fibers and cell shape in comparison to the adjacent, non-transfected cells. The results were then treated similarly to results for the HEK293T cells.
Aggregation assay
MDCK cells were cultured until 70% confluency and then transfected using Lipofectamine 2000. After 4 hours, the cells were scraped off the culture dishes, washed in new medium, counted and transferred in drops of 30 μl (1000 cells/μl) to the inside of a 24-well plate-lid. With PBS in the bottom of the wells, the hanging drops were incubated for 24-48 hours. The drops were then pipetted up and down 10 times with a 200 μl tip and analyzed in a light microscope. For quantification, representative micrographs from a single experiment were used. Quantification of the first two sets of experiments (Fig. 4G,N) was performed as follows: The pictures were overlaid with a 48-square grid. For each picture, the number of squares containing aggregates with at least four cells was divided by the number of squares containing only single cells or aggregates with less than four cells. This yielded an aggregation index. The aggregation indexes from four micrographs per experimental condition were averaged and used for comparison. A standard t-test was performed. For the third set of aggregation assays a modified method was employed because the cell density was higher in all experimental conditions. The micrographs were overlaid with an 80-square grid. Only squares containing cell aggregates with four or more cells that stretched into a neighboring square were counted. The data were then treated similarly to treatment in the first two experimental sets.
Wound assay
Subconfluent C17.2 cells were transfected as above and then allowed to reach 100% confluency. The cells were then treated with 10 μM mitomycin C (Sigma-Aldrich) for 3 hours to arrest the cell cycle. A wound was made through the cells using a 200 μl pipette tip. Medium was changed to serum-reduced (1% FBS) to keep the cells from dividing, and a line was drawn underneath the culture dish perpendicular to the scratch. Pictures were taken just above or below the line under a light phase-contrast microscope, immediately and after 18 hours. For quantification of the results, a representative area was chosen in micrographs taken at 0 hours, and the distance between the edges of the wound were measured. The same area in the micrographs was identified after 18 hours. The measured distance was used as a baseline which, combined with a fixed height, yielded a rectangular field. The number of cells within the field was calculated and then divided by the area.
Immunoprecipitation
Cells grown on a 10-cm culture dish were extracted for 15 minutes in ice-cold lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM sodium chloride, 0.1% SDS, 1 mM EDTA, 1× Roche protease inhibitor cocktail). Then, extracts were cleared by centrifugation at 15,000 g for 5 minutes at 4°C and stored at −80°C until use. The extracts were incubated with anti-HA (Abcam), anti-Rac1 (Upstate) or anti-RhoA (Santa Cruz Biotechnology) antibody for 1 hour in an ice bath. Immunoprecipitates were collected on Protein G Sepharose Fast Flow beads (Amersham Biosciences) by overnight rotation, washed four times with lysis buffer and resuspended in 2× Laemmli sample buffer. Samples were then subjected to SDS-PAGE followed by western blot analysis using anti-HA (Abcam), anti-Vangl2 (R&D Systems), anti-Rac1 (Upstate) or anti-RhoA (Santa Cruz Biotechnology) antibodies. Western blotting was performed as described previously (Bryja et al., 2007).
Pull-down assay
Glutathione-S-transferase (GST)—p21-activated kinase (PAK)—Cdc42/Rac interactive binding domain (CRIB), and GST-RHOtekin recombinant proteins coupled to Sepharose beads were prepared as described previously (Edlund et al., 2002). GST-PAK-CRIB, and GST-RHOtekin pull-down assays for detection of activated Rac1 and RhoA, respectively, were performed as follows: Cells were washed with ice-cold PBS and subsequently allowed to lyse in ice-cold lysis buffer (10 mM Tris-HCl pH 7.5, 110 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 1% Triton X-100, 0.1% SDS, 20 mM β-glycerolphosphate, 1 mM dithiotreitol, complete protease inhibitors) for 5 minutes. Crude cell lysates were spun down in prechilled tubes at 16,000 g for 5 minutes at 4°C. Supernatants (5% saved as input) were supplemented with bait proteins coupled to GST beads, and tubes were incubated rotating end-over-end at 4°C for 15 minutes. Beads were washed three times with washing buffer (lysis buffer without SDS and protease inhibitors) on ice and subsequently mixed with 2× Laemmli buffer. Each sample was boiled for 5 minutes before loading on SDS-PAGE.
