Abstract
Introduction:
Several prior studies suggest that smokeless tobacco use results in less carcinogenic risk than does cigarette smoking. Whether smokers will use smokeless tobacco is unclear, as is the impact of such use on long-term smoking behavior and cessation. It is equally plausible that smokeless tobacco use among smokers could either (a) increase total tobacco exposure and undermine motivation to quit or (b) decrease overall tobacco exposure, motivate smokers to quit, and enhance cessation. Either outcome is of major public health significance.
Methods:
In this small (N = 31), short-term (2 week) pilot study, smokers uninterested in quitting were randomized to (a) receive Ariva or Stonewall (both spitless and smokeless tobacco lozenges) or (b) continue smoking conventional cigarettes.
Results:
Ariva/Stonewall use led to a significant reduction (40%, 95% CI: 24%–55%) in cigarettes per day, no significant increases in total tobacco use (cigarettes + Ariva/Stonewall; p > .05), and significant increases in two measures of readiness to quit, either in the next month (p < .001) or within the next 6 months (p = .04), as well as significant increases in self-efficacy to quit smoking (p < .001). No such changes were found among smokers maintained on conventional cigarettes.
Discussion:
These results suggest no deleterious effect on short-term smoking and quitting behavior among smokers who use smokeless tobacco. More broadly, this study suggests a strong need for a large prospective randomized clinical trial to more accurately assess the long-term viability of smokeless tobacco use as a method for cessation induction among unmotivated smokers.
Introduction
Recent years have seen a significant increase in the development and marketing of potentially reduced exposure products (PREPs), the most recent of which include a number of noncombustible products that provide tobacco in a spitless pouch (such as snus) or lozenge (such as Ariva or Stonewall; Hickman et al., 2004). Many researchers and public health officials question the overall population impact of PREPs (for reviews, see Hatsukami, Benowitz, Rennard, Oncken, & Hecht, 2006; Hatsukami et al., 2008; Pederson & Nelson, 2007). Whether PREPs are a viable or an unacceptable form of tobacco control is not yet known and is of crucial public health significance.
Depending on how PREPs are used in the real world, they could either promote or undermine public health (Warner, 2005). On the one hand, smokers may view these products as an alternative to cessation. This hypothesis is not without parallel, as most evidence has shown that switching to low-tar/low-nicotine cigarettes undermined cessation (Hughes, 2001; Shiffman, Pillitteri, Burton, Rohay, & Gitchell, 2001a, 2001b). Whether the history of low-tar/low-nicotine cigarettes serves as proxy for PREPs is unclear, but there is sufficient cause for concern given the comparable marketing strategies between these products (Hamilton et al., 2004). PREPs could also deter quitting if they are used to circumvent smoking restrictions, and many noncombustible products are marketed with this appeal. Smoking restrictions increase quitting (Farkas, Gilpin, Distefan, & Pierce, 1999; Norman, Ribisl, Howard-Pitney, Howard, & Unger, 2000), and using a PREP to avoid the necessity of going outside or to avoid the experience of withdrawal could maintain nicotine dependence and undermine the beneficial effect of restrictions.
On the other hand, PREPs could promote ultimate cessation if smokers view these products as a step toward quitting. In a population-based sample of more than 450 current smokers, 35% said they would use a cigarette-like PREP device to reduce the risks of smoking, and 28% said they would use it to help them quit (Shiffman, Jarvis, et al., 2007). Other studies have also suggested that PREP interest is greater among smokers interested in quitting (Caraballo, Pederson, & Gupta, 2006; Shiffman, Pillitteri, Burton, & DiMarino, 2004).
Unfortunately, little data exist to link use of smokeless PREPs with smoking behavior and quitting. Several analyses of Swedish smokers, in whom use of smokeless tobacco is prominent, suggest that self-selected use of smokeless tobacco is associated with increased cessation (Furberg et al., 2005, 2007; Gilljam & Galanti, 2003). A better test of the influence of PREPs on smoking and cessation would be through randomized clinical trials, but few such studies exist (Hatsukami et al., 2008). In a recent study on Danish smokers (Tonnesen, Mikkelsen, & Bremann, 2008), 263 smokers were randomized to receive cessation counseling and/or smokeless tobacco. Smokers using smokeless tobacco, relative to control participants, were almost twice as likely to achieve end-of-treatment continuous abstinence. Although abstinence outcomes at other timepoints were nonsignificant, they were all numerically higher in the smokeless group, which argues against an undermining effect. Collectively, these studies suggest that newer smokeless tobacco products might help smokers succeed in their efforts to quit.
