Abstract
The ATP-binding cassette (ABC) transporters are encoded by large gene families in plants. Although these proteins are potentially involved in a number of diverse plant processes, currently, very little is known about their actual functions. In this paper, through a cDNA microarray screening of anonymous cDNA clones from a subtractive library, we identified an Arabidopsis gene (AtPDR12) putatively encoding a member of the pleiotropic drug resistance (PDR) subfamily of ABC transporters. AtPDR12 displayed distinct induction profiles after inoculation of plants with compatible and incompatible fungal pathogens and treatments with salicylic acid, ethylene, or methyl jasmonate. Analysis of AtPDR12 expression in a number of Arabidopsis defense signaling mutants further revealed that salicylic acid accumulation, NPR1 function, and sensitivity to jasmonates and ethylene were all required for pathogen-responsive expression of AtPDR12. Germination assays using seeds from an AtPDR12 insertion line in the presence of sclareol resulted in lower germination rates and much stronger inhibition of root elongation in the AtPDR12 insertion line than in wild-type plants. These results suggest that AtPDR12 may be functionally related to the previously identified ABC transporters SpTUR2 and NpABC1, which transport sclareol. Our data also point to a potential role for terpenoids in the Arabidopsis defensive armory.
Plants have multiple defense mechanisms to fight against microbial pathogens. Some of these defenses involve preformed chemical and physical barriers, which impede pathogen entry into the host plant, whereas others are stimulated in response to pathogen attack and subsequently limit further pathogen growth. Successful recognition of pathogen-derived signals can ultimately result in the hypersensitive response or programmed cell death, which acts to stop the spread of an attempted infection by a biotrophic pathogen. Pathogen challenge also activates a number of signaling pathways that coordinately regulate expression of many genes encoding various transcriptional regulators, enzymes functioning in the synthesis of phytoalexins and other secondary metabolites, pathogenesis-related proteins, and a number of other antimicrobial molecules (Schenk et al., 2000). At least three chemical signal molecules are known to regulate the signaling pathways associated with plant defense responses. These are salicylic acid (SA), jasmonic acid (JA) and its methyl ester, methyl jasmonate (MJ), and ethylene (Dong, 1998; Reymond and Farmer, 1998). Substantial cross talk also occurs among these signaling pathways for mounting a coordinated defense response that may be dependent on the type of the challenging pathogen (for review, see Feys and Parker, 2000; Thomma et al., 2001; Kunkel and Brooks, 2002). The recent use of large-scale gene expression analyses (e.g. cDNA microarrays) suggests that potentially a large number of genes are associated with plant defense responses (Maleck et al., 2000; Schenk et al., 2000). However, so far, only a small number of plant genes identified in these microarray experiments have been functionally characterized at the molecular level.
ATP-binding cassette (ABC)-type membrane proteins (ABC transporters) function as ATP-driven efflux pumps that export a wide variety of compounds (Davies et al., 2000). Although approximately 131 ABC transporters have been identified in Arabidopsis, via sequence similarity to known ABC transporters in other organisms, very little is known about the functions or the substrate specificities of most of these genes (Jasinski et al., 2003). ABC transporters have been associated with various host-pathogen interactions. In plant pathogenic fungi, members of this transporter group play a role in providing resistance to phytoalexins (Nakaune et al., 1998; Urban et al., 1999; Schoonbeek et al., 2001; Fleißner et al., 2002), and to antifungal compounds (Hayashi et al., 2002) or act as novel pathogenicity factors (Urban et al., 1999; Fleißner et al., 2002). The pleiotropic drug resistance (PDR) subfamily of plant ABC transporters also has been implicated in plant defense. For example, the substrate transported by the NpABC1 ABC transporter of Nicotiana plumbaginifolia was found to be an antimicrobial diterpenoid compound sclareol that is excreted onto the leaf surface (Jasinski et al., 2001). A related ABC transporter, SpTUR2 from Spirodela polyrrhiza, with close sequence similarity to NpABC1, could also transport the same substrate when SpTUR2 is constitutively expressed in transgenic Arabidopsis plants (van den Brûle et al., 2002). The Arabidopsis AtPDR12 gene encoding a putative NpABC1 and SpTUR2 homolog also is shown to be responsive to sclareol, indicating that these three proteins are functionally similar (van den Brûle and Smart, 2002).
Our interest is in the identification and functional characterization of genes that are associated with interactions of Arabidopsis with necrotrophic fungal pathogens such as Alternaria brassicicola (Schenk et al., 2000; 2003). To isolate genes that are differentially expressed during this interaction, we employed a cDNA microarray hybridization analysis to screen 2,000 anonymous cDNA clones originating from a subtractive cDNA library prepared from A. brassicicola-inoculated Arabidopsis tissue. This microarray screen identified numerous cDNA clones induced by A. brassicicola. We characterized the defense-associated expression of one of the clones encoding a PDR-like ABC transporter in wild-type and various defense signaling mutants using real-time quantitative (RTQ)-reverse transcriptase (RT)-PCR. These analyses suggested that the induction of this gene by pathogen challenge requires functional SA, JA, and ethylene signaling pathways. Furthermore, analysis of an Arabidopsis line carrying a T-DNA insertion in the AtPDR12 gene showed enhanced susceptibility to sclareol, suggesting that AtPDR12 is possibly a functional homolog of the previously characterized ABC transporters, SpTUR2 and NtPDR12. Overall, our results indicate a potential function for this putative ABC transporter and a role of diterpenoids in the defensive armory of Arabidopsis.
RESULTS
Identification of AtPDR12 by cDNA Microarray Analysis
To identify plant genes that may be specifically induced during the Arabidopsis-A. brassicicola interaction, we first constructed a subtractive cDNA library from Arabidopsis leaf material collected at various time points after inoculation (see “Materials and Methods”). In total, 2,000 anonymous cDNA clones from this library were PCR amplified, and amplification products were spotted in duplicate on microarray slides. These slides were then hybridized simultaneously with Cy3- and Cy5-labeled cDNA probes prepared from two independent biological replicates each of A. brassicicola-inoculated and uninoculated plants and from plants treated with signaling compounds SA, MJ, or ethylene at various time points after inoculation/treatment. These analyses identified 195 cDNAs that were expressed at least 2-fold or higher in RNA samples from inoculated plants. We fully or partially sequenced 53 such clones, which identified 30 unique genes. Seven of these genes had previously described defensive functions. For instance, genes encoding antimicrobial or pathogenesis-related proteins (e.g. PDF1.2 and PR-1) and genes encoding enzymes involved in the synthesis of secondary metabolites/phytoalexins (e.g. a lipoxygenase, a glutathione S-transferase) and defense signaling compounds (e.g. 12-oxophytodienoate reductase) were represented among these genes. The microarray analysis also led to the identification of 23 genes (to be described elsewhere) with either unknown functions or functions that were not previously associated with plant defense responses. One of the cDNAs strongly induced in microarray analysis (i.e. showed >2-fold induction at 3, 6, 12, and 24 h after inoculation) by A. brassicicola was identified as the AtPDR12 gene (At1g15520). BLASTP searches using the deduced amino acid sequence of AtPDR12 demonstrated particularly high amino acid sequence identities between AtPDR12 and annotated PDR-like ABC transporter proteins from other plant species. The amino acid sequence of AtPDR12 was 69%, 68%, and 65% identical to NtPDR12 of tobacco (Nicotiana tabacum), NpABC1 of N. plumbaginifolia, and SpTUR2 of S. polyrrhiza, respectively. The other proteins that also showed particularly high amino acid identities to AtPDR12 were recently annotated PDR subfamily of ABC transporters from rice (Oryza sativa) including PDR7 (CAD59570, 65%), PDR8 (BAB21279, 65%), PDR9 (BAB21276, 67%), PDR10 (BAB21273, 65%), PDR11 (BAB21275%–66%), BAB93292 (63%), and SpTUR2 (65%; see also Jasinski et al., 2003). The analysis of AtPDR12 sequence revealed 12 predicted transmembrane domains that corresponded to the full ABC transporter class. Analysis of deduced amino acid sequence of AtPDR12 using pSORT (available at http://psort.nibb.ac.jp/) analysis also predicted that it was a membrane protein localized either in the plastid or plasma membrane with probability values of 0.7 and 0.64, respectively.
