Abstract
The resorption protection hypothesis, which states that anthocyanins protect foliar nutrient resorption during senescence by shielding photosynthetic tissues from excess light, was tested using wild-type (WT) and anthocyanin-deficient mutants of three deciduous woody species, Cornus sericea, Vaccinium elliottii (Chapmn.), and Viburnum sargentii (Koehne). WT Betula papyrifera (Marsh) was included to compare the senescence performance of a species that does not produce anthocyanins in autumn. Plants were subjected to three environmental regimes during senescence: an outdoor treatment; a 5-d high-stress (high light and low temperature) treatment followed by transfer to a low-stress environment and a low-stress treatment that served as control. In the outdoor treatment, the appearance of anthocyanins in senescing leaves of WT plants was concomitant with the development of photo-inhibition in mutant plants of all three anthocyanin-producing species. In the high-stress environment, WT plants maintained higher photochemical efficiencies than mutants and were able to recover when transferred to the low-stress environment, whereas mutant leaves dropped while still green and displayed signs of irreversible photooxidative damage. Nitrogen resorption efficiencies and proficiencies of all mutants in both stressful treatments were significantly lower than the WT counterparts. B. papyrifera displayed photochemical efficiencies and nitrogen resorption performance comparable with the highest of the anthocyanin-producing species in all three senescing environments, indicating a photoprotective strategy divergent from the other species studied. These results strongly support the resorption protection hypothesis of anthocyanins in senescing leaves.
The role of anthocyanins in plant foliage has long been the subject of study and speculation (for review, see Chalker-Scott, 1999; Steyn et al., 2002). Foliar anthocyanins arise in a great diversity of plant species across a broad range of environments, often occurring in response to environmental stresses such as nutrient deficiency, drought, and low temperature (Steyn et al., 2002). In many species, anthocyanins are produced at specific physiological stages, appearing in expanding, mature, or senescing leaves exposed to high light. Observations over a century ago led to the light screen hypothesis, which states that foliar anthocyanins shade the photosynthetic apparatus from excess light (for review, see Wheldale, 1916).
The resorption protection hypothesis (Hoch et al., 2001) proposed that the shading of photosynthetic tissues by anthocyanins produced during senescence helps protect the plant's ability to resorb foliar nutrients by shielding leaves from potentially harmful light levels. This hypothesis is based on the ideas that senescence-related processes lead to increased vulnerability to damage from visible light, resulting in reduced photosynthetic capacity (photo-inhibition) and that severe photo-inhibition during senescence can significantly affect a plant's ability to resorb foliar nutrients. The capacity of anthocyanins to reduce susceptibility to photo-inhibition via light attenuation is well documented (for recent review, see Steyn et al. (2002), and increased light sensitivity during senescence appears to be common (Kar et al., 1993; Bukhov, 1997; Königer et al., 2000; Polle et al., 2001). However, the existence of a direct association between anthocyanic photoprotection and enhanced nutrient resorption has yet to be established.
Senescence is a highly controlled process by which leaf components are disassembled and mobilized (Noodén et al., 1997). The transfer of nutrients, particularly nitrogen (N) and phosphorus (P), from senescing leaves to perennial tissues for storage (resorption) can commonly account for the majority of these nutrients in overwintering trees (Rosecrance et al., 1998; Niederholzer et al., 2001; Cheng and Fuchigami, 2002) and, therefore, is critical to plant fitness. May and Killingbeck (1992) demonstrated that preventing autumnal resorption in Quercus ilicifolia (Wang.) significantly reduced fitness, as defined by subsequent stem growth, foliar biomass, and fruit production. Similarly, growth and fruit production have been found to be positively correlated to levels of winter reserve N in a variety of perennial species, including apple (Malus domestica; Titus and Kang, 1982), grape (Vitis vinifera; Roubelakis-Angelakis and Kliewer, 1992), pear (Pyrus communis; Sanchez et al., 1990), peach (Prunus persica; Rosecrance et al., 1998), and nectarine (Prunus persica; Tagliavini et al., 1998). In addition, it appears likely that resorption cannot proceed without a competent photosynthetic system to supply the needed energy for catabolic activity and photosynthate to drive the nutrient-laden translocation stream (Chapin and Moilanen, 1991; May and Killingbeck, 1992). Clearly, photoprotection during senescence can impact plant fitness.
In this study, wild-type (WT) and anthocyanin-deficient mutants of three deciduous woody species, Cornus sericea, Vaccinium elliottii (Chapmn.), and Viburnum sargentii (Koehne), were used to test the resorption protection hypothesis of anthocyanins in senescing foliage. These mutants allowed for the comparison of photosynthetic and resorption performance between anthocyanic and nonanthocyanic leaves of similar age and light exposure within and across three different senescing environments. Betula papyrifera (Marsh) was included in this study to compare the senescence activity of a species that does not produce anthocyanins in autumn. This study focuses on N resorption because N is the most common growth-limiting nutrient in ecosystems (Vitousek and Howarth, 1991), and the resorption of N and P are significantly correlated over a broad range of perennial genera (Aerts, 1996; Killingbeck, 1996).
RESULTS
Resorption proficiencies (levels to which N is reduced in senescing leaves, i.e. greater proficiency = lower content) and efficiencies (percentage of N recovered from senescing leaves) of plants from the three environmental regimes are indicated in Table I. Within the low-stress environment, resorption proficiencies and efficiencies of mutant plants relative to the WT counterparts were higher for V. elliottii, comparable for C. sericea, and lower for V. sargentii. In contrast to the low-stress environment, outdoor and high-stress regime resorption proficiencies and efficiencies were significantly lower for mutant relative to WT plants of all three anthocyanin-producing species. Although the resorption performance of mutant V. sargentii was lower than WT plants in the low-stress environment, resorption proficiencies and efficiencies of these plants in the low-stress environment were significantly higher than in the outdoor and high-stress regimes. B. papyrifera displayed resorption proficiencies and efficiencies equivalent to the highest of the anthocyanin-producing species in all three environments.