A particularly compelling question is how smokeless tobacco might influence smoking behavior and cessation among smokers who are not interested in quitting, for whom novel methods for cessation induction are necessary. To date, there has never been a randomized clinical trial, among unmotivated smokers, testing smokeless tobacco use and its influence on smoking behavior and cessation. This article presents a pilot randomized trial testing Ariva and Stonewall, two products that are conceptually equivalent (both smokeless and spitless tobacco lozenges), versus conventional cigarettes.
Methods
Participants
Participants were recruited through local media advertising and flyers, using the general message “smokers needed to test new and potentially safer tobacco product.” Participants were eligible for study entry if they (a) were aged 18–65 years, (b) were daily smokers of at least 10 cigarettes/day on average for at least 1 year, (c) had no recent history of cardiovascular distress (heart attack in past year; arrhythmia, uncontrolled hypertension), (d) were neither pregnant nor breast feeding, (e) had no intention to quit smoking in the next month (≤7 on a 0–10 scale), (f) had no use of non-cigarette tobacco (cigars, chewing tobacco) in the past 6 months, (g) had lifetime nonuse of any PREP, and (h) had an absence of any major current psychiatric impairment, including current alcohol/drug abuse and dependence.
Procedures
Within the baseline visit, eligible participants were told of the study purpose: to measure changes in smoking behavior while using the new tobacco product. Consenting individuals were randomized to receive Ariva/Stonewall (n = 19; hereafter referred to as PREP group) or conventional cigarettes (n = 12; hereafter referred to as control group), with recurrent laboratory visits at 1 and 2 weeks following the baseline visit. Participants were randomized in a 2:1 (approximate) ratio with the goal of attaining a larger sample of PREP users. Smokers in the PREP group who smoked 1 or less pack/day received Ariva and those who smoked more than 1 pack/day received Stonewall, consistent with marketing claims that the former is intended for lighter smokers and the latter is intended for heavier smokers. However, PREP participants were able to switch products during the study if they chose to do so, based on self-report of product liking/preference and/or adverse events.
Participants in both study groups were instructed to use the intended products, either PREP or conventional cigarettes, for 2 weeks. Ariva and Stonewall were provided in their original packaging, and participants were provided with added material from each product’s marketing themes, described briefly in the following. No further information on either product was provided. PREP group participants were asked to “substitute these products for smoking as much as they can tolerate.” However, in an effort to provide some structure, and to increase the likelihood that participants used these products, participants were advised to use them at least every 2 hr. There was no requirement to abstain entirely from conventional cigarettes, since this was one study outcome. Ariva and Stonewall were provided free of charge, and participants were oversupplied with the product (150% of cigarettes/day), so as to allow for any potential increase in units of PREP used per day relative to cigarettes smoked per day. Participants in both groups were provided up to $100 in study compensation; control group participants were provided with added compensation to equate for free tobacco products (we did not want smoking behavior to be a function of free tobacco product).
Upon completion of the study, all participants were provided with cessation resources if they were interested. All participants were debriefed and told that there is no safe tobacco product and that the best thing they can do for their health is to quit entirely. No funding or product support for this study was provided by the tobacco industry, and all procedures were approved by the Medical University of South Carolina Institutional Review Board.
Ariva and Stonewall
Ariva (marketed by Star Scientific, Petersburg, VA) is a hard lozenge containing both tobacco and nicotine (1.5 mg). It is marketed (www.dissolvabletobacco.com) as having “no smoke or tar” and having “the lowest level of TSNAs [tobacco-specific nitrosamines] of any smokeless tobacco product marketed in the US,” a finding based on independent evaluation (Hatsukami, Ebbert, Feuer, Stepanov, & Hecht, 2007; Stepanov, Jensen, Hatsukami, & Hecht, 2006). It was developed to “give adult smokers a smoke/tar free alternative that is effective and satisfying” and to “create a tobacco product with the lowest levels of the leading toxins.” It is “made from select premium tobacco that is cured using a patented process that prevents the formation of one of the leading cancer causing compounds (TSNAs).” It dissolves in the mouth and requires no spitting and should be used “when you can’t smoke.”