Vangl2 RT-PCR in HEK293T and MDCK cells
Vangl2 gene expression was investigated by real-time RT-PCR (iQ5 Real-Time Detection System, Bio-Rad Laboratories, Sundbyberg, Sweden) in control transfected and Vangl2-overexpressing cells. The iScript One-Step RT-PCR kit (Bio-Rad) with SYBR green was utilized for the reactions according to the manufacturer's instructions. All reactions were performed both with and without added reverse transcriptase. The end product was run on a 2% agarose gel.
Identification of transgenic, loop-tail embryos and in situ hybridization
The expression cassette, hnestin 1852/tk promoter Vangl2 ORF was used for pronuclear injection of fertilized mouse oocytes. Oocytes were used to generate transgenic mice using standard techniques. Pregnant dams with embryos of E8.5 or E9.5 were sacrificed by spinal dislocation, and the embryos were rapidly dissected out. Yolk sac DNA was used to genotype transgenic and loop-tail embryos. PCR to identify transgenics was performed with a sense primer complementary to human nestin intron 2 combined with an antisense primer complementary to the Vangl2 ORF. Loop-tail embryos were identified by PCR performed with primers against a microsatellite sequence for the lpt locus. Vangl2 mRNA expression was investigated by digoxigenin in situ hybridization. A standard protocol from the manufacturer (Roche) was used with some modifications. The riboprobe template was generated by PCR from the Vangl2 CDS (16-455 bp). Animals were treated according to European Communities Council Guidelines (Directive 86/609/EEC).
Immunohistochemistry
Immunohistochemistry was performed as previously described (Shariatmadari et al., 2005). Primary antibodies used were anti-β-catenin (BD Biosciences) at a 1:200 dilution, anti-N-cadherin at a 1:100 dilution, anti-Rac1 at a 1:200 dilution, anti-Scrb1 at a 1:50 dilution (all from Santa Cruz Biotechnology) and anti-Rac1 (Upstate) at a 1:100 dilution.
Acknowledgments
We wish to thank Ola Hermanson for valuable comments on the manuscript, KCTT for excellent technical support, Eva Lundberg for secretarial assistance, and Beston Nore (KFC, Enheten för molekykär cellbiologi och genterapivetenskap. Karolinska Institutet), Alan Hall (Memorial Sloan-Kettering Cancer Center, NY) and Lelita T. Braiterman for plasmids. This study was supported by grants from the Swedish Foundation of Strategic Research, Swedish Research Council, Barncancerfonden, Stiftelsen Frimurare Barnhuset, and Sällskapet Barnavård. G.S was supported by Knut & Alice Wallenberg Foundation (KAW 2008.0149), Swedish Research Council (K2008-68P-20810-01-4, K2008-68X-20805-01-4) and Åhlén Foundation. V.B. is supported by the EMBO Installation Grant programme and the grant of Ministry of Education, Youth and Sports of the Czech Republic (MSM0021622430). Z.H. is supported by the European Research Universities (LERU) and T.R. by a grant from Märta och Gunnar V. Philipsons Stiftelse. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. E.A. was supported by the Swedish Research Council (VR2008:2811 and DBRM), Norwegian Research Council and Karolinska Institutet. Deposited in PMC for release after 12 months.