Table I.
Changes in leaf N content, leaf mass per area (LMA), and anthocyanin reflectance indices for mutant and wild-type plants of each anthocyanin-producing species and Betula papyrifera within three different environmental regimes
Means with the same letter are not significantly different at α = 0.05 within each treatment. Data are means ± se (n = 6).
| Species/Treatment/Plant | Presenescent N Content | Senesced N Content (Resorption Proficiency) | Resorption Efficiency | Presenescent LMA | Senesced LMA | Change in LMA | Senesced Anthocyanin Index |
|---|---|---|---|---|---|---|---|
| g m-2 | % | g m-2 | % | (R550)-1 - (R700)-1 | |||
| Low stress | |||||||
| C. sericea | |||||||
| WT | 0.89 ± 0.01d | 0.16 ± 0.01a | 82 ± 1.1a | 60.3 ± 1.1d | 46.2 ± 1.8b | -23 | 18.9 ± 0.4a |
| Mutant | 1.13 ± 0.02a | 0.17 ± 0.01ab | 85 ± 0.9a | 70.1 ± 3.9b | 49.2 ± 2.7b | -29 | 0.52 ± 0.08ba |
| V. elliottii | |||||||
| WT | 0.93 ± 0.01cd | 0.22 ± 0.01c | 77 ± 0.7b | 74.3 ± 1.8b | 67.0 ± 1.7a | -10 | 19.0 ± 0.9a |
| Mutant | 0.60 ± 0.02e | 0.16 ± 0.01ab | 73 ± 1.4c | 63.3 ± 0.54cd | 53.7 ± 1.3b | -15 | 0.54 ± 0.06ba |
| V. sargentii | |||||||
| WT | 0.90 ± 0.05d | 0.19 ± 0.01bc | 79 ± 1.2b | 59.7 ± 1.2d | 46.7 ± 3.4b | -22 | 19.8 ± 1.1a |
| Mutant | 1.09 ± 0.04ab | 0.38 ± 0.03d | 65 ± 1.5d | 92.0 ± 2.6a | 69.0 ± 4.9a | -25 | 0.38 ± 0.1ba |
| B. papyrifera | |||||||
| WT | 1.00 ± 0.06bc | 0.16 ± 0.01ab | 84 ± 0.7a | 69.0 ± 1.9bc | 49.0 ± 1.4b | -29 | 0.42 ± 0.1ba |
| Outdoor | |||||||
| C. sericea | |||||||
| WT | 0.92 ± 0.04d | 0.19 ± 0.01a | 79 ± 0.9a | 93.0 ± 0.6bc | 64.2 ± 3.9e | -31 | 19.7 ± 0.5b |
| Mutant | 1.02 ± 0.05cd | 0.40 ± 0.03c | 61 ± 0.6c | 93.7 ± 4.5bc | 101.8 ± 4.4bc | 9b | 0.87 ± 0.08ca |
| V. elliottii | |||||||
| WT | 1.20 ± 0.05ab | 0.28 ± 0.01b | 76 ± 1.3ab | 123.0 ± 5.1a | 104.5 ± 2.5b | -15 | 22.1 ± 0.5a |
| Mutant | 0.73 ± 0.02e | 0.40 ± 0.02c | 45 ± 1.1d | 92.0 ± 3.7c | 93.8 ± 2.7c | 2b | 0.72 ± 0.08ca |
| V. sargentii | |||||||
| WT | 1.14 ± 0.06abc | 0.31 ± 0.02b | 73 ± 1.6b | 95.3 ± 3.1bc | 65.0 ± 4.8e | -32 | 20.4 ± 1.1ab |
| Mutant | 1.24 ± 0.02a | 0.76 ± 0.02d | 39 ± 0.8e | 122.3 ± 0.8a | 120.8 ± 3.5a | -1b | 0.49 ± 0.2ca |
| B. papyrifera | |||||||
| WT | 1.11 ± 0.03bc | 0.23 ± 0.01a | 79 ± 1.3a | 102.3 ± 2.7b | 81.8 ± 1.9d | -20 | 0.29 ± 0.09ca |
| High stress | |||||||
| C. sericea | |||||||
| WT | 0.90 ± 0.02c | 0.23 ± 0.03ab | 75 ± 2.0ab | 62.7 ± 1.8c | 48.3 ± 3.0d | -23 | 19.8 ± 0.9a |
| Mutant | 1.10 ± 0.02a | 0.36 ± 0.02c | 67 ± 1.4c | 69.8 ± 3.0b | 60.2 ± 3.1c | -14 | 0.68 ± 0.2ba |
| V. elliottii | |||||||
| WT | 0.89 ± 0.02c | 0.19 ± 0.01a | 79 ± 1.0a | 70.7 ± 1.8b | 63.5 ± 1.8bc | -10 | 20.1 ± 0.4a |
| Mutant | 0.65 ± 0.04d | 0.39 ± 0.01c | 40 ± 2.1d | 63.8 ± 1.5c | 66.1 ± 1.3b | 4b | 0.58 ± 0.1ba |
| V. sargentii | |||||||
| WT | 0.87 ± 0.05c | 0.28 ± 0.01b | 68 ± 1.3bc | 58.6 ± 1.4c | 49.3 ± 1.2d | -16 | 18.5 ± 0.7a |
| Mutant | 1.11 ± 0.04a | 0.84 ± 0.03d | 24 ± 0.9e | 90.7 ± 2.2a | 76.8 ± 6.4a | -15 | 0.24 ± 0.06ba |
| B. papyrifera | |||||||
| WT | 1.02 ± 0.05b | 0.21 ± 0.03a | 80 ± 1.5a | 73.3 ± 2.4b | 50.2 ± 1.1d | -32 | 0.23 ± 0.6ba |
aAnthocyanin values are not significantly different than presenescent, anthocyanin-free leaves.
b Change in LMA is not significantly different from zero at α = 0.05.