Stonewall is marketed with nearly the same claims as Ariva (same Web site as aforementioned) but delivers more nicotine (4 mg) and is marketed for heavier smokers. Both Ariva and Stonewall are available in flavors of Wintergreen and Java, both of which were available to study participants.
Assessments
The assessment protocol included standard questions on demographics and lifetime smoking, as well as weekly measurement of nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991; Shiffman, Waters, & Hickcox, 2004), withdrawal (Hughes & Hatsukami, 1986), self-efficacy (Velicer, DiClemente, Rossi, & Prochaska, 1990), and motivation to quit (Biener & Abrams, 1991; Prochaska, Velicer, DiClemente, & Fava, 1988). Participants completed a timeline followback at each visit, indicating daily cigarette and PREP use. As we could find no established measure to assess attitudes and beliefs toward PREPs, we adapted various items from previous literature (Biener, Bogen, & Connolly, 2007; Hund et al., 2006; O’Connor, Hyland, Giovino, Fong, & Cummings, 2005; Shiffman, Gitchell, Rohay, Hellebusch, & Kemper, 2007). Pictures of various PREP products were shown as an aid to orient participants as they completed these assessments. We further asked PREP participants about the manner in which they used Ariva/Stonewall (e.g., to avoid smoking restrictions, cut down on cigarette smoking). As prior studies have examined toxicant exposure (Hecht et al., 2007; Mendoza-Baumgart et al., 2007), these outcomes were not included here; however, participants provided a breath sample for carbon monoxide (CO) testing at each visit.
Data analyses
Baseline demographics and smoking history were compared for between-group differences (PREP vs. control), using a chi-square test, t test, and, where appropriate, Mann–Whitney U test. We did not make explicit comparisons between Ariva versus Stonewall because this was not our study focus and also because the limited sample size prohibited such comparison. Main outcomes (cigarettes/tobacco units per day, CO, readiness to quit, and self-efficacy) were assessed using a generalized estimation equation (GEE) approach (Liang & Zeger, 1986), with study group as a between-subjects factor and each outcome over time as a within-subjects factor, and their interaction, all adjusted for baseline values. Cigarettes per day were an average of the 7 days prior, assessed at each visit. Similarly, total tobacco units per day represented a weekly average of cigarettes + PREP products. Attitudes toward PREPs were similarly analyzed with a series of GEEs, each using a binary logistic approach for dichotomous outcomes. Across 93 potential study visits (31 participants × 3 visits each), only 1 was missed. For this individual, it was assumed that no changes were made for any outcome variable (last datapoint carried forward).
Results
Sample characteristics
Within a 2-month period, 113 smokers responded to our recruitment strategies, of whom 34 (30%) were consented and 31 (27%) enrolled. One individual dropped out of the study prematurely. Participants were primarily men (61%), Caucasian (81%), and with a mean age of 40.4 years (SD = 14.4). They smoked on average 23.5 cigarettes/day (SD = 8.9) and were of moderate nicotine dependence. Few (10%) had ever heard of any PREP product, and although nearly half (48%) lived with a smoker, only 39% reported having no restrictions on indoor smoking within their household. Complete demographics and smoking history are presented in Table 1; there were no differences between PREP versus control groups. Within the PREP group, 5 participants used Ariva and 14 used Stonewall, 4 of whom later switched to Ariva.
Table 1.