AtPDR12 Is a Pathogen-, SA-, MJ-, and Ethylene-Responsive Putative ABC Transporter Gene
Searching the Arabidopsis genome for AtPDR12-like sequences revealed at least 13 other PDR-like ABC transporter genes with strong nucleotide sequence similarities (75%–85%) to the AtPDR12 gene. This indicated the likelihood that cross-hybridization from such related sequences could complicate the confirmation of microarray results by any other hybridization-based procedure. Therefore, we used RTQ-RT-PCR with AtPDR12-specific primers for subsequent expression analyses. RTQ-RT-PCR is a specific and sensitive method that is now widely used particularly for transcript profiling of individual members of gene families in plants (Charrier et al., 2002; Orsel et al., 2002; Brown et al., 2003; Mladek et al., 2003; Schenk et al., 2003; Shimada et al., 2003). Using this method, we first confirmed the pathogen responsiveness of AtPDR12 after inoculation with the incompatible fungal pathogen A. brassicicola. Plants were grown in controlled environment rooms under a light regime of 8 h of light/16 h of dark and sampled at various time points after inoculations/treatments. In this sampling regime, 0 h corresponded to a time point of 1 h after the start of the light period. Inoculations of leaves with the incompatible fungal pathogen A. brassicicola demonstrated maximal AtPDR12 expression, reaching a sharp peak of 105-fold induction at 12 h after inoculation (Fig. 1A). Similarly, inoculation of leaves with the compatible fungal pathogen S. sclerotiorum resulted in strong induction of AtPDR12 as early as 3 h after inoculation (Fig. 1B), whereas inoculation of roots with the compatible fungal pathogen F. oxysporum induced the AtPDR12 expression at relatively late time points (Fig. 1C). Finally, inoculations with the compatible bacterial pathogen P. syringae pv tomato DC3000 also resulted in induction of this gene (Fig. 1D). These results suggested that AtPDR12 is a pathogen-responsive ABC transporter gene.
Figure 1.
Induction ratio (inoculated/mock) of AtPDR12 determined by RTQ-RT-PCR after inoculation of Arabidopsis plants with the incompatible fungal pathogen A. brassicicola (A) and the compatible fungal pathogens Sclerotinia sclerotiorum (B), Fusarium oxysporum (C), and the compatible bacterial pathogen Pseudomonas syringae pv tomato DC3000. Data shown for each time point were obtained from two independently replicated experiments for A. brassicicola. The highest fold induction values (i.e. 105-, 371-, 11-, and 68-fold) observed also were shown.
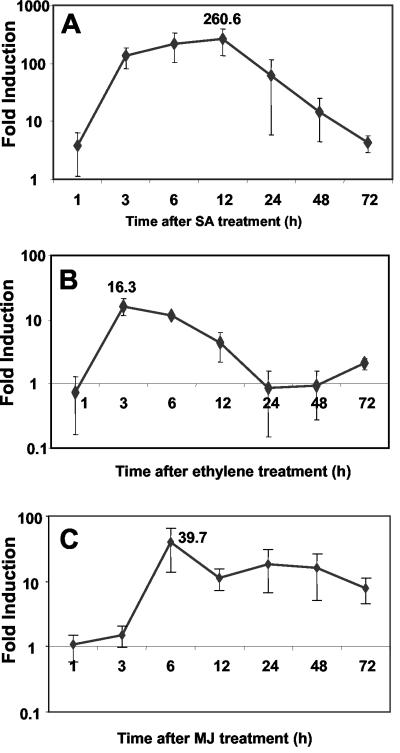
We also tested the induction patterns of the AtPDR12 gene after treatments with SA, MJ, and ethylene using plants grown and sampled under identical conditions as those used in pathogen inoculation assays. In these experiments, SA treatment strongly induced expression of AtPDR12 up to 260-fold at 12 h after treatment (Fig. 2A). Similarly, treatments with ethylene and MJ resulted in a 16- and a 40-fold induction of this gene as early as 3 and 6 h after treatment, respectively (Fig. 2, B and C). Overall, these results suggested that AtPDR12 was coordinately regulated by multiple defense-associated signals with distinct induction kinetics.
Figure 2.
Induction ratios (treated to mock) of AtPDR12 determined by RTQ-RT-PCR after treatment of Arabidopsis plants with SA (A), ethylene (B), and MJ (C) compared with untreated control plants. Data for each time point were obtained from at least two independently replicated experiments. The highest fold-induction values (260.6-, 16.3-, and 39.7-fold) observed during the time course were also shown for each treatment. Please note that the y axis in A differs from those in B and C.
Interestingly, although we observed strong and consistent induction of this gene by pathogen inoculation or treatments with chemical signal compounds, fold-induction values observed for some time points were more variable than the others (for instance, see the 1-, 24-, and 48-h time points after SA, ethylene, and MJ treatments in Fig. 2). To better understand the possible reason(s) underlying this phenomenon, we examined the transcript abundance of AtPDR12 in uninoculated plants grown and sampled under identical growth conditions using two independently replicated experiments (i.e. RNA collected from plants grown and inoculated in separate occasions). This expression profiling revealed steadily reduced levels of the AtPDR12 transcript abundance during the light period, suggesting an influence of the diurnal cycle on AtPDR12 expression (Fig. 3). Interestingly, the time points with more variable induction values coincided with the early hours of light period during which we observed a rapid reduction in the AtPDR12 transcript abundance.
Figure 3.
Diurnal changes at the transcript abundance of AtPDR12 determined by RTQ-RT-PCR in Arabidopsis. Data shown for each time point were obtained from two independently replicated experiments.
Tissue-Specific Expression of AtPDR12 in Arabidopsis
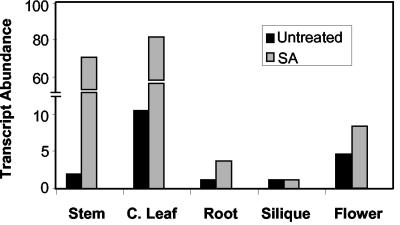
We also analyzed the transcript abundance of AtPDR12 in various Arabidopsis tissues taken from the plants at flowering and seed-forming stage. Varying levels of AtPDR12 transcripts were detected in stems, cauline leaves, roots, developing siliques, and flowers, with expression in stems and cauline leaves being significantly higher than in other tissues (Fig. 4). Treating the aboveground organs of these plants with SA induced AtPDR12 expression in the stems, cauline leaves, and the roots, whereas it did not have any effect on AtPDR12 transcript levels in siliques and flowers (Fig. 4).
Figure 4.