LMA of WT plants decreased within all three senescing environments (Table I). Mutant LMA also decreased within the low-stress environment and in the high-stress regime for mutant C. sericea and V. sargentii. LMA did not change significantly for all mutants within the outdoor environment and for mutant V. elliottii within the high-stress regime.
Anthocyanin reflectance indices, which were used to measure foliar anthocyanin levels, were not significantly different for senesced leaves among WT plants of the three anthocyanin-producing species within the low-stress treatment (Table I). The only significant difference in anthocyanin levels within all treatments was in V. elliottii WT plants in the outdoor treatment, which produced somewhat higher values than the other plant types. Because WT plants were not moved into the low- and high-stress treatments until the accumulation of anthocyanins, the absence of significant differences in anthocyanin levels between treatments might be expected. No anthocyanin accumulation was observed in senesced leaves of mutant and B. papyrifera plants because anthocyanin reflectance indices from these plants were within the range obtained from presenescent, anthocyanin-free leaves of both WT and mutant plants.
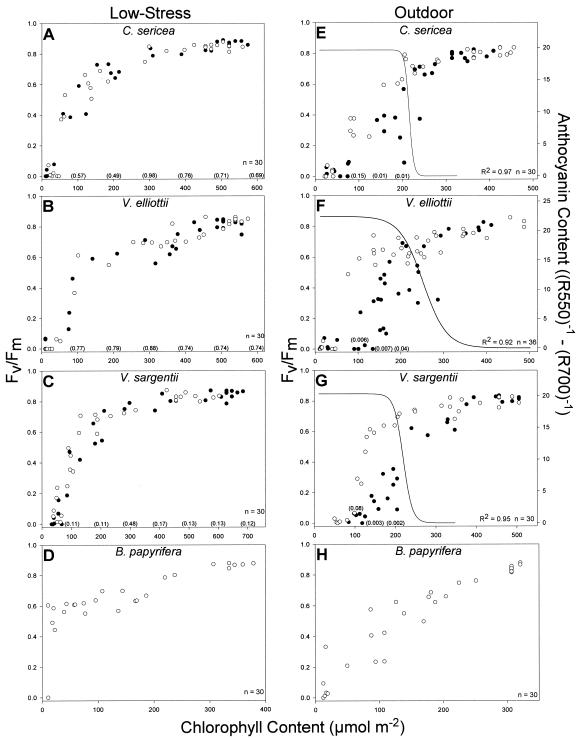
The relationships between chlorophyll (Chl) content and photochemical efficiency (Fv/Fm) for the seven plant types in the low-stress treatment are shown in Figure 1, A to D. Photochemical efficiencies as a function of Chl content were not significantly different between WT and anthocyanin-deficient mutants within each species, based on quadratic lines fitted to each plant type (P values indicated in Fig. 1, A–C). For the three anthocyanin-producing species, this relationship can generally be described as a gradual loss of Fv/Fm until Chl content falls below approximately 150 to 200 μmol m–2, when Fv/Fm drops off dramatically until both values approach zero. In B. papyrifera, the gradual loss of Fv/Fm above approximately 200 μmol m–2 Chl content was similar to the anthocyanin-producing species. However, below approximately 200 μmol m–2 Chl content, B. papyrifera displayed greater stability, maintaining a relatively high Fv/Fm until Chl levels became nearly zero.
Figure 1.
Progression during senescence of the relationship between Fv/Fm and Chl content for mutant (•) and WT (○) plants of each anthocyanin-producing species and B. papyrifera for the low-stress (A–D) and outdoor (E–H) treatments. P values for differences in Fv/Fm between mutant and WT regressions are indicated parenthetically on the graphs at intervals of 100 μmol m–2 Chl content for the low-stress treatment and 50 μmol m–2 Chl content for non-senesced anthocyanic leaves for the outdoor treatment. The development of anthocyanins in senescing leaves of WT plants from the outdoor treatment is indicated on the graphs of the anthocyanin-producing species by a three-parameter sigmoid line: [y = a/1 + e ^ – (x – x0/b)], representing the leaf reflectance index (R550)–1 – (R700)–1 for each data point on the graphs.
In contrast to the low-stress senescing environment, Fv/Fm as a function of Chl content in plants grown in the outdoor treatment was significantly different between WT and mutant plants for all three anthocyanin-producing species (P values indicated in Fig. 1, E–G). These statistical inferences are based on differences in lines fitted to each plant type for data representing the period when anthocyanic photoprotection would normally be relevant (non-senesced leaves with Chl contents below the center point [x0] of the sigmoid curve representing the development of anthocyanins in WT plants). x0 (μmol m–2 Chl content) of the sigmoid curves were 215.5 for C. sericea, 251 for V. elliottii, and 218.1 for V. sargentii. In all three species, significantly lower photochemical efficiencies appeared in mutant plants when Chl content dropped below approximately 250 μmol m–2. As Chl declines, anthocyanins appear very near the onset of significantly lower photochemical efficiencies in mutants relative to WT plants. The rapid accumulation of anthocyanins in relation to changes in Chl content detected here are similar to that observed in other studies (Moore, 1965; Sanger, 1971). As in the low-stress treatment, the decrease in Fv/Fm of B. papyrifera was more linear than that of the three anthocyanin-producing species, although the rate of decline was faster in the outdoor treatment. When viewed on the basis of Chl content, the Fv/Fm of B. papyrifera in the outdoor treatment was higher than WT plants of the other species, not dropping below an Fv/Fm of 0.2 until Chl content was <50 μmol m–2.
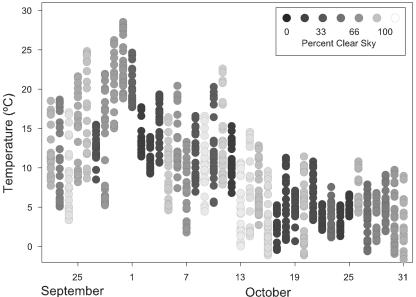
Plants in the outdoor treatment were exposed to conditions that would have likely caused photooxidative stress (Wise, 1995), providing an effective test for the photoprotective effects of anthocyanins on nutrient recovery in senescing leaves. Figure 2 indicates that a number of highly photo-inhibitory days (high light and low temperatures) were experienced during the time of leaf nutrient recovery before the collection of senesced leaves, which took place between October 21 and November 5. Between September 22 and October 21, 15 d were both bright (>60% clear sky) and had temperatures <10°C at 9:00 am. Six of these days had 9:00 am temperatures <5°C.
