Abstract
Introduction:
Emerging research suggests potential effects of the menstrual cycle on various aspects of smoking behavior in women, but results to date have been mixed. The present study sought to explore the influence of menstrual cycle phase on reactivity to smoking in vivo and stressful imagery cues in a sample of non–treatment-seeking women smokers.
Methods:
Via a within-subjects design, nicotine-dependent women (N = 37) participated in a series of four cue reactivity sessions, each during a distinct biologically verified phase of the menstrual cycle (early follicular [EF], mid-follicular [MF], mid-luteal [ML], and late luteal [LL]). Subjective (Questionnaire of Smoking Urges–Brief; QSU-B) and physiological (skin conductance and heart rate) measures of craving and reactivity were collected and compared across phases.
Results:
Subjective reactive craving (QSU-B) to smoking in vivo cues varied significantly across the menstrual cycle (p = .02) and was higher in both EF and MF phases versus ML and LL phases, but this finding was not sustained when controlling for reactivity to neutral cues. Heart rate reactivity to stressful imagery cues (p = .01) and skin conductance reactivity to smoking in vivo cues (p = .05) varied significantly across the menstrual cycle upon controlling for reactivity to neutral cues, with highest reactivity during the MF phase.
Discussion:
Menstrual cycle phase may have an effect on reactivity to smoking-related and stressful cues among women smokers. These findings contribute to an expanding literature, suggesting menstrual cycle effects on smoking behaviors in women.
Introduction
One hypothesized contributor to gender differences in smoking-related behaviors is the potential influence of hormonal variations during the menstrual cycle in women (Allen, Bade, Center, Finstad, & Hatsukami, 2008; Allen, Hatsukami, Christianson, & Brown, 2000). Higher smoking rates have been observed during the luteal (i.e., premenstrual) phase in several (Craig, Parrott, & Coomber, 1992; Marks, Hair, Klock, Ginsburg, & Pomerleau, 1994; Mello, Mendelson, & Palmieri, 1987; Snively, Ahijevych, Bernhard, & Wewers, 2000; Steinberg & Cherek, 1989) but not all (Allen, Hatsukami, Christianson, & Nelson, 1996; Pomerleau, Cole, Lumley, Marks, & Pomerleau, 1994) studies. Additionally, some studies have suggested that nicotine withdrawal and craving may be more severe during the luteal phase (see Carpenter, Upadhyaya, LaRowe, Saladin, & Brady, 2006, for review).
Minimal research in this area has focused on cue reactivity, a laboratory procedure used to study responses to smoking-related and negative affect/stress cues. Only one prior study has explored smoking cue reactivity and menstrual cycle (Franklin et al., 2004). In this study of 41 treatment-seeking women smokers, subjective craving in response to smoking-related cues was greater among women in the luteal phase (n = 24) than those in the follicular phase (n = 17). While these findings suggest that women in the luteal phase may be especially responsive to smoking-related cues, they should be interpreted with caution because menstrual phase was assessed retrospectively via self-report alone.
Given the suggestive results of prior investigations, the present study sought to explore the influence of menstrual cycle phase on smoking cue reactivity with additional methodological rigor, including prospective biological verification of phase, inclusion of both smoking-related and stressful cues, and use of multiple measures of cue reactivity. It was hypothesized that women would be more reactive to cues during the luteal phase of the menstrual cycle.
Methods
Participants
Non–treatment-seeking female smokers (≥10 cigarettes/day) ages 18–40 years, having regular menstrual cycles between 25 and 35 days and not taking hormonal contraception or replacement, were recruited from the community. Current major comorbid psychiatric or substance use disorders (including premenstrual dysphoric disorder) were exclusionary (First, Spitzer, Gibbon, & Williams, 2002).