The relative transcript abundance of AtPDR12 in various Arabidopsis tissues collected from the plants at flowering and seed setting stage. Black and gray bars, AtPDR12 transcript levels in untreated and SA-treated (aboveground organs) plants, respectively. The relative transcript abundance shown here for each tissue was normalized using actin expression (see “Materials and Methods”). The transcript abundance values calculated based on 25S RNA levels in these tissues also produced similar results (data not shown).
Pathogen-Responsive Expression of AtPDR12 Requires EDS5 and NPR1
To further characterize which signaling pathway(s) plays a major role in regulating AtPDR12 expression, we examined the induction kinetics of AtPDR12 in various Arabidopsis signaling mutants at 5 and 15 h after A. brassicicola inoculation (Fig. 5A). To reliably detect potential quantitative differences in AtPDR12 expression in each of the mutants, we replicated these inoculation experiments at least three times at different occasions. Because our analyses showed that the expression of AtPDR12 was more strongly induced by SA than by any other chemical treatment, it is expected that the SA signaling pathway would have particular importance for the induction of AtPDR12 after pathogen challenge. To examine the role of SA accumulation in regulating AtPDR12 expression, we analyzed the induction patterns of AtPDR12 in the eds5 and npr1 mutants at 5 and 15 h after inoculation with A. brassicicola. The pathogen-induced expression of AtPDR12 was clearly reduced at the 5-h time point in the eds5 mutant as compared with induction of AtPDR12 in pathogen inoculated wild-type plants (Fig. 5A). The eds5 mutant is known to have reduced levels of SA accumulation; thus, the abolishment of AtPDR12 expression in this mutant at a relatively early stage of infection suggests that pathogen-responsive expression of AtPDR12 may require accumulation of sufficient amount of SA. Despite this initial delay, expression of AtPDR12 was restored to wild-type levels at a later time point (≥15 h) in eds5. Consistent with this observation, treatment of the eds5 plants with SA demonstrated AtPDR12 induction, which is similar to the levels normally observed in SA-treated wild-type plants (data not shown).
Figure 5.
The average fold induction of AtPDR12 determined by RTQ-RT-PCR in wild-type and defense signaling mutants at 5 and 15 h after inoculation with A. brassicicola. ** and *, Statistically significant differences between the wild-type and mutant expression values of AtPDR12 at P > 0.01 and P > 0.05, respectively (A). The fold induction of AtPDR12 in npr1 and wild-type (Columbia [Col-0]) plants treated for 12 h with SA (B). Relative transcript levels of AtPDR12 measured by RTQ-RT-PCR analysis in leaf tissue of wild type and the cpr5 mutant plants inoculated (In) with A. brassicicola (5 and 15 h after inoculation) compared with mock-inoculated (M) tissue (C). Each bar = average expression value from three to four independently replicated inoculation experiments. **, Significant difference between mock-inoculated wild-type and mock-inoculated cpr5 plants at both 5- and 15-h time points at P > 0.01.
We further examined whether pathogen-responsive expression of AtPDR12 requires a functional NPR1 protein. As shown in Figure 5A, the AtPDR12 expression was significantly reduced at both 5 and 15 h after inoculation with A. brassicicola in this mutant, suggesting that a functional NPR1 protein is required for pathogen responsiveness of AtPDR12. We subsequently treated both the wild-type and npr1 plants with SA and sampled the plants 12 h after treatment because the results presented in Figure 2A showed that AtPDR12 expression was maximal in SA-treated wild-type plants around this time point. These assays did not reveal any significant difference in AtPDR12 expression between the treated wild type and the npr1 mutant (Fig. 5B), suggesting that SA may regulate the expression of this gene in an NPR1-independent manner, although NPR1 is required for pathogen-responsive expression of AtPDR12.
In an effort to determine the presence of any conserved promoter sequence elements that may be responsible in mediating the SA-responsive expression of AtPDR12, we examined approximately 1 kb of the putative AtPDR12 promoter sequence using the PLACE database (available at http://www.dna.affrc.go.jp/htdocs/PLACE/). This analysis identified four W boxes (TTGAC), two ASF-1 elements (TGACG), and three GT-1 (GAAAAA) motifs in the AtPDR12 promoter. All these motifs are conserved sequence elements commonly found in the promoters of SA-regulated genes (Du and Chen, 2000; Klinedinst et al., 2000; Maleck et al., 2000; Yu et al., 2001). Relatively high incidence of these sequence elements in the AtPDR12 promoter is, therefore, in line with the observation that AtPDR12 is an SA-responsive gene. Further experimental evidence may be required to establish definite links between these putative promoter elements and their role(s) in regulation of AtPDR12 expression in response to an SA signal.
Pathogen-Responsive Expression of AtPDR12 Requires Functional Jasmonate and Ethylene Signaling Pathways
To determine whether the other major signaling pathways play any role in regulation of expression, we tested the pathogen responsiveness of the AtPDR12 gene in jasmonate and ethylene signaling mutants. Low or no significant induction of this ABC transporter gene was observed in ethylene signaling mutants (etr1 and ein2) and in jasmonate signaling mutant (jar1) at 5 h after inoculation as compared with the wild-type plants (Fig. 5A). Despite this initial delay in induction, 15 h after pathogen challenge, AtPDR12 was induced in etr1 and ein2 to levels that were not significantly different from those observed in wild-type plants. Taken together, these results suggested that, in addition to SA, both JA- and ethylene dependent-signaling pathways are required for rapid induction of this gene after pathogen challenge.
Finally, we examined the transcript abundance of AtPDR12 in the cpr5 mutant. Interestingly, the AtPDR12 transcript levels in the uninoculated cpr5 mutant were as high as AtPDR12 expression observed in the locally inoculated tissue of wild-type plants (Fig. 5C). Hence, similar to constitutive expression of other defense genes such as PR1 and PDF1.2 (Bowling et al., 1997), the AtPDR12 appears to be constitutively expressed in the cpr5 mutant. Inoculation of the cpr5 plants with A. brassicicola did not have any further effect on already elevated levels of AtPDR12 at any of the time points examined (Fig. 5C).
AtPDR12 Insertion Line Displays Enhanced Susceptibility to the Diterpenoid Sclareol
The close similarity of the AtPDR12 protein to the related ABC transporters SpTUR2 and NpABC1 implicated in sclareol transport has led to the suggestion that AtPDR12 may also function in transporting sclareol or related terpenoids (van den Brûle and Smart, 2002). Supporting this hypothesis, van den Brûle and Smart (2002) found that the treatment with sclareol induces the expression of AtPDR12. Our results from RTQ-RT-PCR experiments also confirmed the sclareol response of AtPDR12 because we found a 926-fold induction of AtPDR12 in plants floated for 24 h on a water solution containing 100 μm sclareol (Fig. 6). Spraying the leaves of Arabidopsis plants with the same sclareol solution did not have any effect on the expression of AtPDR12 (data not shown). Furthermore, sclareol treatment induced the expression from various other defense-associated genes (Fig. 6), indicating that sclareol may act as a signal for defense gene induction in Arabidopsis, although we cannot rule out the possibility that the induced gene expression observed could be due to a secondary effect (e.g. stress) of this treatment.
Figure 6.
The induction ratio (treated to untreated) of AtPDR12 and defense-related genes in the plants treated with the diterpenoid compound sclareol in Arabidopsis as determined by RTQ-RT-PCR analysis. Arabidopsis plants were floated on sterile water containing 100 μm sclareol for 24 h before RNA isolations. Control plants were similarly floated on sterile water containing 0.06% (v/v) dimethyl sulfoxide (DMSO). Lectin, gene encoding lectin-like protein; hevein, gene encoding hevein-like protein; B. chitinase, gene encoding basic chitinase.