Figure 2.
Vertical point plot of hourly temperatures and each day's percentage clear sky (indicated by black circle shading) for the outdoor treatment for the period of September 22 through October 31.
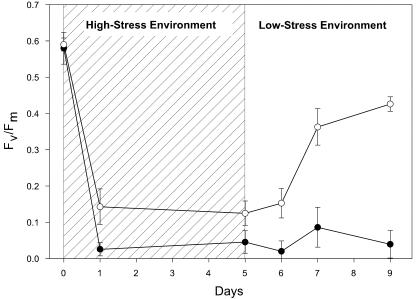
Effects of the high-stress treatment on WT and mutant V. elliottii are displayed in Figure 3. Although both WT and mutant plants showed a decrease in Fv/Fm because of the high-stress (high light/low temperature) environment, WT V. elliottii plants maintained a significantly higher Fv/Fm. After transfer of the plants from the high- to low-stress environment, WT plants were able to recover from the photooxidative damage over a period of 4 d and return to Fv/Fm levels that were similar to WT plants of comparable Chl content from the low-stress treatment.
Figure 3.
Time course for changes in Fv/Fm for mutant (•) and WT (○) plants of V. elliottii during high-stress treatment and recovery period. Data are means ± SEM (n = 6).
Leaves of mutant plants subjected to the high-stress treatment dropped from the plants while still green and often exhibited necrotic lesions similar to those observed by Karpinski et al. (1999), indicating irreversible photooxidative damage. Although only V. elliottii were measured at intervals of adequate frequency to effectively display the effects of the high-stress treatment on the decline and recovery of Fv/Fm, the responses of C. sericea and V. sargentii to the high-stress treatment were comparable with V. elliottii as indicated by analogous findings of: (a) irreversible photooxidative damage in mutant plants, (b) similar differences in resorption performance between mutant and WT plants, and (c) higher Fv/Fm measurements for WT plants in the high-stress environment and during recovery (data for C. sericea and V. sargentii not shown).
DISCUSSION
This study provides the first experimental evidence that anthocyanins facilitate the recovery of foliar nutrients by protecting senescing leaves from excess light. The association between lower Fv/Fm and lower resorption performance in mutant versus WT plants of all three anthocyanin-producing species within the two stressful environments, together with the comparable values of these measurements between mutant and WT plants in the low-stress treatment, strongly supports the resorption protection hypothesis of anthocyanins in senescing leaves. The close relationship between the appearance of anthocyanins in senescing leaves of WT plants in the outdoor treatment and the development of photo-inhibition in mutant plants suggests an important period of physiological change within the senescing leaves of these species, where the need for the additional photoprotection provided by anthocyanins becomes critical. The increased resorption performance of WT versus mutant plants subjected to the high-stress treatment is further evidence of the ability of anthocyanins to reduce the potential for oxidative damage via light attenuation (Steyn et al., 2002), which allowed WT plants to withstand the high-stress environment until conditions were satisfactory for the completion of senescence.
The low-stress treatment provided a functional control to the high-stress and outdoor treatments, demonstrating that in the absence of photo-inhibitory conditions, mutant and WT plants were similar in Fv/Fm and resorption performance during senescence. The one exception to this, the mutant clone of V. sargentii, displayed photochemical efficiencies similar to WT plants as Chl levels declined, yet demonstrated significantly lower resorption proficiency (higher N content in senesced leaves). The fact that the lower resorption proficiency of V. sargentii mutants was not associated with differences in Fv/Fm relative to WT plants in the low-stress treatment may indicate that factors other than photoprotection were involved in the lower resorption activity. One factor that likely contributed to differences in resorption proficiency between mutant and WT V. sargentii plants was the large discrepancy in presenescent LMA, which typically would result in a higher minimum potential N content during senescence. Although mutants of this species displayed lower resorption performance than WT plants in the low-stress treatment, the large differences in resorption proficiencies and efficiencies between V. sargentii mutants in the low-stress treatment and those in the high-stress and outdoor treatments are consistent with the reduced photoprotective capacities of the other anthocyanindeficient phenotypes examined in this study.
The association between anthocyanic photoprotection and resorption performance in this study is compatible with the concept that source-sink relationships may strongly influence resorption activity. The presence of strong sinks such as new growth (Nambiar and Fife, 1987), developing fruit (Chapin and Wardlaw, 1988), and catkins (Chapin and Moilanen, 1991) have been shown to increase nutrient resorption from leaves. Similarly, reducing the source of photosynthate by shading senescing leaves has been found to reduce the amount of nutrients recovered from these leaves (Chapin and Moilanen, 1991; May and Killingbeck, 1992). In this study, the loss of Fv/Fm in mutant plants reduced the source of photosynthate needed to drive the translocation stream exporting nutrients from leaves and to supply energy for the many senescence-related processes.
For this study, resorption proficiency (level to which N is reduced in senesced leaves) was probably more indicative of senescence performance than was resorption efficiency (percentage of N recovered from senescing leaves). The focus of these experiments was to determine the extent and consequences of anthocyanic photoprotection during senescence, as measured by Fv/Fm and resorption performance during the later portion of senescence, which is the period when anthocyanins are present. Although both measurements have weaknesses, the results of this study suggest that resorption proficiency more accurately reflect a plant's ability to maintain photosynthetic activity and nutrient export late into senescence as photosynthetic instabilities continue to increase. Resorption efficiency, on the other hand, appears to be more commonly affected by differences in presenescent N content between plant types, as they were in this study. For example, in the low-stress treatment, V. elliottii mutants displayed significantly better resorption proficiency (0.16 g N m–2) than did the WT counterpart (0.22 g N m–2). In contrast, the resorption efficiencies of these same plants would indicate better resorption performance by WT (77%) versus mutant plants (73%), due to the 55% greater presenescent leaf N content of WT plants. In general, leaf N content slowly declines from peak values in early to mid summer until the onset of senescence, when nutrients are exported more rapidly (Chapin and Moilanen, 1991; Collier and Thibodeau, 1995; Königer et al., 2000). A source of error within the presenescent N content measurements is variation in timing of presenescent nutrient resorption between plant types, leading to disparities in presenescent values between plants that had exported different amounts of N by the time of sampling.