Procedure
Eligible participants attended four laboratory-based cue reactivity sessions, timed to coincide with four distinct menstrual cycle phases: (a) early follicular (EF), timed 1–3 days following the onset of menses (see below); (b) mid-follicular (MF), 7–10 days following the onset of menses; (c) mid-luteal (ML), 6–9 days following ovulation; and (d) late luteal (LL), 10–13 days following ovulation. Phase distinctions were based on associated changes in ovarian hormones within each (Guyton & Hall, 2000; Marks, Pomerleau, & Pomerleau, 1999), though prior related research has yielded no definitive phase definitions (Carpenter et al., 2006). To minimize potential order effects, the testing sequence was randomized to four sequences: EF first (n = 11), MF first (n = 11), ML first (n = 9), and LL first (n = 6). Via telephone contact, participants reported onset of menses; thereafter, EF and MF phases were identified. Home testing kits (Clearplan Easy; Unipath Diagnostics Company, Englewood Cliffs, NJ) were used to monitor for luteinizing hormone level surge, which denoted ovulation and allowed for the identification of ML and LL phases.
After smoking 45–60 min prior to the procedure, participants were exposed to four cues (90 s each, counterbalanced and separated by 10-min nature slide show). Active cues consisted of (a) in vivo manipulation of smoking paraphernalia and (b) imagery-based script of personalized stress-inducing event. Inactive/control cues consisted of (a) in vivo manipulation of pencil and eraser and (b) imagery-based script of relaxing event. Our prior work has shown these active cues to reliably induce craving relative to control cues (Carpenter et al., 2009; LaRowe, Saladin, Carpenter, & Upadhyaya, 2007). Physiological reactivity measures were collected prior to and throughout cue administration. Subjective craving measures were collected before and after cue administration. Sessions lasted about 120 min.
Measures
The Questionnaire of Smoking Urges–Brief (QSU-B; Cox, Tiffany, & Christen, 2001) was used to measure subjective craving/reactivity. The QSU-B includes two factors: Factor 1 assesses positive reinforcement (i.e., hedonic craving) and Factor 2 assesses negative reinforcement (i.e., alleviation of negative affect/withdrawal). Physiological assessment included heart rate and skin conductance (LaRowe et al., 2007). Participants completed daily self-report smoking diaries throughout study participation.
Analyses
Outcome variables were assessed for normality, and log10, square root, or inverse transformations were applied as appropriate. Findings with p value <.05 were considered significant, and those with p value between .10 and .05 were considered borderline significant.
Analyses first focused on measures of craving/reactivity in response to the active cues in isolation. Subsequent analyses controlled for response to the appropriate control cue. Subjective craving was defined as the postcue QSU-B score. Physiological cue reactivity was defined as the percent change from baseline in response to the cue via the following formula: ([maximum value during cue − precue value]/precue value) × 100.
Cycle effects were initially analyzed by comparing measures of reactivity across four phases (EF, MF, ML, and LL). They were then analyzed with phases collapsed into two categories: follicular and luteal. Post-hoc pairwise t tests were performed as appropriate to identify specific phase effects. Covariance pattern models in SAS proc mixed were used to account for repeated measures within participants over multiple sessions/timepoints. All analyses controlled for cue presentation order.
Similar methods were employed to analyze phase effects on smoking behavior. Participant self-report smoking diary data for the day preceding and the day following each phase-determined session were used as an index of smoking during the corresponding phase. Smoking data corresponding to the day of each cue reactivity session were not included in the analysis, given the likelihood that the 2-hr cue session altered spontaneous smoking behavior.
Results
Participants
Thirty-seven women participated in the study. Average age (mean ± SE) was 30.4 ± 1.1 years. They smoked 18.1 ± 1.2 cigarettes/day and had been regular smokers for 11.2 ± 1.0 years. Among the participants, 78% were White, 62% were employed, 67% had schooling beyond high school, and 56% had Fagerström Test for Nicotine Dependence score ≥6.
Smoking cue
Subjective.