Sample characteristics
| PREP, n = 19 | Control, n = 12 | |
| Age in years, M (SD) | 42.2 (14.1) | 37.6 (15.1) |
| Caucasian, n (%) | 16 (84) | 9 (75) |
| Male, n (%) | 12 (63) | 7 (58) |
| Employed full/part time, n (%) | 8 (42) | 5 (42) |
| High school education or more, n (%) | 17 (89) | 9 (75) |
| Smoking history | ||
| Cigarettes/day—weekday, M (SD) | 24.4 (10.2) | 22.0 (6.5) |
| Cigarettes/day—weekend, M (SD) | 26.0 (11.9) | 24.3 (5.0) |
| Age started smoking regularly, M (SD) | 16.0 (3.0) | 15.7 (2.3) |
| No. of prior quit attempts, M (SD) | 1.5 (1.1) | 1.8 (2.9) |
| Ever heard of any PREP product,a n (%) | 2 (11) | 1 (8) |
| Live with a smoker, n (%) | 10 (53) | 5 (42) |
| Smoking restrictions at home | ||
| No smoking at all indoors, n (%) | 9 (47) | 6 (50) |
| Restrictedb smoking indoors, n (%) | 3 (16) | 1 (8) |
| Unrestricted smoking indoors, n (%) | 7 (37) | 5 (42) |
| Usual brand of cigarettes | ||
| Regular, n (%) | 14 (74) | 8 (67) |
| Light, n (%) | 5 (26) | 3 (25) |
| Ultra light, n (%) | 0 | 1 (8) |
| Nicotine dependencec, M (SD) | ||
| FTND | 5.9 (2.1) | 4.9 (2.0) |
| NDSS | −0.19 (0.99) | −0.04 (0.77) |
Note. FTND = Fagerström Test for Nicotine Dependence; NDSS = Nicotine Dependence Syndrome Scale; PREP = potentially reduced exposure product.
Each participant was provided with a menu of PREP products available at the time of study initiation; awareness based on yes/no.
Restricted to time, place, or both.
High scores on both FTND (Heatherton et al., 1991) and NDSS (Shiffman, Waters, et al., 2004) indicate greater dependence.
Smoking behavior and PREP use
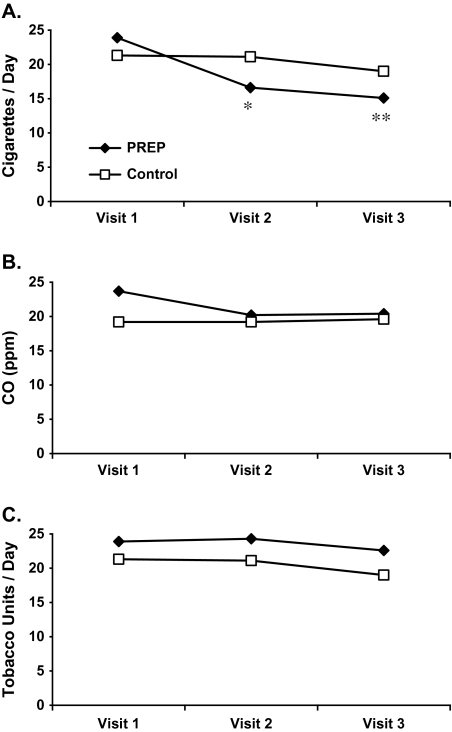
Participants using either Ariva or Stonewall reported a significant reduction in cigarettes smoked per day at both Visit 2 and Visit 3 (Figure 1A), amounting to a 40% reduction (95% CI: 24%–55%) in the 2-week study period. Participants smoking their own cigarettes had also reported a reduction (11%), but nonsignificantly (95% CI: −6% to 28%), and the overall interaction for Group × Time was significant (p < .001). However, there were no significant group, time, or interaction effects on CO (Figure 1B). PREP participants used an average of 7.7 (SE = 1.7) and 7.5 (1.2) Ariva/Stonewall lozenges per day during Week 2 and Week 3, respectively. When combined with cigarettes, total tobacco units per day remained relatively stable in both groups, with no group, time, or interaction effects present (Figure 1C).
Figure 1.
(A) Changes in cigarettes per day. Significant Group × Time interaction (p < .001) (*significantly different from Visit 1, p = .002; **significantly different from Visit 1, p < .001). (B) Changes in carbon monoxide and (C) changes in tobacco units per day.
PREP participants were asked how they used the Ariva/Stonewall. Half (50%) stated that they used their PREP product “more than a few times” or “frequently” to cut down on their cigarettes smoked, whereas only 39% used it to cope with or avoid smoking restrictions. Use of PREP was more predominant to avoid smoking restrictions at work (44%) versus at home (33%).
There were no changes in withdrawal or craving in either group during the course of the study (data not shown). Participants in both groups reported a nominal and nonsignificant decrease in withdrawal over time.