To gain some insights into the role of AtPDR12, we analyzed an Arabidopsis insertion line (SALK_005635) in which the AtPDR12 genomic sequence was disrupted with a T-DNA insertion. We first confirmed the predicted location of T-DNA insertion within the second putative transmembrane domain from the carboxyl terminus of (Fig. 7, A and B) of AtPDR12 by a DNA sequencing strategy (see “Materials and Methods”). To test whether plants from this insertion line would have enhanced susceptibility to compounds containing an indole ring or SA, we germinated the seeds from wild type and the AtPDR12 insertion line on plant growth medium containing indole acetic acid, abscisic acid, sclareolide, sclareol, Rhodamine 6G, or SA. At 3 d after germination on medium containing 50 μm sclareol, seeds from the AtPDR12 insertion line showed only 52% of the wild-type germination rate. Higher sclareol concentrations (e.g. 100, 200, and 300 μm) affected the germination rates of both the wild-type and AtPDR12 insertion line plants. Particularly, at 300 μm sclareol concentrations, none of the seeds from AtPDR12 insertion line was germinated at 3 d postincubation, whereas a substantial portion of the wild-type plants germinated (i.e. radicle emerged from the seeds; Figs. 8A and 9), but these wild-type seedlings showed extremely slow growth rates. Seeds from both wild type and the AtPDR12 insertion line germinated at similar rates and grew normally on plant growth medium devoid of sclareol (Figs. 8, A and B, and 9). In addition, germinated seedlings from the AtPDR12 insertion line showed a reduced root length after germination as compared with wild-type plants treated similarly. This effect was quantifiable after germination of seeds on a medium containing 50 μm sclareol (Fig. 8B) Germination assays using other indole ring-containing compounds or SA did not reveal any difference between wild type and insertion line, at least at the concentrations tested (data not shown), suggesting that the observed effect was specific to sclareol. Overall, this result suggested that AtPDR12 is functionally related to NpABC1 and SpTUR2, which were previously shown to transport sclareol (Jasinski et al., 2001; van den Brûle and Smart, 2002).
Figure 7.
Schematic representation of the genomic organization of AtPDR12 (A). The overall predicted structure of AtPDR12 demonstrating the transmembrane domains and the ABC domain, consisting of an ABC signature and Walker A and B motifs (B, adapted from Davies and Coleman, 2000). The confirmed location of the T-DNA insertion in the AtPDR12 insertion line is also indicated in both A and B.
Figure 8.
Effect of sclareol on germination rate (A) and root lengths (B) in wild-type (Col-0) and AtPDR12 insertion line plants (AtPDR12Ins). Germination rates of seeds (40 seeds each) at various sclareol concentrations were scored 3 d after the plates were placed in a germination chamber. The root lengths of the seedlings from wild type and the AtPDR12 insertion line germinated on a medium containing either 50 μm sclareol (50 s) or no sclareol (0 s) were measured 3 to 6 d after the plates were placed into a germination chamber and incubated vertically under continuous diffuse light at 24°C.
Figure 9.
Effect of sclareol on the development of plants from wild-type (Col-0) and the AtPDR12 insertion line at 6 d after the plates were placed into a germination cabinet. The seeds were germinated on plates containing either 0, 100, 200, or 300 μm sclareol and grown vertically under continuous diffuse light at 24°C.
Disease Resistance Response of the AtPDR12 Insertion Line
If the putative substrate transported by AtPDR12 acts as an endogenous signal for defense gene induction and/or has direct antimicrobial effects on pathogen growth, then the possibility exists that the plants with an inactive copy of this gene would have compromised (i.e. enhanced susceptibility) disease resistance response. To test this hypothesis, we performed two independently replicated inoculation experiments on wild-type and AtPDR12 insertion line plants using fungal (F. oxysporum) and bacterial (P. syringae pv tomato DC3000) pathogens of Arabidopsis. However, the results from these inoculation experiments did not show any altered susceptibility in AtPDR12 insertion line plants (data not shown). Similarly, inoculation with incompatible strains of A. brassicicola and P. syringae pv tomato 1065 did not reveal any enhanced sensitivity to these pathogens, measured as trypan blue staining of the inoculated leaves (data not shown). Taken together, these results suggest that the basal plant defenses operating against at least these two pathogens were still sufficiently intact in the AtPDR12 insertion line plants, although further testing against various other pathogens may be warranted before any conclusions can be made about the role of this gene in plant defense.
DISCUSSION
Recently, cDNA microarray analyses have been successfully used in Arabidopsis to identify genes associated with various physiological conditions, such as plant responses to biotic and abiotic stresses (Maleck et al., 2000; Schenk et al., 2000; Desikan et al., 2001; Seki et al., 2001). However, application of this technology is somewhat limited in most other plant species where well-characterized expressed sequence tag (EST) collections have not yet been assembled fully. Even in plants with large numbers of EST collections, it is possible that certain genes with low or unique expression patterns will not be represented in the available EST collections (Richmond and Somerville, 2000). In the present paper, the screening strategy (microarray analysis of anonymous cDNA clones from a subtractive library) was not dependent on the availability of any of the previously assembled EST collections or prior sequence information. The genes found to be induced after microarray hybridization analysis were partially or fully sequenced and identities of these genes determined. This permitted identification of novel genes that were not previously reported to be associated with plant defense responses.
One of the pathogen-inducible genes identified in this analysis was the AtPDR12 gene, encoding a PDR-like ABC transporter protein. To date, the AtPDR12 gene has not been represented in any of the EST collections available from Arabidopsis, possibly due to very low transcript levels of this gene in the absence of an inducing agent such as pathogen challenge. The PDR subfamily of Arabidopsis contains 13 genes with high DNA sequence homology (Jasinski et al., 2003). Therefore, we used RTQ-RT-PCR to analyze the transcript abundance of this gene because this technique was demonstrated to be particularly suitable for specific quantification of expression from multigene family members with low transcript abundance and/or high sequence similarity (Charrier et al., 2002; Orsel et al., 2002; Mladek et al., 2003; Schenk et al., 2003; Shimada et al., 2003). We first confirmed the pathogen-responsive expression of this gene in time course experiments after inoculation with compatible and incompatible pathogens and exposure to the defense-associated chemical signals, SA, ethylene, and MJ. AtPDR12 expression is induced in response to challenge by various pathogens with distinct induction kinetics, suggesting that it has a role in plant defense. van den Brûle and Smart (2002) have examined the expression patterns of the AtPDR12 gene after treatment with a number of other inducers such as ABA, epibrassinolide, 2,4-dichlorophenoxyacetic acid, NaCl, cold, low light, and dark. Interestingly, none of these stress factors induced the expression of this gene, further suggesting that AtPDR12 is not a general stress response gene but specifically responds to pathogen infection and defense-associated chemical signals. The responses of the previously characterized AtPDR12 homologs (NpABC1 and SpTUR2) to pathogen infection are not known. However, the NtPDR1 gene, another close homolog of AtPDR12 in tobacco, has been reported to be responsive to flagellin from P. syringae pv tomato (Sasabe et al., 2002).