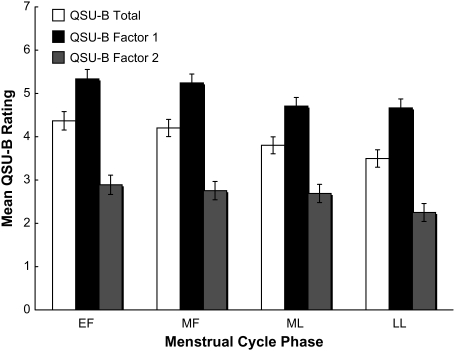
Subjective craving in response to smoking in vivo cues varied across the cycle, QSU-B total score F(3, 84.3) = 2.54, p = .02; QSU-B Factor 1 score F(3, 84.2) = 2.26, p = .09; and QSU-B Factor 2 score F(3, 54,4) = 2.57, p = .06; see Figure 1. Post-hoc t tests between EF and other phases revealed that subjective craving was higher during EF than during LL, total t(84.4) = 1.71, p = .02; Factor 1 t(83.4) = 1.72, p = .09; and Factor 2 t(79.0) = 2.18, p = .03. When phases were collapsed into two categories, greater craving was noted in the follicular phase, total F(1, 84.5) = 6.70, p = .01 and Factor 1 F(1, 86.8) = 6.79, p = .01. When controlling for response to the neutral in vivo cue, phase effects on subjective craving were no longer significant.
Figure 1.
Mean (least square ± SE) subjective craving response to smoking in vivo cues across menstrual cycle phases, controlling for order effects.
Physiological.
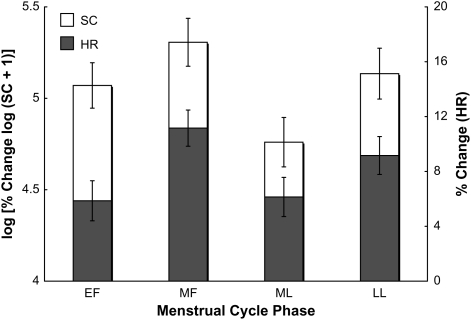
When controlling for response to the neutral in vivo cue, a marginal phase effect on skin conductance was noted, F(3, 36.2) = 2.84, p = .05, with highest response in the MF phase (Figure 2). Post-hoc t tests between MF and other phases revealed that skin conductance differences were most notable between MF and ML, t(49.1) = 2.84, p = .01. No phase effect was observed for heart rate response to in vivo cues.
Figure 2.
Mean (least square ± SE) skin conductance (SC) response to smoking in vivo cues and heart rate (HR) response to stressful imagery cues across menstrual cycle phases, controlling for order effects. Log (% change log[SC + 1]) was analyzed to obtain near normality of regression residuals. Menstrual cycle phase effect on SC p = .05 and effect on HR p = .01 when controlling for reactivity to neutral cues. EF = early follicular; MF = mid-follicular; ML = mid-luteal; LL = late luteal.
Stressful script cue
Subjective.
No significant phase effects on subjective response to script cues were observed. When phases were collapsed into two categories, women in the follicular phase had marginally more negative affect/withdrawal craving, QSU-B Factor 2 F(1, 39.8) = 3.47, p = .07]. When controlling for response to the neutral/relaxing script cue, no phase effects on subjective craving were significant.
Physiological.
Heart rate response to the stressful script cue varied marginally across the cycle, with highest response in the MF phase, F(3, 56.8) = 2.49, p = .07. When phases were collapsed into two categories, women in the follicular phase had a greater heart rate response, F(1, 51.3) = 4.51, p = .04. When controlling for the effects of the neutral/relaxing script cue, heart rate response varied across the cycle, with highest response in the MF phase, F(1, 59.6) = 3.82, p = .01; Figure 2. Post-hoc t tests between MF and other phases revealed that heart rate differences were most notable between MF and ML, t(76.1) = 2.54, p = .01, and between MF and EF, t(76.0) = 2.55, p = .01. No phase effects on skin conductance response to the script cues were observed.
Smoking behavior
No differences in smoking behavior across the cycle were observed, regardless of measurement with four, F(3, 49.5) = 0.98, p = .41, or two, F(1, 48.1) = 0.92, p = .34, phases.