Motivation to quit
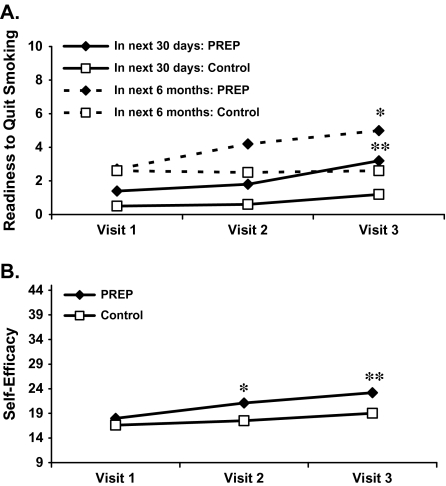
Readiness to quit (0–10 scale) in the next 30 days and within the next 6 months increased significantly among PREP participants but not among control participants (Figure 2A). The overall Group × Time interaction was significant for intentions to quit both in the next 30 days (p < .001) and within the next 6 months (p = .04). In terms of stage of change movement, 53% of participants in the PREP group progressed, 37% did not change, and 11% regressed. Comparable numbers from control participants were 42%, 48%, and 10%, respectively. Confidence (self-efficacy) in quitting also showed a significant Group × Time interaction (p < .001; Figure 2B), such that confidence increased significantly over time within the PREP group only. One participant within each group reported a quit attempt over the entire study period. Four PREP participants reported seeking information about smoking cessation versus zero control participants.
Figure 2.
(A) Changes in readiness to quit (0–10 scale). Significant Group × Time interaction for readiness to quit both in the next month (p < .001) and within the next 6 months (p = .04) (*significantly different from Visit 1, p < .01; **significantly different from Visit 1, p = .03). (B) Changes in self-efficacy to quit (9–45). Significant Group × Time interaction (p < .001) (*significantly different from Visit 1, p < .05; **significantly different from Visit 1, p < .001).
Attitudes toward PREPs
All participants were asked about their attitudes toward smokeless, spitless PREPs in general (Table 2). Most smokers viewed these types of products as safer than conventional cigarettes, and these beliefs did not significantly vary by time or group. After using Ariva/Stonewall for 2 weeks, PREP participants were significantly more likely to change their opinion in favor of using such PREPs for purposes to reduce smoking (p = .01) and to avoid smoking restrictions (p = .005). At the end of the study, PREP participants were twice as likely to express intentions to purchase these products as were control participants (67% vs. 33%), although this difference was only marginally significant (p = .09).
Table 2.
Attitudes toward PREPsa
| PREP |
Control |
|||
| Visit 1b (%) | Visit 3c (%) | Visit 1 (%) | Visit 3 (%) | |
| Compared with cigarettes, how risky would this PREP be for your health? | ||||
| Less risky | 67 | 83 | 92 | 75 |
| Equally risky | 33 | 16 | 8 | 25 |
| More risky | 0 | 0 | 0 | 0 |
| Switching to this PREP would lower risk ford | ||||
| Cancer | 68 | 83 | 92 | 92 |
| Heart disease | 68 | 78 | 75 | 83 |
| Others around me | 90 | 83 | 100 | 92 |
| I would use this PREP toe | ||||
| Reduce smoking | 37 | 74 | 50 | 42 |
| Quit smoking | 68 | 58 | 25 | 25 |
| Cope with smoking restrictions | 32 | 68 | 25 | 58 |
| How likely are you to buy this PREP?f | 32 | 67 | 42 | 33 |
Note. PREP = potentially reduced exposure product.
Regarding smokeless, spitless tobacco lozenges/pouches in general (not brand specific).
Prior to PREP use.
Following 2 weeks of PREP use.
% moderately/strongly agree.
% yes; options are not mutually exclusive.
% somewhat/very likely.
During each follow-up visit, PREP participants were asked to rate how they liked Ariva/Stonewall on a scale of 0–10 (absolute liking, not in reference to cigarettes). Average likeability was moderate at both Visit 2 (M = 4.5, SE = .7) and Visit 3 (M = 4.9, SE = .7). By Visit 3, and in comparison with regular cigarettes, 56% reported liking Ariva/Stonewall less than cigarettes; 28%, about the same; and 17%, more than cigarettes.
Adverse events
Within the PREP group, 12 participants (63%) reported a total of 20 adverse events, of which 14 (70%) were rated (participant reported) as mild and 6 (30%) were moderate. The most common events were nausea (n = 9), hiccups (n = 4), and insomnia (n = 3).