The lowest N content means of senesced leaves in this study were comprised of seven different values ranging from 0.16 to 0.19 g N m–2, which are comparable with the ≈0.20 g N m–2 described by Killingbeck (1996) as the current best estimate of ultimate potential resorption. Several factors probably contributed to these means, which are somewhat lower than the lowest means commonly found by others (0.36–0.43 g N m–2; for review, see Aerts, 1996; Killingbeck, 1996). First, the near-optimum senescing climate of the low-stress environment likely contributed to the low N content in senesced leaves. Nine of the 10 lowest senesced N content means in this study came from plants that had finished senescing in the low-stress environment. In addition, leaves of these nine plant types were adapted to the light environment of the shade structure and, therefore, possessed lower LMA and N content than full-sun leaves, a characteristic that may have facilitated greater resorption proficiency measured on a per-area basis. In addition, the age of the plants and the culture method used in this study likely played a role in the low senesced N content observed. Plants were container grown and relatively young (2–4 years of age), whereas plants examined in the reviewed studies were growing in natural ecosystems and, in general, were much older and larger. In a study of ramets from a single Populus tremuloides clone that ranged in age from 4 to 15 years, Killingbeck et al. (1990) found that N resorption proficiency and efficiency decreased as plant height and age increased, suggesting that resorption is partially a function of plant size and/or age. Characteristics that may potentially arise because of the container environment, such as higher rates of root respiration and increased root to shoot ratios, could also increase resorption proficiency through improved sink strength for photosynthate and foliar N during senescence.
The reductions in LMA observed in WT plants within each treatment are comparable with other studies where decreases in LMA ranged from 16% to 42% (Oland, 1963; Adams et al., 1990; Collier and Thibodeau, 1995; Polle et al., 2001). Senescence-related reductions in LMA may be expected because senescence activity normally results in an increasingly carbon-starved environment within the leaf (Buchanan et al., 2000). For example, as photosynthetic capacity progressively declines during senescence, N is exported in organic form as amino acids (e.g. Gln and Asn), nutrient export requires the continued phloem loading of sugars, and the numerous senescence-related catabolic activities impose energy demands upon the leaf (Feller and Fischer, 1994). Cumulatively, senescence-related processes result in a net decline in leaf carbon and, therefore, lower LMA. The lack of change in LMA of mutant plants in the outdoor treatment despite a measurable amount of resorption activity, which might otherwise be expected to reduce LMA, may be the result of an increase in foliar starch content. An accumulation of starch within leaves has been linked to a variety of stresses that increased reactive oxygen species formation, including excess light (Layne and Flore, 1995; Kadlecek et al., 2001), nutrient deficiency (Godde and Hefer, 1994), and ozone (Schiffgens-Gruber and Lutz, 1992; Ranieri et al., 2001). Oxidative stress resulting from the reduced photoprotective capacity of anthocyanin-deficient mutants in the outdoor treatment could well have caused starch accumulation in chloroplasts, explaining the absence of LMA reduction in senescent leaves. Photooxidative damage within the high-stress treatment quickly overwhelmed the photosynthetic apparatus of mutants, precluding the production of starch, which could account for the reduction in LMA observed in two mutants within this treatment.
Although relatively large differences in resorption were observed between mutant and WT plants within the outdoor and high-stress treatments, this effect is not applicable to every leaf on the plant. Because photo-inhibition is a high-light phenomenon, we anticipate that this mechanism is only protective for leaves that experience full sunlight for prolonged periods. Only leaves that were exposed to direct light were used in this study. The reduced production or absence of anthocyanins in shaded leaves within the canopy of senescing plants (Kozlowski and Pallardy, 1997) suggests a diminished need for these pigments in reduced light. This supports the conclusions of Feild et al. (2001), who found no difference in N recovery between anthocyanic sun leaves and nonanthocyanic shade leaves of C. sericea. We conclude that internal shading would be predicted to improve resorption performance in the absence of anthocyanins, somewhat diluting the overall effectiveness of this mechanism on a whole-plant basis.
Alternative explanations for the differences between mutant and WT plants observed in this study may reside in possible pleiotropic effects of the anthocyanin-deficient mutations. No inherent weaknesses of the anthocyanin-deficient mutants were evident because differences in overall vigor were not perceived between mutant and WT plants within each species. One theory concerning the presence of autumnal anthocyanins suggests that these pigments increase the freezing tolerance of leaves (for review, see Chalker-Scott, 1999). However, it is improbable that increased freezing-tolerance is germane to this study because the temperature in the high-stress treatment did not drop below 2°C, and only light frosts (–1.1°C) were experienced in the outdoor treatment until the evening of October 31, by which time almost all of the senesced leaves had been collected. In addition, the fact that the three mutants used in this study represent distinct mutation events in separate species reduces the likelihood that similar pleiotropic effects would exist in all mutants.
Although the evidence presented here strongly supports the resorption protection hypothesis of anthocyanins in senescing leaves, anthocyanins are only one of many different means of photoprotection during senescence. The anthocyanin-producing species and B. papyrifera examined in this study demonstrated divergent photoprotective strategies that were equivalent in terms of resorption performance. Thus, species that do not utilize autumnal anthocyanins have likely evolved a number of photoprotective combinations that make anthocyanins unnecessary in senescing leaves.