Discussion
Results suggest that some components of reactivity to smoking in vivo and stressful imagery cues may vary across the menstrual cycle in women smokers. Contrary to our hypothesis, the significant findings, while modest, are consistently in the direction of greater reactivity during the follicular phase. These findings contribute to a mixed literature. Several studies have failed to demonstrate phase effects on craving in nonabstinent women smokers (Allen, Hatsukami, Christianson, & Nelson, 1999; Pomerleau, Garcia, Pomerleau, & Cameron, 1992; Pomerleau, Mehringer, Marks, Downey, & Pomerleau, 2000; Snively et al., 2000), and others have suggested increased craving in the luteal or late luteal phase (Allen et al., 1996; Franklin et al., 2004).
Inconsistency in results across studies may reflect differences in design and/or methodology. First, prior studies have utilized a variety of retrospective self-report methods to determine menstrual phase. Second, some studies have incorporated within-subjects designs, while others have made between-subjects comparisons. Third, instruments and methods for measurement and analysis of craving and cue reactivity have varied across studies. Fourth, potentially confounding inclusion criteria (e.g., psychiatric comorbidity, such as premenstrual dysphoric disorder) have not been consistent between studies.
Investigation of potential menstrual phase effects on smoking cessation outcome is also relevant, given that cue reactivity has repeatedly been found to predict smoking cessation outcome (e.g., Payne, Smith, Adams, & Diefenbach, 2006). However, the few studies that have experimentally examined this relationship have yielded mixed results (Allen, Allen, Lunos, & Hatsukami, 2009; Allen et al., 2008; Carpenter, Saladin, Leinbach, LaRowe, & Upadhyaya, 2008; Franklin et al., 2008). Our finding that smoking behavior did not vary across the cycle contributes to a similarly inconsistent literature.
Heightened craving and cue reactivity during the follicular phase, as is suggested by the present results, may in part reflect underlying hormonal activity. Fluctuations in estrogen and progesterone levels across the menstrual cycle may play a key role in modulating craving. Prior research has demonstrated that administration of progesterone during the follicular phase attenuates craving for cigarettes in female smokers (Sofuoglu, Babb, & Hatsukami, 2001). This is congruent with basic science (Feltenstein & See, 2007) and clinical research (Sinha et al., 2007) examining cocaine use and ovarian hormone levels, which suggests that progesterone reduces cocaine cue reactivity and intake. To clarify the possible relationship between ovarian hormones and craving/cue reactivity, future prospective studies should include ovarian hormone level measurement.
The present findings should be considered in light of limitations. First, participants smoked 45–60 min prior to each session, creating a partially sated condition that may have dampened overall cue reactivity. Second, a multiple-item craving instrument (QSU-B) was used in lieu of a single item measure. Over repeated administration, participant fatigue/boredom may have reduced careful completion of self-report assessments. Third, and most importantly, the relatively small sample size affected the overall power of the study. The relatively modest results likely reflect this power limitation. Nonetheless, the present study incorporated a prospective design with biological phase verification and yielded findings that provide a novel addition to the developing literature on menstrual cycle effects on smoking behaviors in women.
Conclusions
The present findings suggest an effect of menstrual cycle phase on some aspects of reactivity to smoking and stressful cues. Contrary to our hypothesis, reactivity may be heightened during the follicular phase. In the context of prior research, this may suggest that fluctuations in ovarian hormone levels underlie these changes in reactivity. Future studies should directly investigate the effect of ovarian hormone levels on various aspects of smoking behavior, cigarette craving, and cue reactivity in women smokers.
Funding
This research was supported by National Institute on Drug Abuse grants P50 DA016511 (HPU, component PI; Drs. Kathleen T. Brady and Ronald See, Center PIs), K12 DA000357 (KMG), and K23 DA020482 (MJC) as well as United States Public Health Service grant M01 RR01070 (Medical University of South Carolina Clinical and Translational Research Center).