References
- Akasaki T., Koga H., Sumimoto H. (1999). Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J. Biol. Chem. 274, 18055-18059 [DOI] [PubMed] [Google Scholar]
- Braga V. M., Machesky L. M., Hall A., Hotchin N. A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 137, 1421-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V., Schulte G., Rawal N., Grahn A., Arenas E. (2007). Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci. 120, 586-595 [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F., Concha M. L., Takeuchi M., Ueno N., Wilson S. W., Tada M. (2003). Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development 130, 4037-4046 [DOI] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M., Schier A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O. A., Chiariello M., Yu J. C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J. S. (1995). The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81, 1137-1146 [DOI] [PubMed] [Google Scholar]
- Darken R. S., Scola A. M., Rakeman A. S., Das G., Mlodzik M., Wilson P. A. (2002). The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 21, 976-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund S., Landstrom M., Heldin C. H., Aspenstrom P. (2002). Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell 13, 902-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M., Weber U., Strutt D. I., Mlodzik M. (2000). Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 10, 979-988 [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. (1988). Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol. Cell. Biol. 8, 3235-3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R. S., Quinlan M. P. (1998). Identification of a novel mechanism of regulation of the adherens junction by E1A, Rac1, and cortical actin filaments that contributes to tumor progression. Cell Growth Differ. 9, 905-918 [PubMed] [Google Scholar]
- Goto T., Keller R. (2002). The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 247, 165-181 [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Nobes C. D. (2000). Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355, 965-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T. S., Nelson W. J. (1998). Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142, 85-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans D., Hack I., Frotscher M., Taylor V., Kemler R. (2005). Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev. Dyn. 233, 528-539 [DOI] [PubMed] [Google Scholar]
- Kallay L. M., McNickle A., Brennwald P. J., Hubbard A. L., Braiterman L. T. (2006). Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J. Cell Biochem. 99, 647-664 [DOI] [PubMed] [Google Scholar]
- Katoh M. (2002). Structure and expression of Strabismus 1 gene on human chromosome 1q21-q23. Int. J. Oncol. 20, 1197-1203 [PubMed] [Google Scholar]
- Kibar Z., Vogan K. J., Groulx N., Justice M. J., Underhill D. A., Gros P. (2001). Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251-255 [DOI] [PubMed] [Google Scholar]
- Kim G. H., Han J. K. (2005). JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev. Dyn. 232, 958-968 [DOI] [PubMed] [Google Scholar]
- Lee J. M., Dedhar S., Kalluri R., Thompson E. W. (2006). The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 172, 973-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon G., Auffray C., Polymeropoulos M., Soares M. B. (1996). The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33, 151-152 [DOI] [PubMed] [Google Scholar]
- Lothian C., Lendahl U. (1997). An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur. J. Neurosci. 9, 452-462 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Sans N., Huss D., Kach J., Dickman J. D., Forge A., Rachel R. A., Copeland N. G., Jenkins N. A., Bogani D., et al. (2006). Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265-5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J. N., Doudney K., Paternotte C., Copp A. J., Stanier P. (2001a). Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 10, 2593-2601 [DOI] [PubMed] [Google Scholar]
- Murdoch J. N., Rachel R. A., Shah S., Beermann F., Stanier P., Mason C. A., Copp A. J. (2001b). Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics 78, 55-63 [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235-1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio N., Feiguin F., Boutros M., Eaton S., Mlodzik M. (1999). The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 18, 4669-4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P. (1999). Rac regulates the stability of the adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene 18, 6434-6442 [DOI] [PubMed] [Google Scholar]
- Ren X. D., Schwartz M. A. (2000). Determination of GTP loading on Rho. Methods Enzymol. 325, 264-272 [DOI] [PubMed] [Google Scholar]
- Schambony A., Wedlich D. (2007). Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12, 779-792 [DOI] [PubMed] [Google Scholar]
- Shariatmadari M., Peyronnet J., Papachristou P., Horn Z., Sousa K., Arenas E., Ringstedt T. (2005). Increased Wnt levels in the neural tube impair the function of adherens junctions during neurulation.. Mol. Cell. Neurosci. 30, 437-451 [DOI] [PubMed] [Google Scholar]
- Strutt D. I., Weber U., Mlodzik M. (1997). The role of RhoA in tissue polarity and Frizzled signalling. Nature 387, 292-295 [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Nakabayashi J., Sakaguchi T., Yamamoto T. S., Takahashi H., Takeda H., Ueno N. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674-679 [DOI] [PubMed] [Google Scholar]
- Torban E., Wang H. J., Patenaude A. M., Riccomagno M., Daniels E., Epstein D., Gros P. (2007). Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr. Patterns 7, 346-354 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. (2003). Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680-685 [DOI] [PubMed] [Google Scholar]
- Williams C. L. (2003). The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell Signal 15, 1071-1080 [DOI] [PubMed] [Google Scholar]
- Wong L. L., Adler P. N. (1993). Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123, 209-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P., Copp A. J. (1999). Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev. Dyn. 215, 273-283 [DOI] [PubMed] [Google Scholar]