Discussion
The current study examined short-term changes in smoking behavior and proxy measures of cessation as a function of smokeless tobacco use (Ariva/Stonewall) among smokers not wanting to quit. With minimal instructions on how to use Ariva or Stonewall, most smokers made a partial substitution of their regular cigarettes. Smoking (cigarettes/day) significantly decreased (40%) over the 2-week study period, but overall total tobacco units per day (cigarettes + Ariva/Stonewall) remained fairly stable. This suggests that Ariva and Stonewall are effective products to curb withdrawal and craving. In support of this interpretation, we found no changes in overall craving or withdrawal as smokers substituted Ariva/Stonewall for cigarettes, which is generally consistent with reports from others (Blank, Sams, Weaver, & Eissenberg, 2008; Kotlyar et al., 2007; Mendoza-Baumgart et al., 2007). However, although cigarettes per day significantly decreased among smokers who used Ariva/Stonewall, reduction in CO was less striking (6%), suggesting partial compensation (e.g., inhaling deeper, more frequent puffs) and/or problems with the use of CO as a biomarker of tobacco exposure in this population (see following).
We found no evidence that smokeless tobacco (Ariva or Stonewall) undermines quitting. To the contrary, readiness to quit (in the next 1 month and within the next 6 months) significantly increased among smokers who used a smokeless tobacco product relative to those who continued to smoke conventional cigarettes. No group differences were noted for stage of change movement. Confidence in quitting smoking also significantly increased within the smokeless tobacco group only. Each of these measures (readiness to quit and self-efficacy) is predictive of smoking cessation (Carpenter, Hughes, Solomon, & Callas, 2004; Gwaltney, Metrik, Kahler, & Shiffman, 2009). Thus, our data support the notion that Ariva or Stonewall, and perhaps smokeless tobacco in general, could serve as a catalyst to increase motivation among smokers not wanting to quit. This is consistent with the only published randomized clinical trial of smokeless tobacco among smokers wanting to quit (Tonnesen et al., 2008), which found mixed but generally supportive evidence that smokeless tobacco promotes cessation.
The overall population impact of smokeless tobacco products, and PREPs in general, is unclear. Although PREPs are not yet popular among smokers, some indicators suggest they will be. Recent studies estimate that ever use of any PREP is between 4% and 10% but that consumer interest is much higher (50%–77%; Hund et al., 2006; Parascandola, Hurd, & Augustson, 2008). Many smokers believe that these products are safer than conventional cigarettes (Biener et al., 2007; Hamilton et al., 2004; O’Connor et al., 2005). Thus, the allure of a “safe(r)” tobacco product offers intuitive appeal for many smokers, and it is likely that the product’s popularity will increase as palatability increases. Within our study, palatability was mixed, and this may in part be a consequence of giving a smokeless tobacco product to cigarette smokers who are accustomed to the oral sensorimotor aspects of cigarette smoking. It is doubtful that a smokeless tobacco product could ever serve as a total substitute for cigarettes among a majority of smokers. However, the amount of substitution among those smokers who choose to use smokeless products is not insignificant.
Risks and benefits of smokeless tobacco at the individual level will need to be carefully evaluated and balanced with broader population needs (Warner, 2005). Even if a product could indeed be shown to be safe(r), the potential for large increases in usage could actually produce net harm to the population if significant numbers of smokers use it. Some tobacco control advocates have pointed to Sweden, where cessation (Furberg et al., 2005, 2007) and smoking mortality (Foulds, Ramstrom, Burke, & Fagerstrom, 2003) have been linked to increases in smokeless tobacco use, as evidence that smokeless tobacco could promote public health among smokers. Others doubt that the Swedish experience could translate to U.S. smokers (Zhu et al., 2009). Clearly, more research is needed to determine (a) if and how smokers use smokeless tobacco in the real world, (b) the long-term impact of such usage on smoking behavior, and (c) its ultimate impact on cigarette and tobacco cessation. The important challenge is that these issues be examined soon before novel smokeless tobacco products, and PREPs in general, reach wide popularity.