MATERIALS AND METHODS
Plant Material
The Vaccinium elliottii (Chapmn.) anthocyanin-deficient mutants used in this study were a full-sib population of V. elliottii homozygous for the y (yellow leaf) allele (Lyrene, 1988). The other two anthocyanin-deficient mutants were clonally propagated individuals of Cornus sericea `Flaviramea' L. and Viburnum sargentii `Flavum' (Koehne). Lyrene (1988) found the yellow leaf phenotype to be inherited as a single recessive allele (y), and unpublished data (D. Kromm, personal communication) indicate that the anthocyanin-deficient phenotypes of the C. sericea and V. sargentii mutants are inherited in the same fashion.
Mutant V. elliottii were obtained from Paul Lyrene (University of Florida, Gainesville). WT V. elliottii were purchased from Mail-Order Natives (Lee, FL). Both V. elliottii varieties were potted into 7.6-L (no. 2) containers filled with peat moss. WT and mutant plants of C. sericea were obtained from Gro-Plant Nursery (Princeton, WI). All C. sericea were potted in 11.4-L (no. 3) containers filled with composted bark-based media. WT and mutant V. sargentii were purchased balled and burlaped from Beaver Creek Nursery (Poplar Grove, IL). All V. sargentii were potted into 26.5-L (no. 7) containers filled with Metro-Mix soil-less potting medium (Scotts-Sierra Horticultural Products, Marysville, OH.). Betula papyrifera (Marsh) `Renaissance Reflections' were obtained from Evergreen Nursery (Sturgeon Bay, WI) and grown in 7.6-L (no. 2) containers filled with Metro-Mix soil-less potting medium.
Plants were potted before the appearance of leaves in the spring of 2002 and grown in two environments until the start of experiments in autumn. Plants for the outdoor treatment were grown in an outdoor cold frame throughout the growing season. Plants for use in the two controlled environments were acclimated to the light levels of these treatments by growing outdoors in a 3.7- × 4.9- × 2.4-m wood-framed structure covered with 55% polypropylene shade cloth (Cornelia Textiles, Cornelia, GA) until movement to the growth chambers in fall. All plants were fertilized three times at 4-week intervals starting in May with 200 mg N L–1 from 20N-20P-20K water-soluble fertilizer (Peter's Professional, Scotts-Sierra Horticultural Products).
Treatments
Mutant and WT plants of each species were moved together into the controlled environments when anthocyanins appeared in WT plants: October 16 for C. sericea, October 21 for V. sargentii, and October 23 for V. elliottii. Plants of B. papyrifera were moved into the controlled environments on October 21 when Chl content reached the level at which anthocyanins appeared in WT plants of the anthocyanin production species, approximately 200 to 250 μmol m–2. Plants were randomized within each environment.
Three replicates of each of the seven plant varieties were tested within three different environments during senescence as follows.
Outdoor
An outdoor cold frame in Madison, WI exposed to full sunlight and ambient temperatures throughout the 2002 growing season and autumn senescence period was utilized. Temperature was recorded hourly using an Optic Stowaway Temperature Logger (model WTA08-05 + 37, Onset Computer Corp., Pocasset, MA) fastened inside of a 15.2-cm diameter white polyvinyl chloride pipe end cap (Lasco Fittings Inc., Brownsville, TN) positioned 1 m above the ground. Daily percentage clear sky data were obtained from the University of Wisconsin Automated Weather Observation Network (Madison).
Low Stress
A low-stress senescing environment was created in a 9.5-m2 plant growth room at the University of Wisconsin Biotron facility (Madison): 10-h day length, 380 μmol m–2 s–2 light, 20°C day temperature, and the night temperature was stepped down at approximately 3°C h–1 to 4°C before stepping back up to 20°C when the next day cycle began.
High Stress
A 3.3-m2 walk-in growth chamber (model PGW-136C, Percival Scientific, Boone, IA) was used for the high-stress treatment. The high-stress environment consisted of a 10-h day length, 900 μmol m–2 s–2 light, 3°C day temperature, and 2°C night temperature. The high-stress treatment was given to each species for 5 d before the plants were moved to the low-stress environment in the Biotron facility for the remainder of the senescence period. The light source for both controlled environments was supplied by a combination of cool-white fluorescent (1,500 W very high output) and incandescent bulbs. Photosynthetic photon flux densities were measured with a quantum meter (LI-COR Inc., Lincoln, NE).
Sampling
Leaves for use in the study were selected in early August. For each plant type, leaves of similar Chl content (within 40 μmol m–2 Chl) were identified using a Minolta SPAD-502 meter (Minolta Corp., Osaka) as described below, and the nodes were labeled with 3.8- × 2.4-cm marking tags (Avery Dennison Corp., Pasadena, CA). Only undamaged leaves that were positioned on the plant to be completely exposed to direct light were chosen.
Presenescent values for Chl fluorescence and anthocyanin, Chl and N contents were determined by sampling on August 28 for the outdoor treatment and September 3 for the two controlled environment treatments. Senesced leaves were collected from V. sargentii and C. sericea by tethering leaves to the adjacent stem using 3.8- × 2.4-cm marking tags (Avery Dennison Corp.) tied to the leaf petiole. Due to the extremely short petiole of V. elliottii leaves, senescent leaves of this species were collected daily by placing a piece of paper beneath the selected branches and tapping the stem to dislodge any weakly attached leaves.
Chl Fluorescence Measurements
After the presenescent sampling, Chl fluorescence was measured at approximately 2-week intervals until the time that anthocyanins began to appear in WT plants, after which samples were taken at intervals of approximately 1 week, except for the high-stress treatment measurements as described in Figure 3. At each sampling date, two leaves from each of the three replicates of each plant type were removed for analysis.
An Opti-Sciences OS5-FL modulated Chl fluorometer using FL-DC dark adaptation cuvettes (Opti-Sciences, Tyngsboro, MA) was used for all measurements. Photo-inhibition of PSII was measured as a decrease in Fv/Fm. Leaves were dark adapted for 30 min at room temperature before Fv/Fm measurements were taken. Dark adaptation was initiated within 10 min of removing leaves from the plant. All Fv/Fm measurements were performed between 11 am and 12 pm. For plants grown outdoors, Fv/Fm measurements were only taken on days that were mostly clear (>60% clear sky), with the exception of senesced leaves, which were analyzed on the day they were collected.