The quantification of the transcript abundance of AtPDR12 also revealed that this gene was responsive to diurnal cycles with steadily reduced transcript levels in uninoculated plants at time points where we observed higher variability in induction ratios. It is possible that transcription factors involved in regulating the expression of AtPDR12 under these seemingly different conditions may be competing, contributing to the variation we observed for certain time points. Other examples of pathogen-responsive genes that are also diurnally regulated are known (Schaffer et al., 2001). In addition, Logemann and Hahlbrock (2002) recently have identified promoter elements known as ACGT-containing elements in the promoter of acyl-CoA oxidase gene of parsley (Petroselinum crispum), which inversely regulate the light and pathogen responsiveness of this gene. Interestingly, the AtPDR12 promoter contains putative ACGT-containing elements that may also be involved in regulating the expression of this gene, although further experimentation is required to test this possibility.
In addition to pathogen signals, AtPDR12 was also responsive to defense-associated chemical signals SA, ethylene, and MJ. SA-induced expression of AtPDR12 was substantially stronger and sustained for longer periods than the ethylene- and MJ-induced expression of AtPDR12, suggesting that SA may have a predominant effect on the regulation of AtPDR12. The strong effect of SA on the induction of this gene was also evident in various tissues (except siliques) analyzed. Similar to AtPDR12, AtPDR12-homologous NtPDR12 and NpABC1 were found to be responsive to MJ (Sasabe et al., 2002; Grec et al., 2003). The involvement of both SA and JA signaling pathways in the regulation of AtPDR12 was particularly evident in the cpr5 mutant because we found constitutive AtPDR12 expression in the absence of any inoculation or treatment in this mutant. Previously, cpr5 is shown to constitutively express other SA- and JA-inducible defense genes in Arabidopsis (Bowling et al., 1997). The abolishment of early SA inducibility of AtPDR12 in eds5 and npr1 mutants further implicated the significance of the SA signaling pathway in regulation of this gene. The restoration of AtPDR12 expression in the eds5 mutant at 15 h after inoculation suggests that this induction may be occurring via either delayed accumulation of SA that gradually reaches to a level sufficient for gene induction or synthesis of SA occurred via an alternative pathway. It is also possible that the JA signaling pathway, which is still intact in eds5, may have contributed to this induction.
The NPR1-independent induction of AtPDR12 after SA treatment is in agreement with the notion that an SA-dependent, NPR1-independent defense pathway is functional in Arabidopsis. The presence of this pathway was first proposed based on the observations that the loss-of-function mutations in NPR1 did not result in complete loss of PR gene expression (Rate et al., 1999). Second, PR gene expression in the mpk4 mutant of Arabidopsis is dependent on SA but is independent of NPR1 (Petersen et al., 2000). Third, in the cpr5 mutant, the constitutive expression of PR-1 is dependent on SA and is only partially suppressed by NPR1, indicating the existence of an SA-mediated, NPR1-independent response in this mutant (Clarke et al., 2000). Similarly, in recently isolated ssi2 (Shah et al., 2001) and agd2 (Rate and Greenberg, 2001), mutant resistance against P. syringae is mediated by an SA-dependent and NPR1-independent signaling pathway. Finally, induction of a class of genes, NIR (NPR1 independent response), by Peronospora parasitica requires SA but not NPR1 (Rairdan and Delaney, 2002). Interestingly, the NIR genes still require a functional NPR1 protein for full induction by isonicotinic acid. Similar to the NIR class genes, it is possible that the maximal induction of AtPDR12 by SA may be occurring simultaneously both via NPR1-dependent and -independent signaling pathways. Further investigation of this phenomenon requires the analysis of double mutants defective for both SA accumulation and NPR1 function.
Although AtPDR12 is predominantly regulated by the SA signaling pathway, our results from mutant analyses indicated that the functional JA and ethylene pathways are also required for full and/or early induction of this gene. Defense gene induction through overlapping action of SA, JA, and ethylene signaling pathways also has been shown in various Arabidopsis mutants. For instance, defense gene expression and resistance against pathogens are regulated synergistically by SA and JA/ethylene pathways in the hrl1 mutant (Devadas et al., 2002). More interestingly, the resistance response observed in cir1 against P. syringae, which occurs via an SA-dependent, NPR1-independent signaling pathway, was reduced in cir1jar1 and cir2ein2 double mutants, suggesting that in addition to SA, JA and ethylene sensitivity were required for cir1-mediated resistance against this bacterial pathogen (Murray et al., 2002). The regulation of AtPDR12 expression by multiple signaling pathways and its early induction in the defense response may suggest that the putative transporter encoded by the AtPDR12 gene may play some roles in plant defense.
Recent studies have revealed that ABC transporters from Arabidopsis can be involved in a variety of functions (Coleman et al., 1997; Sidler et al., 1998; Gaedeke et al., 2001; Noh et al., 2001; Li et al., 2002). However, so far in Arabidopsis, none of the genes encoding PDR-like ABC transporters has been functionally characterized. One putative function ascribed to AtPDR12, based on its close sequence homology to other known ABC transporters, is the transport of substrates such as diterpenoids (van den Brûle and Smart, 2002). Our results using an AtPDR12 T-DNA insertion line suggested that the presence of a functional AtPDR12 protein could overcome sclareol toxicity possibly by actively transporting this compound out of the cells. Due to the location of the T-DNA insertion within a predicted transmembrane domain of AtPDR12, it is likely that AtPDR12 may still be transcribed, but a fully functional protein may not form. In fact, transcript levels determined with RTQ-RT-PCR primers that are specific to AtPDR12 sequences further upstream from the T-DNA insertion were similar in the SA-treated insertion line to those of SA-treated wild-type plants (data not shown). Sclareol is known to be toxic to plant and fungal cells (Bailey et al., 1974; Cutler et al., 1977; Jasinski et al., 2001; van den Brûle et al., 2002). If AtPDR12 could transport sclareol, then an enhanced sensitivity to this compound would be expected in the AtPDR12 insertion line. Alternatively, although it is less likely, the AtPDR12 insertion line may have increased sclareol uptake and/or increased intracellular sensitivity to this compound.
Although enhanced susceptibility to sclareol observed in the AtPDR12 insertion line indicates potential involvement of AtPDR12 in diterpenoid transport, so far there has been no report of diterpenoids or sclareol synthesized in Arabidopsis. In contrast, both monoterpenes and sesquiterpenes are synthesized in Arabidopsis (Chen et al., 2003). Recent in silico analysis of the Arabidopsis genome, however, has identified several genes with DNA sequence similarities to the diterpenoid synthase genes from other organisms (Aubourg et al., 2002). Considering the fact that the ABC transporters may act on a broad range of substrates, the substrate transported by this gene could be a related diterpenoid associated with plant defense. Assuming that AtPDR12 functions in terpenoid transport, the transported compound may also have defense-inducing activities such as an endogenous signal. Our results showed that the diterpenoid sclareol could activate defense gene expression in Arabidopsis. This is consistent with a recent report by Seo et al. (2003), who showed that a sclareol-related diterpenoid (WAK-1) isolated from tobacco acts as an endogenous signal for activation of basic PR genes in tobacco. Although these authors have observed an enhanced resistance to tobacco mosaic virus infection in the detached leaves fed with WAK-1 through their petioles, this treatment probably may have increased the endogenous concentrations of WAK-1 to levels that are not normally sustained naturally in the plant tissue. Our results did not suggest any altered susceptibility of AtPDR12 insertion line plants after testing against fungal and bacterial pathogens, suggesting that the insertion in AtPDR12 did not have any significant or detectable effect on defense responses that are effective against the pathogens tested. However, more comprehensive testing of AtPDR12 insertion and overexpression lines against various pathogens might be warranted before any conclusions could be made about the potential role of the AtPDR12 gene in plant defense.