As a pilot study, the current investigation was not designed as a complete test of smokeless tobacco and its impact on smoking. As such, there are notable limitations within. In addition to the limited sample size, the lack of placebo control (there is no known placebo for Ariva/Stonewall), and a fairly short study period, limitations include a lack of rigorous biological verification of tobacco exposure. Our study collected CO, which (unlike cotinine, nicotine, anabasine, and anatabine) is the only biomarker of tobacco exposure that is sensitive to smokeless versus smoked tobacco (thiocyanate is another such biomarker but is often influenced by diet; Sherer, 2006). However, CO is sensitive to only recent smoking behavior (Shields, 2002), and it is unclear how continuous assessment of tobacco exposure among smokers who concurrently use smokeless and smoked tobacco could be done effectively. Another limitation herein is that we assessed motivation to quit cigarette smoking but not motivation to quit all tobacco products. Although readiness to quit smoking increased among users of Ariva/Stonewall, the clinical interpretation of this increase would likely vary if these same individuals intended to quit/continue smokeless tobacco use. These limitations aside, we believe that this is only the third study (Mendoza-Baumgart et al., 2007; Tonnesen et al., 2008) to test directly (i.e., through randomized methods) the impact of smokeless tobacco among smokers, only the second (Tonnesen et al.) to report on prospective changes in smoking behavior and cessation, and the first to do so among smokers unmotivated to quit.
In sum, results from the current study suggest no deleterious effect on smoking and quitting behavior among smokers who do not wish to quit but who use smokeless tobacco. Smokeless tobacco could potentially serve as a method for cessation induction among unmotivated smokers. However, this notion can only be resolved with additional larger studies that directly test the long-term consequences of smokeless tobacco use. Until then, the tobacco control community will require sustained commitment toward complete abstinence from all tobacco.
Funding
This research was supported in part by the National Institute on Drug Abuse grants K23 DA020482 (MJC) and K12 DA000357 (KMG).
Declaration of Interests
No conflicts are declared for MJC. KMG receives research support from Pfizer, Inc.
Supplementary Material
Acknowledgments
The authors thank Liz Byrd, Amy Boatright, and Nicola Thornley for their assistance with study procedures and data collection.
References
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Biener L, Bogen K, Connolly G. Impact of corrective health information on consumers’ perceptions of “reduced exposure” tobacco products. Tobacco Control. 2007;16:306–311. doi: 10.1136/tc.2006.019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Sams C, Weaver MF, Eissenberg T. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine & Tobacco Research. 2008;10:417–421. doi: 10.1080/14622200801901880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RS, Pederson LL, Gupta N. New tobacco products: Do smokers like them? Tobacco Control. 2006;15:39–44. doi: 10.1136/tc.2005.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tobacco Control. 1999;8:261–265. doi: 10.1136/tc.8.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Bulik C, Lerman C, Lichtenstein P, Pedersen N, Sullivan P. Is Swedish snus associated with smoking initiation or smoking cessation? Tobacco Control. 2005;14:422–424. doi: 10.1136/tc.2005.012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Lichtenstein P, Pedersen NL, Bulik CM, Lerman C, Sullivan PF. Snus use and other correlates of smoking cessation in the Swedish Twin Registry. Psychological Medicine. 2007;38:1299–1308. doi: 10.1017/S0033291707002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilljam H, Galanti MR. Role of snus (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction. 2003;98:1183–1189. doi: 10.1046/j.1360-0443.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: A meta-analysis. Psychology of Addictive Behaviors. 2009;23:56–66. doi: 10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WL, Norton GD, Ouellette TK, Rhodes WM, Kling R, Connolly GN. Smokers’ responses to advertisements for regular and light cigarettes and potential reduced-exposure tobacco products. Nicotine & Tobacco Research. 2004;6(Suppl. 3):S353–S362. doi: 10.1080/14622200412331320752. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine & Tobacco Research. 2006;8:169–191. doi: 10.1080/14622200600576628. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products: New delivery systems. American Journal of Preventive Medicine. 2007;33:S368–S378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Joseph AM, LeSage M, Jensen J, Murphy SE, Pentel PR, et al. Developing the science base for reducing tobacco harm. Nicotine & Tobacco Research. 2008;9:S537–S553. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K.-O. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, et al. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiology Biomarkers & Prevention. 2007;16:1567–1572. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- Hickman N, Klonoff EA, Landrine H, Kashima K, Parekh B, Fernandez S, et al. Preliminary investigation of the advertising and availability of PREPs, the new “safe” tobacco products. Journal of Behavioral Medicine. 2004;27:413–424. doi: 10.1023/b:jobm.0000042413.69425.aa. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Do “light” cigarettes undermine cessation? Tobacco Control. 2001;10:ip41–ip42. doi: 10.1136/tc.10.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hund LM, Farrelly MC, Allen JA, Chou RH, St. Claire AW, Vallone DM, et al. Findings and implications from a national study on potential reduced exposure products (PREPs) Nicotine & Tobacco Research. 2006;8:791–797. doi: 10.1080/14622200601004042. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li Z, Pentel PR, Barnett BC, Feuer RM, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tobacco Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Mendoza-Baumgart MI, Tulunay OE, Hecht SS, Zhang Y, Murphy S, Le C, et al. Pilot study on lower nitrosamine smokeless tobacco products compared with medicinal nicotine. Nicotine & Tobacco Research. 2007;9:1309–1323. doi: 10.1080/14622200701704228. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Ribisl KM, Howard-Pitney B, Howard KA, Unger JB. The relationship between home smoking bans and exposure to state tobacco control efforts and smoking behaviors. American Journal of Health Promotion. 2000;15:81–88. doi: 10.4278/0890-1171-15.2.81. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Hyland A, Giovino GA, Fong GT, Cummings KM. Smoker awareness of and beliefs about supposedly less harmful tobacco products. American Journal of Preventive Medicine. 2005;29:85–90. doi: 10.1016/j.amepre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Parascandola M, Hurd AL, Augustson E. Consumer awareness and attitudes related to new potential reduced-exposure tobacco products. American Journal of Health Behavior. 2008;32:431–437. doi: 10.5555/ajhb.2008.32.4.431. [DOI] [PubMed] [Google Scholar]
- Pederson LL, Nelson D. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: Communication implications. Nicotine & Tobacco Research. 2007;9:525–534. doi: 10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF, DiClemente CC, Fava JL. Measuring processes of change: Applications to the cessation of smoking. Journal of Consulting and Clinical Psychology. 1988;56:520–528. doi: 10.1037//0022-006x.56.4.520. [DOI] [PubMed] [Google Scholar]
- Sherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Health Psychology. 2006;3:563–581. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Shields PG. Tobacco smoking, harm reduction, and biomarkers. Journal of the National Cancer Institute. 2002;94:1435–1444. doi: 10.1093/jnci/94.19.1435. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gitchell J, Rohay JM, Hellebusch SJ, Kemper KE. Smokers’ preferences for medicinal nicotine vs smokeless tobacco. American Journal of Health Behavior. 2007;31:462–472. doi: 10.5555/ajhb.2007.31.5.462. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Jarvis MJ, Pillitteri JL, Di Marino ME, Gitchell JG, Kemper KE. UK smokers’ and ex-smokers’ reactions to cigarettes promising reduced risk. Addiction. 2007;102:156–160. doi: 10.1111/j.1360-0443.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, DiMarino ME. Smoker and ex-smoker reactions to cigarettes claiming reduced risk. Tobacco Control. 2004;13:78–84. doi: 10.1136/tc.2003.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Effect of health messages about “light” and “ultra light” cigarettes on beliefs and quitting intent. Tobacco Control. 2001a;10:i24–i32. doi: 10.1136/tc.10.suppl_1.i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers’ beliefs about “light” and “ultra light” cigarettes. Tobacco Control. 2001b;10:i17–i23. doi: 10.1136/tc.10.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–347. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, Mikkelsen K, Bremann L. Smoking cessation with smokeless tobacco and group therapy: An open, randomized, controlled trial. Nicotine & Tobacco Research. 2008;10:1365–1372. doi: 10.1080/14622200802238969. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Warner KE. Will the next generation of “safer” cigarettes be safer? Journal of Pediatric Hematology/Oncology. 2005;27:543–550. doi: 10.1097/01.mph.0000184574.00717.6c. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wang JB, Hartman A, Zhuang Y, Gamst A, Gibson JT, et al. Quitting cigarettes completely or switching to smokeless: Do U.S. data replicate the Swedish results. Tobacco Control. 2009;18:82–87. doi: 10.1136/tc.2008.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.