Pigment Analysis
For each leaf that had been analyzed for Chl fluorescence, leaf Chl content was determined using a Minolta SPAD-502 meter. A SPAD value was obtained for each leaf by taking the average of four readings, one from each quadrant of the leaf. To calibrate the meter to the species being used, Chl content was measured using the dimethyl sulfoxide Chl extraction technique of Hiscox and Israelstam (1979) and compared with the SPAD meter value after translation into leaf Chl content using the exponential and polynomial equations described by Markwell et al. (1995). The exponential equation: Chl (micromoles per meter squared) = 10(M ^ 0.265) (Markwell et al., 1995) was most accurate, being within 5% of the dimethyl sulfoxide-determined value, except when leaf Chl content became very low (<100 μmol m–2).
After Chl content measurement, each leaf's anthocyanin reflectance indices were determined nondestructively using a LI-COR LI-1800 spectrora-diometer equipped with an integrating sphere LI-1800-IS as described by Gitelson et al. (2001). An anthocyanin reflectance index in the form (R550)–1 – (R700)–1 was used to estimate foliar anthocyanin levels (Gitelson et al., 2001). A three-parameter sigmoid line [y = a/1 + e ^ – (x – x0/b)] was generated for each anthocyanin-producing species with data from the outdoor treatment.
Leaf N Analysis
N content was determined for presenescent and senesced samples of each plant type in all environments. Two samples/plant were analyzed, with each sample consisting of one leaf for B. papyrifera, C. sericea, and V. sargentii and eight leaves for the small-leaved V. elliottii. The same leaves used for presenescent and senesced Chl fluorescence and anthocyanin measurements were used for N analysis, and in the case of V. elliottii, six additional leaves were added to provide adequate tissue for analysis.
Leaf samples were digested using the Kjeldahl method (H2SO4), and total N concentration (percentage) was determined by flow injection spectrometer (Lachat Instruments, Milwaukee, WI). Results were converted to N content (grams per meter squared). Before analysis, petioles were removed, leaf area was measured using an LI-3100 area meter, and the leaves were oven dried at 60°C and weighed.
Data Analysis
Resorption proficiency is defined as the level to which a nutrient is reduced in a senescing leaf, reported here for N as content (grams per meter squared). Resorption efficiency is defined as the percentage of a nutrient recovered from a senescing leaf and is calculated here as: [(N content in presenescent leaf) – (N content in senesced leaf)/(N content in presenescent leaf)] × 100. LMA is reported as grams per meter squared.
Significant differences in photochemical efficiencies as a function of leaf Chl content were determined by least square means comparisons along fitted lines using SAS proc mixed (SAS Software version 8.0, SAS Institute, Cary, NC; Littell et al., 1996) with a “repeated” measures statement to account for covariances between measurements made on the same individuals and/or the same sampling dates. For the low-stress treatment, data for each species were fitted with a quadratic line function, and significant differences between mutant and WT lines were determined at intervals of 100 μmol m–2 Chl content. Data for the outdoor treatment were more complex in shape for mutant plants, which precluded the use of a single fitted line that could be properly analyzed for significant differences across the entire length of the graph. Therefore, for the outdoor treatment only, data from leaves that would normally be affected by anthocyanic photoprotection were analyzed (non-senesced leaves that were below the Chl content defined by x0 representing WT anthocyanin levels for each species). Lines fitted to data within this range were analyzed for significant differences at intervals of 50 μmol m–2 Chl content. Means of presenescent and senesced LMA and N content, resorption efficiency, and senesced anthocyanin reflectance indices were subjected to ANOVA using SAS proc mixed. Leaves and plants were considered random in determining the expected mean squares and appropriate F test in the ANOVA. Mean comparisons were made using Fisher's protected lsd test (α = 0.05).
Acknowledgments
The authors express their sincere appreciation to John Stier (Department of Horticulture, University of Wisconsin, Madison), John Norman (Department of Soil Science, University of Wisconsin, Madison), and Palle Pedersen (Department of Agronomy, Iowa State University, Ames) for their generosity in supplying equipment to this project. We also thank Peter Crump (Department of Computing and Biometry, University of Wisconsin, Madison) and Palle Pedersen for their help with statistical analysis and Thomas Sharkey (Department of Botany, University of Wisconsin, Madison) for technical assistance. We are grateful to Ronald Amos (Evergreen Nursery, Sturgeon Bay, WI) and Paul Lyrene (Department of Horticultural Sciences, University of Florida, Gainesville) for supplying plant material for the study.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027631.