Determining the exact substrate of AtPDR12 protein along with its subcellular localization would also reveal more information about the putative function of this ABC transporter protein. Overexpression of AtPDR12 in transgenic plants and evaluation of diterpenoid partitioning and composition could be useful to determine the precise role of this ABC transporter.
MATERIALS AND METHODS
Plant Inoculations/Treatments
For the construction of a subtracted cDNA library, Arabidopsis (Col-0) plants were grown in a growth chamber (20°C daytime temperature and 18°C night temperature with a 12-h photoperiod [100 μE m–2 s–1]). Four week-old plants were inoculated with Alternaria brassicicola (strain MUCL 20297) by applying 5-μL drops of a spore suspension (5 × 105 spores mL–1in distilled water) on four to six lower rosette leaves. The plants were placed in a propagator flat with a clear polystyrene lid and kept at high humidity to stimulate infection by hyphal germlings. Control plants were not inoculated, but were otherwise treated the same way. At 3, 6, 9, 12, 18, 24, 48, 56, and 64 h after inoculation, leaves from infected plants (both treated and non-treated leaves from the same plant) were collected. For cDNA microarray and/or RTQ-RT-PCR experiments, seeds of Arabidopsis ecotype Col-0 (wild type) and of defense signaling mutants npr1 (Arabidopsis Biological Resource Center stock no. CS3726), eds5 (CS3735), cpr5 (CS3770), jarl (CS8072), ein2 (CS8844), and etr1 (CS273; all in Col-0 background) were planted in autoclaved soil and cold treated at 4°C for 4 d. Plant were then grown to eight- to 12-leaf stage in controlled environment rooms with a photoperiod of 8 h light (180 μE m–2 s–1 at 24°C with 75% relative humidity and at 20°C/95% relative humidity at night time) and treated with either chemical signal compounds or fungal pathogens. For treatments with SA, plants were sprayed with a 5 mm solution (pH 7) containing 0.1% (w/v) ethanol. MJ treatment was carried out by taping a cotton ball containing 200 μL of a 0.5% (w/v) solution in ethanol onto the wall of a 20-L airtight container. For fungal inoculations, 5-μL drops of a spore suspension (5 × 105 spores mL–1 in water) of A. brassicicola (isolate UQ4273, freshly grown on agar plates containing clarified vegetable juice [Campbell's, Camden, NJ]) were pipetted onto two to four leaves per plant. Agar blocks (4 × 4 mm) containing a lawn of fungal hyphae were used for inoculation with Sclerotinia sclerotiorum (UQ isolate 3833 kindly provided by Ken C. Goulter, Cooperative Research Centre for Tropical Plant Protection, Queensland, Australia) grown on agar plates containing oat flakes. Fusarium oxysporum (isolate 5176 kindly provided by Roger Shivas of Department of Primary Industries, Queensland, Australia) was maintained on either sterile filter paper or on carnation leaf agar and grown on one-half-strength potato dextrose agar for approximately 1 week before inoculations. Five plugs were taken from these plates under sterile conditions with a cork borer and placed into 50 mL of potato dextrose broth containing 100 μg mL–1 streptomycin and 15 μg mL–1 tetracycline. The flasks containing the inoculum were placed onto a shaker for approximately 3 to 4 d and drained through Miracloth (Calbiochem, San Diego) before quantification with a hemocytometer. A spore concentration of 1 × 106 spores mL–1, diluted with sterile distilled water, was used to inoculate the plants. The plants used in the inoculation were grown in autoclaved sandy soil for approximately 3 to 4 weeks before inoculation. Before inoculations, the plants were carefully removed from the soil, and the roots were rinsed with sterile water and then dipped for 20 s into the fungal inoculum. The inoculated plants were sealed with a clear autoclave bag in the first 2 d postinoculation. Ninety plants from each of the wild-type and AtPDR12Ins plants were inoculated in two independent inoculation experiments. The disease symptoms were scored approximately 8 to 11 d postinoculation by counting the number of dead leaves and wilted plants. The amount of fungal mass in inoculated plants was also estimated using RT-Q-RT-PCR using a primer pair (5′-CGCCAGAGGACCCCTAAAC-3′ and 5′-ATCGATGCCAGAACCAAGAGA-3′) specific (i.e. no homology to Arabidopsis sequences) for amplification of the 18S transcribed internal spacer (GenBank accession no. AY237110). The amount of fungal RNA in the plant tissue was normalized using expression from the Arabidopsis β-actin genes as described below.
The bacterial pathogen Pseudomonas syringae pv tomato DC3000 (compatible strain) and P. syringae pv tomato 1065 (incompatible strain) kindly provided by Peter Mohr (Deakin University, Victoria, Australia) were grown on King's B media at pH 7.0 (10 g L–1 Bacto peptone, 1.5 g L–1 K2HPO4, and 15 g L–1 glycerol) containing 50 μg mL–1 each of rifampicin and kanamycin for DC3000, whereas only rifampicin was used for 1065. The cultures were shaken for 2 d at 28°C in the dark and resuspended in sterile 10 mm MgSO4 to the OD600 value of 0.05. The inoculum was then injected into the extracellular spaces of leaves from 3- to 4-week-old plants using a 10-cc syringe. Three leaf discs (6 mm in diameter) from each plant were collected beginning from d 1 of the inoculation and for each of the following 3 to 4 d. The bacterial growth was quantified according to Mohr and Cahill (2003).
For sclareol treatments, plants (six- to eight-leaf stage) were floated (roots were immersed into the solution while the abaxial surfaces of the lower leaves were in contact with the solution) on sterile water containing 100 μm sclareol (dissolved in DMSO, Aldrich, Milwaukee, WI) for 24 h before collection of leaves for RNA isolations. Control plants were floated on sterile water containing 0.06% to 0.25% (v/v) DMSO. Control plants were grown in parallel and treated exactly the same way as described above except for the addition of fungal inoculum or chemicals.
For sclareol resistance assays, seeds from wild type and AtPDR12 T-DNA insertion line (40 each) were germinated on petri plates incubated vertically under continuous light at 24°C on one-half-strength B5 medium (Sigma, St. Louis) containing various concentrations of sclareol for 7 d, and root and plant growth were observed daily. Germination assays were also conducted in the presence of (3aR)(+) sclareolide (100 μm; Aldrich Chemicals), indole-3-acetic acid (1 μm; Sigma), abscisic acid (50 μm; mixed isomers, Sigma), SA (100 μm), or rhodamine 6G (1 μm; Sigma). The concentrations of sclareol and sclareolide used in these experiments were in the range of those reported by van den Brûle et al. (2002) in the analysis of Arabidopsis lines overexpressing the SpTUR2 gene. However, we used 3- to 10-fold higher concentrations of rhodamine 6G and indole-3-acetic acid than those used by van den Brûle et al. (2002) because these authors observed no significant root inhibition with these chemicals at the concentrations tested.