Declaration of Interests
KMG has received research support from Pfizer, Inc. (medication and placebo supply for National Institutes of Health–funded research). Over the past 2 years, HPU has been a consultant and/or advisory board member of Eli Lilly and Company and Shire Pharmaceuticals. HPU is an ex-stockholder of New River Pharmaceutical Company; was on the Speakers’ Bureau of Shire Pharmaceuticals and Pfizer, Inc.; and has received research support from Cephalon, Inc., Eli Lilly and Company, and Pfizer, Inc. HPU recently became an employee of, and holds stock in, Eli Lilly and Company. The other investigators deny any potential conflicts of interest.
Supplementary Material
Acknowledgments
The authors thank Christine Horne, Ashley McCullough, Erin Klintworth, Gina Frattarolli, and the Medical University of South Carolina Clinical and Translational Research Center staff for their invaluable contributions to completion of this project.
References
- Allen S, Allen A, Lunos S, Hatsukami D. Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addictive Behaviors. 2009;34:928–931. doi: 10.1016/j.addbeh.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenopausal symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine & Tobacco Research. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- Allen S, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. Journal of Substance Abuse. 1996;8:303–319. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- Allen S, Hatsukami D, Christianson D, Nelson D. Withdrawal and pre-menopausal symptomatology during the menstrual cycle in short-term smoking abstinence: Effects of menstrual cycle on smoking abstinence. Nicotine & Tobacco Research. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, DeSantis SD, Gray KM, LaRowe SD, Upadhyaya HP. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addictive Behaviors. 2009;34:536–541. doi: 10.1016/j.addbeh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, Leinbach AS, LaRowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: A pilot feasibility study. Journal of Women's Health (Larchmt) 2008;17:293–301. doi: 10.1089/jwh.2007.0415. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine & Tobacco Research. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Cox L, Tiffany S, Christen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Craig D, Parrott A, Coomber J. Smoking cessation in women: Effects of the menstrual cycle. International Journal of the Addictions. 1992;27:697–706. doi: 10.3109/10826089209068761. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RW. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug and Alcohol Dependence. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for axis I disorders, patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Franklin T, Napier K, Ehrman R, Gariti P, O'Brien C, Childress A. Retrospective study: Influence of menstrual cycle on cue-induced cigarette craving. Nicotine & Tobacco Research. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O'Brien CP, et al. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: A retrospective analysis. Journal of Women's Health (Larchmt) 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton A, Hall J. Textbook of medical physiology. 10th ed. Philadelphia: Saunders; 2000. [Google Scholar]
- LaRowe S, Saladin M, Carpenter M, Upadhyaya H. Reactivity to nicotine cues over repeated cue reactivity sessions. Addictive Behaviors. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J, Hair C, Klock S, Ginsburg B, Pomerleau C. Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. Journal of Substance Abuse. 1994;6:235–243. doi: 10.1016/s0899-3289(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Marks J, Pomerleau C, Pomerleau O. Effects of menstrual phase on reactivity to nicotine. Addictive Behaviors. 1999;24:127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Mello N, Mendelson J, Palmieri S. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addictive Behaviors. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Pomerleau C, Garcia A, Pomerleau O, Cameron O. The effects of menstrual phase and nicotine abstinence on nicotine intake and on biochemical and subjective measures in women smokers: A preliminary report. Psychoneuroendocrinology. 1992;17:627–638. doi: 10.1016/0306-4530(92)90021-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau C, Mehringer A, Marks J, Downey K, Pomerleau O. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addictive Behaviors. 2000;25:483–497. doi: 10.1016/s0306-4603(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. Journal of Substance Abuse. 1994;6:227–234. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: Implications for relapse susceptibility. Experimental and Clinical Psychopharmacology. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Snively T, Ahijevych K, Bernhard L, Wewers M. Smoking behavior, dysphoric states and the menstrual cycle: Results from the single smoking sessions and the natural environment. Psychoneuroendocrinology. 2000;25:677–691. doi: 10.1016/s0306-4530(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: Effects on smoking behavior in women. Pharmacology, Biochemistry and Behavior. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Steinberg J, Cherek D. Menstrual cycle and cigarette smoking behavior. Addictive Behaviors. 1989;14:173–179. doi: 10.1016/0306-4603(89)90045-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.