References
- Adams IIIWW, Winter K, Schreiber U, Schramel P (1990) Photosynthesis and chlorophyll fluorescence characteristics in relationship to changes in pigment and element composition of leaves of Platanus occidentalis L. during autumnal leaf senescence. Plant Physiol 93: 1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84: 597–608 [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- Bukhov NG (1997) Leaf senescence: an evaluation of limiting steps in photosynthesis by means of chlorophyll fluorescence-quenching coefficients and P700 redox changes in leaves. Russ J Plant Physiol 44: 303–310 [Google Scholar]
- Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70: 1–9 [Google Scholar]
- Chapin IIIFS, Moilanen L (1991) Nutritional controls over nitrogen and phosphorus resorption from Alaskan birch leaves. Ecology 72: 709–715 [Google Scholar]
- Chapin IIIFS, Wardlaw IF (1988) Effect of phosphorus deficiency on source-sink interactions between the flag leaf and developing grain of barley. J Exp Bot 39: 165–177 [Google Scholar]
- Cheng L, Fuchigami LG (2002) Growth of young apple trees in relation to reserve nitrogen and carbohydrates. Tree Physiol 22: 1297–1303 [DOI] [PubMed] [Google Scholar]
- Collier DE, Thibodeau BA (1995) Changes in respiration and chemical content during autumnal senescence of Populus tremuloides and Quercus rubra leaves. Tree Physiol 15: 759–764 [DOI] [PubMed] [Google Scholar]
- Feild TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn: the role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127: 566–574 [PMC free article] [PubMed] [Google Scholar]
- Feller U, Fischer A (1994) Nitrogen metabolism in senescing leaves. Crit Rev Plant Sci 13: 241–273 [Google Scholar]
- Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol 74: 38–45 [DOI] [PubMed] [Google Scholar]
- Godde D, Hefer M (1994) Photoinhibition and light-dependent turnover of the D1 reaction-centre polypeptide of photosystem II are enhanced by mineral-stress conditions. Planta 193: 290–299 [Google Scholar]
- Hiscox JD, Israelstam FG (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bo 57: 1332–1334 [Google Scholar]
- Hoch WA, Zeldin EL, McCown BH (2001) Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol 21: 1–8 [DOI] [PubMed] [Google Scholar]
- Kadlecek P, Ticha I, Haisel D, Capkova V, Shafer C (2001) Importance of in vitro pretreatment for ex vitro acclimatization and growth. Pant Sci 161: 95–701 [Google Scholar]
- Kar M, Streb P, Hertwig B, Feierabend J (1993) Sensitivity to photodamage increases during senescence in excised leaves. J Plant Physiol 141: 538–544 [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–656 [DOI] [PubMed] [Google Scholar]
- Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77: 1716–1727 [Google Scholar]
- Killingbeck KT, May JD, Nyman S (1990) Foliar senescence in an aspen (Populus tremuloides) clone: the response of element resorption to interramet variation and timing of abscission. Can J For Res 20: 1156–1164 [Google Scholar]
- Königer M, Harris GC, Kibler E (2000) Seasonal changes in the physiology of shade leaves of Acer saccharum. J Plant Physiol 157: 627–636 [Google Scholar]
- Kozlowski TT, Pallardy SG (1997) Physiology of Woody Plants. Academic Press, Sand Diego, pp 159–172
- Layne DR, Flore JA (1995) End-product inhibition of photosynthesis in Prunus cerasus L. in response to whole-plant source-sink manipulation. J Am Soc Hortic Sci 120: 583–599 [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS System for Mixed Models. SAS Institute Inc., Cary, NC
- Lyrene PM (1988) An allele for anthocyanin-deficient foliage, buds and fruit in Vaccinium elliottii. J Hered 79: 80–82 [Google Scholar]
- Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res 46: 467–472 [DOI] [PubMed] [Google Scholar]
- May JD, Killingbeck KT (1992) Effects of preventing nutrient resorption on plant fitness and foliar nutrient dynamics. Ecology 73: 1868–1878 [Google Scholar]
- Moore KG (1965) Senescence in leaves of Acer pseudoplatanus L. and Parthenocissus tricuspidata Planch. Ann Bot 29: 433–444 [Google Scholar]
- Nambiar EKS, Fife DN (1987) Growth and nutrient retranslocation in needles of radiata pine in relation to nitrogen supply. Ann Bot 60: 147–156 [Google Scholar]
- Niederholzer FJA, DeJong TM, Saenz JL, Muraoka TT, Weinbaum SA (2001) Effectiveness of fall versus spring soil fertilization of field-grown peach trees. J Am Soc Hortic Sci 125: 644–648 [Google Scholar]
- Noodén LD, Guiamét JJ, John I (1997) Senescence mechanisms. Physiol Plant 101: 746–753 [Google Scholar]
- Oland K (1963) Changes in the content of dry matter and major elements of apple foliage during senescence and abscission. Physiol Plant 16: 682–694 [Google Scholar]
- Polle A, Schwanz P, Rudolf C (2001) Developmental and seasonal changes of stress responsiveness in beech leaves (Fagus sylvatica L.). Plant Cell Environ 24: 821–829 [Google Scholar]
- Ranieri A, Giuntini D, Ferraro F, Nali C, Baldan B, Lorenzini G, Soldatini GF (2001) Chronic ozone fumigation induces alterations in thylakoid functionality and composition in two poplar clones. Plant Physiol Biochem 39: 999–1008 [Google Scholar]
- Rosecrance RC, Johnson RS, Weinbaum SA (1998) The effect of timing of post-harvest foliar urea sprays on nitrogen absorption and partitioning in peach and nectarine trees. J Hortic Sci Biotechnol 73: 856–861 [Google Scholar]
- Roubelakis-Angelakis KA, Kliewer WM (1992) Nitrogen metabolism in grapevine. Hortic Rev 10: 407–452 [Google Scholar]
- Sanchez EE, Righetti TL, Sugar D, Lombard PB (1990) Responses of “Comice” pear tree to postharvest urea spray. J Hortic Sci 65: 541–546 [Google Scholar]
- Sanger JE (1971) Quantitative investigations of leaf pigments from their inception in buds through autumn coloration to decomposition in falling leaves. Ecology 52: 1075–1089 [Google Scholar]
- Schiffgens-Gruber A, Lutz C (1992) Ultrastructure of mesophyll cell chloroplasts of spruce needles exposed to O3, SO2 and NO2 alone and in combination. Environ Exp Bot 3: 243–254 [Google Scholar]
- Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155: 349–361 [DOI] [PubMed] [Google Scholar]
- Tagliavini M, Millard P, Quartieri M (1998) Storage of foliar-absorbed nitrogen and remobilization for spring growth in young nectarine (Prunus persica var. nectarina) trees. Tree Physiol 18: 203–207 [DOI] [PubMed] [Google Scholar]
- Titus JS, Kang S (1982) Nitrogen metabolism, translocation and recycling in apple trees. Hortic Rev 4: 204–246 [Google Scholar]
- Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13: 87–115 [Google Scholar]
- Wheldale M (1916) The Anthocyanin Pigments of Plants. Cambridge University Press, London, pp 126–141
- Wise RW (1995) Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45: 79–97 [DOI] [PubMed] [Google Scholar]