Construction of a Subtractive cDNA Library
Total RNA was extracted by the phenol-LiCl method according to Eggermont et al. (1996) from the leaf samples frozen in liquid nitrogen. RNA isolated from infected plants collected at different time points was pooled together. Using the SMART cDNA Synthesis Kit (CLONTECH Laboratories, Palo Alto, CA) full-length, double-stranded cDNA was generated starting from the pool of total RNA isolated from infected plants and from total RNA isolated from control plants. Differentially expressed transcripts (present in the RNA pool from infected leaves but reduced or absent in the control RNA) were selected and amplified from the full-length cDNA using the PCR-Select cDNA Subtraction Kit (CLONTECH Laboratories). The differentially expressed sequences in the subtracted cDNA pool were cloned into an equimolar mixture of pCR2.1 and pCR-Blunt plasmids (Invitrogen, Carlsbad, CA) to create a subtracted cDNA library.
cDNA Microarray Analysis
A total of 2,000 plasmid clones from the subtracted cDNA library were collected, and the cDNA inserts were amplified by PCR. For control purposes, cDNA inserts representing negative controls (i.e. not induced after pathogen challenge) and cDNA inserts representing positive controls from the EST collection described by Schenk et al. (2000) were included. In total, 2,400 PCR products were ethanol precipitated, redissolved in 50% (w/v) DMSO, and arrayed onto Corning slides. Each PCR product was represented at least two times on each slide. cDNA synthesis, labeling reactions, microarray hybridizations, and microarray data analyses were carried out as described previously (Schenk et al., 2000, 2003).
RTQ-RT-PCR
Total RNA (500 ng–5 μg) isolated from treated and control plants was denatured at 70°C for 5 min followed by a quick chill on ice in a 13-μL reaction containing 10 ng of anchored oligo(dT) (23 mers), 4.5 ng of random hexamer primers (Invitrogen), and 0.5 μL of 20 mm dNTPs. After the addition of 4 μL of 5× reaction buffer (Invitrogen) and 2 μL of 0.1 m dithiothreitol, the reaction was preheated to 42°C for 2 min before adding 1 μL (200 units) of Superscript II RT (Invitrogen) followed by incubation at 42°C for another 50 min. After terminating the reaction at 70°C for 15 min, the resulting cDNA was subsequently taken up in a volume of 500 μL, and PCR fragments were amplified by using gene-specific primers designed from the coding sequence and over a RNA splice junction of ATPDR12 (5′-CTTTCGCTCAGGTTTTCATCG-3′ and 5′-CTTCACCGCCGTCCACTC-3′) using the Primer Express 1.5 software (Perkin-Elmer Applied Biosystems, Foster City, CA). The following similarly designed primer pairs were used to detect expression from PDF1.2 (At5g44420, 5′-TTGCTGCTTTCGACGCA-3′ and 5′-TGTCCCACTTGGCTTCTCG-3′) and from the genes encoding a lectin-like protein (At3g15356, 5′-GTTTCGTCTCTGGGTCATGGA-3′ and 5′-GCAGCAACTTGTTATTCCTTGGA-3′), a hevein-like protein (At3g04720, 5′-TGCTACATCCAAATCCAAGCCT-3′ and 5′-CGGCAAGTGTTTAAGGGTGAAG-3′), and a basic chitinase (At3g12500, 5′-ATCAGCGCTGCAAAGTCCTTC-3′ and 5′-GTGCTGTAGCCCATCCACCTG-3′). RTQ-RT-PCR using the ABI PRISM 7700 Sequence Detector and SYBR Green Master Mix (Perkin-Elmer Applied Biosystems) was carried out using primers at a final concentration of 0.28 μm each and 1 μL of cDNA (the equivalent of 10 ng of total RNA) as template. PCR cycling conditions comprised an initial polymerase activation step at 95°C for 10 min followed by 45 cycles at 95°C for 15 s and 59°C for 1 min. Real-time DNA amplification was monitored and analyzed using the Sequence Detector 1.7 program (Perkin-Elmer Applied Biosystems). Differences in cycle numbers during the linear amplification phase between samples containing cDNA from treated and untreated plants were used to determine differential gene expression, each cycle representing a 2-fold change in template abundance. Expression detected from three β-actin genes of Arabidopsis, β-Actin-2 (At3g18780), β-Actin-7 (At5g09810), and β-Actin-8 (At1g49240) with universal actin forward primer 5′-AGTGGTCGTACAACCGGTATTGT-3′ and specific reverse primers 5′-GATGGCATGGAGGAAGAGAGAAAC-3′, 5′-GAGGAAGAGCATTCCCCTCGTA-3′, and 5′-GAGGATAGCATGTGGAACTGAGAA-3′, respectively, were used as combined internal standards to normalize small differences in template amounts. Furthermore, transcript abundance at different tissues was also assayed for the 25S rRNA gene (At3g41950) of Arabidopsis using the following primers (5′-TTAACAGCCTGCCCACCCTGG-3′ and 5′-ATCCATTTTGCCGACTTCCC-3′).
All cDNA samples were assayed in duplicate, with cycle threshold (Ct) values generally within 0.5 to 1 cycles of each other. The following statistical analysis of RT-QPCR was performed on experiments replicated more than two times (e.g. AtPDR12 expression in defense signaling mutants). First, the δCt values were calculated from the average Ct value (PCR cycle no. during exponential amplification) for each sample that was assayed in duplicate, where δCt = Ct (AtPDR12) – Ct (actin) (normalized to actin). The δδCt value was obtained where δδCt = δCt (treatment) – δCt (control). This was used to determine the mean δδCt and its se from three to four replicates. Data are presented as the treatment (T) to control (C) ratios where T:C = 2–δδCt (ABI700 Sequence detection system, User Bulletin no. 2, ABI, Sunnyvale, CA). In graphical representations, error bars were determined by calculating the difference between the mean ratio (determined from δδCt) and the mean ratio ± se (determined from [δδCt + se] and [δδCt – se]). For the analysis of defense signaling mutants, Student's t test was performed on the δδCt values to identify induction ratios that were significantly different to those from wild-type plants.
Characterization of the AtPDR12 T-DNA Insertion Line
The location of the T-DNA insertion in the AtPDR12 insertion line (available through Arabidopsis Stock Centre, Ohio State University, Columbus) was originally identified at the Salk Institute Genomic Analysis Laboratory (La Jolla, CA) using the protocol described by Siebert et al. (1995). The location of the T-DNA insertion in the AtPDR12 insertion line (SALK_005635) was further verified by using a nested PCR approach with an AtPDR12 (5′-GATTTCTTGGATACAAAATGGAGGGA-3′) and TDNA-specific (LBa1 5′-TGGTTCACGTAGTGGGCCATCG-3′ and LBa1 5′-TGGTTCACGTAGTGGGCCATCG-3′) primers as recommended by the Salk Institute Genomic Analysis Laboratory (see http://signal.salk.edu/tdna_FAQs.html), where this insertion line was generated.
Acknowledgments
We thank Iain Wilson for his help in preparation of microarray slides; B. Simpson for advice on RTQ-RT-PCR analysis; Ken Goulter, Peter Mohr, and Roger Shivas for S. sclerotiorum, P. syringae pv tomato, and F. oxysporumstrains, respectively; Peter Mohr, Roger Shivas, and Leanne Forsyth for advice on pathogen inoculations; and the Arabidopsis Stock Centre (Ohio State University, Columbus) for seeds of the AtPDR12 insertion line and mutant lines of Arabidopsis plants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.024182.
This work was supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (postdoctoral fellowship to I.A.M.A.P.) and by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen and the Cooperative Research Centre for Tropical Plant Protection (grant to I.A.M.A.P. supporting a research stay at the University of Queensland, Australia).
References
- Aubourg S, Lecharny A, Bohlman J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genome 267: 730–745 [DOI] [PubMed] [Google Scholar]
- Bailey JA, Vincent GG, Burden RS (1974) Diterpenes from Nicotiana glutinosa and their effect on fungal growth. J Gen Microbiol 85: 57–64 [DOI] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Kazan K, McGrath K, Maclean DJ, Manners JM (2003) A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 132: 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier B, Champion A, Henry I, Martin K (2002) Expression profiling of the whole Arabidopsis shaggy-like kinase multigene family by real-time reverse transcriptase-polymerase chain reaction. Plant Physiol 130: 577–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tholl D, D'Auria JC, Farooq A, Pichersky E (2003) Biosynthesis and emission of terpenoids volatiles from Arabidopsis flowers. Plant Cell 15: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel F, Dong X (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JOD, Blake-Kaiff MMA, Davies TGE (1997) Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci 2: 144–151 [Google Scholar]
- Cutler HG, Reid W, Deletang J (1977) Plant growth inhibiting properties of diterpenes from tobacco. Plant Cell Physiol 18: 711–714 [Google Scholar]
- Davies TGE, Coleman JOD (2000) The Arabidopsis thaliana ATP-binding cassette proteins: an emerging superfamily. Plant Cell Environ 23: 431–443 [Google Scholar]
- Desikan RA-H, Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. Plant J 30: 467–480 [DOI] [PubMed] [Google Scholar]
- Dong X (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1: 316–323 [DOI] [PubMed] [Google Scholar]
- Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24: 837–847 [DOI] [PubMed] [Google Scholar]
- Eggermont K, Goderis IJ, Broekaert WF (1996) High-throughput RNA extraction from plant samples based on homogenisation by reciprocal shaking in the presence of a mixture of sand and glass beads. Plant Mol Biol Rep 14: 273–279 [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Fleißner A, Sopalla C, Weldring K-M (2002) An ATP-binding cassette multidrug-resistance transporter is necessary for tolerance of Gibberela pulicaris to phytoalexins and virulence on potato tubers. Mol Plant-Microbe Interact 15: 102–108 [DOI] [PubMed] [Google Scholar]
- Gaedeke N, Klein M, Kolukisaoglu U, Forestier C, Muller A, Ansorge M, Becker D, Mamnun Y, Kuchler K, Schulz B et al. (2001) The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J 20: 1875–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grec S, Vanham D, de Ribaucourt JC, Purnelle B, Boutry M (2003) Identification of regulatory sequence elements within the transcription promoter region of NpABC1, a gene encoding a plant ABC transporter induced by diterpenes. Plant J 35: 237–250 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Schoonbeek HJ, Waard MA (2002) Bcmfs1, a novel major facilitator superfamily transporter from Botrytis cinerea, provides tolerance towards the natural toxic compounds camptothecin and cercosporin and towards fungicides. Appl Environ Microbiol 68: 4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski M, Ducos E, Martinoia E, Boutry M (2003) The ATP-binding cassette transporters: structure, function and gene family comparison between rice and Arabidopsis. Plant Physiol 131: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13: 1095–1107 [PMC free article] [PubMed] [Google Scholar]
- Klinedinst S, Pascuzzi P, Redman J, Desai M, Arias JR (2000) A xenobiotic-stress-activated transcription factor and its cognate target genes are preferentially expressed in root tip meristems. Plant Mol Biol 42: 679–688 [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM (2002) Crosstalk between signalling pathways in pathogen defence. Current Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Li L, He Z, Pandey GK, Tsuchiya T, Luan S (2002) Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277: 5360–5368 [DOI] [PubMed] [Google Scholar]
- Logemann E, Hahlbrock K (2002) Crosstalk among stress responses in plants: pathogen defense overrides UV protection through an inversely regulated ACE/ACE type of light-responsive gene promoter unit. Proc Natl Acad Sci USA 99: 2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis during systemic acquired resistance. Nat Genet 26: 403–410 [DOI] [PubMed] [Google Scholar]
- Mladek C, Guger K, Hauser M-T (2003) Identification and characterization of the ARIADNE gene family in Arabidopsis. A group of putative E3 ligases. Plant Physiol 131: 27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PG, Cahill DM (2003) Abscisic acid influence the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30: 461–469 [DOI] [PubMed] [Google Scholar]
- Murray SL, Thomson C, Chini A, Read ND, Loake GJ (2002) Characterization of a novel, defense-related Arabidopsis mutant, cir1, isolated by luciferase imaging. Mol Plant-Microbe Interact 15: 557–566 [DOI] [PubMed] [Google Scholar]
- Nakaune R, Adachi K, Nawata O, Tomiyama M, Akutsu K, Hibi T (1998) A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl Environ Microbiol 64: 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis: structure and gene expression. Plant Physiol 129: 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE et al. (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Rairdan GJ, Delaney TP (2002) Role of salicylic acid and NIM1/NPR1 in race-specific resistance in Arabidopsis. Genetics 161: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman G, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signalling pathway in controlling cell death, defenses and cell growth. Plant Cell 11: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate DN, Greenberg JT (2001) The Arabidopsis aberrant growth and death 2 mutant shows resistance to Pseudomonas syringae and reveals a role for NPR1 in suppressing hypersensitive cell death. Plant J 27: 203–211 [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defence gene expression. Curr Opin Plant Biol 1: 401–411 [DOI] [PubMed] [Google Scholar]
- Richmond T, Somerville S (2000) Chasing the dream: plant EST microarrays. Curr Opin Plant Biol 3: 108–116 [DOI] [PubMed] [Google Scholar]
- Sasabe M, Toyoda K, Shiraishi T, Inagaki Y, Ichinose Y (2002) cDNA cloning and characterization of tobacco ABC transporter: NtPDR1 is a novel elicitor-responsive gene. FEBS Lett 518: 164–168 [DOI] [PubMed] [Google Scholar]
- Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Manners JM, Anderson JP, Simpson B, Wilson I, Somerville SC, Maclean DJ (2003) Systemic gene expression in Arabidopsis during an incompatible interaction with Alternaria brassicicola. Plant Physiol 132: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by cDNA microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonbeek H, Del Sorbo G, De Waard MA (2001) The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol Plant-Microbe Interact 14: 562–571 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Microarray monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA. Plant Cell 13: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Seto H, Koshino H, Yoshida S, Ohashi Y (2003) A diterpene as an endogenous signal for the activation of defense responses to infection with tobacco mosaic virus and wounding in tobacco. Plant Cell 15: 863–873 [PMC free article] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Nandi A, Klessig DF (2001) A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J 25: 563–574 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Goda H, Nakamura A, Takatsuto S, Fujioka S, Yoshida S (2003) Organ-specific expression of Brassinosteroid-biosynthetic genes and distribution of endogenous Brassinosteroids in Arabidopsis. Plant Physiol 131: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert PD, Chenchik A, Kellogg DE, Lukyanov KA, Lukyanov SA (1995) An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res 23: 1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler M, Hassa P, Hasan S, Ringli C, Dudler R (1998) Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 10: 1623–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Penninckx IA, Broekaert WF, Cammue BP (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Urban M, Bhargava T, Hamer JE (1999) An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J 18: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brûle S, Müller A, Fleming AJ, Smart CC (2002) The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. Plant J 30: 649–662 [DOI] [PubMed] [Google Scholar]
- van den Brûle S, Smart CC (2002) The Plant PDR family of ABC transporters. Planta 216: 95–106 [DOI] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13: 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]