Abstract
To assess the variability in accuracy of contrast media introduction, leakage, required time and patient discomfort in four different centres, each using a different image-guided glenohumeral injection technique. Each centre included 25 consecutive patients. The ultrasound-guided anterior (USa) and posterior approach (USp), fluoroscopic-guided anterior (FLa) and posterior (FLp) approach were used. Number of injection attempts, effect of contrast leakage on diagnostic quality, and total room, radiologist and procedure times were measured. Pain was documented with a visual analogue scale (VAS) pain score. Access to the joint was achieved in all patients. A successful first attempt significantly occurred more often with US (94%) than with fluoroscopic guidance (72%). Leakage of contrast medium did not cause interpretative difficulties. With US guidance mean room, procedure and radiologist times were significantly shorter (p < 0.001). The USa approach was rated with the lowest pre- and post-injection VAS scores. The four image-guided injection techniques are successful in injection of contrast material into the glenohumeral joint. US-guided injections and especially the anterior approach are significantly less time consuming, more successful on the first attempt, cause less patient discomfort and obviate the need for radiation and iodine contrast.
Keywords: Ultrasound, Fluoroscopy, Shoulder, Image-guided injection, MR arthrography
Introduction
MR arthrography is frequently performed in the assessment of shoulder problems [1]. Intra-articular injection of a gadolinium chelate or a saline solution improves the detection of rotator cuff tears and demonstration of the labrum [1].
Deposition of contrast media into the glenohumeral joint may be a challenge for some radiologists. According to the numerous publications on this subject, it is still an item of investigation [2–20]. There are various techniques from blind [4,17,19] to image guided [2,3,10,12]. Glenohumeral injections are generally performed using an anterior approach under fluoroscopic guidance, which was first described in 1933 by Oberholzer [21] and modified by Schneider [22]. Other methods for intra-articular injection of the glenohumeral joint have been described, e.g., fluoroscopically guided using a posterior approach [9, 10], palpation directed [4, 19], ultrasound (US) [2, 3, 14], CT [11] and MR guided [7, 20]. These studies deal mostly with the techniques and/or their feasibility. Nowadays there is growing interest in evaluating and improving radiologists’ interpretive performance and efficiency in delivering patient care. However, little is known about differences in accuracy of contrast injection, the effect of contrast leakage on image interpretation [19], the time taken and the discomfort experienced by the patient [23, 24] among different radiologists using different techniques.
The purpose of our study was to prospectively assess the variation in accuracy, effect of leakage of contrast medium on diagnostic quality, the time required and the discomfort experienced by the patient for the introduction of contrast material into the glenohumeral joint with four different frequently applied image-guided (fluoroscopy and US) injection techniques.
Materials and methods
Patients
A prospective study was performed in four centres each including 25 consecutive patients with shoulder instability who were referred for MR arthrography by orthopaedic surgeons. Patient characteristics are illustrated in Table 1. Patients who had previously undergone shoulder surgery were excluded. Institutional review board approval and verbal consent were obtained.
Table 1.
Four patient groups
| Patients | Gender | Age | Sidedness | |||
|---|---|---|---|---|---|---|
| No. | M | F | Mean/median (range) | L | R | |
| FLa | 25 | 17 | 7 | 39/44 (14–69) | 9 | 16 |
| FLp | 25 | 14 | 11 | 44/44 (28–62) | 14 | 11 |
| USa | 25 | 16 | 9 | 35/34 (18–59) | 9 | 16 |
| USp | 25 | 19 | 6 | 36/37 (16–69) | 10 | 15 |
Number of patients per group, gender, age and sidedness of the punctured shoulders are displayed
USa: US-guided anterior injection, USp: US-guided posterior injection, FLa: fluoroscopic-guided anterior injection, FLp: fluoroscopic-guided posterior injection
Four radiologists in four different centres were deliberately chosen because each routinely used a different approach for glenohumeral injection. In this way we were able to audit and compare the daily practice of various glenohumeral humeral injection techniques applied by experienced musculoskeletal radiologists (>9 years of experience each). In each centre a different intra-articular approach was applied by an experienced musculoskeletal radiologist, fluoroscopic-guided anterior (FLa), fluoroscopic-guided posterior (FLp), US-guided anterior (USa) and US-guided posterior (USp).
All patients underwent intra-articular injection of gadoterate meglumine (gadoteric acid) 0.0025 mmol/ml (Artirem®; Guerbet S.A., Villepinte, France), which is a dilution (1:200) of Dotarem® (Gd-DOTA) provided in pre-filled syringes of 20 ml, until intra-articular pressure rose or up to a maximum of 20 ml.
Injection techniques
All radiologists used the technique and materials with which they were familiar. The FLp and Fla injections were performed with a 1.2 mm × 88.9-mm spinal needle (18-gauge, 3.5 inch) and a 0.9 × 90-mm spinal needle (20 gauge), respectively, and the US-guided injections with a 0.8 × 50-mm bevelled needle (21-gauge) without a stylet.
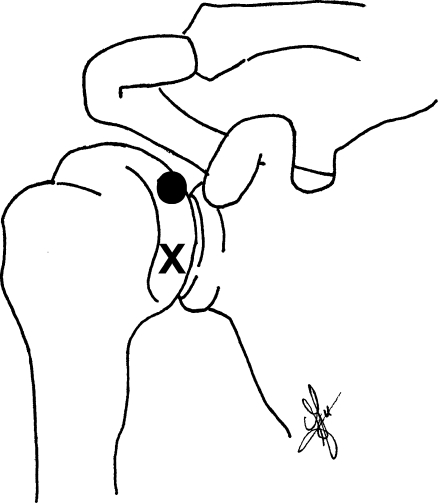
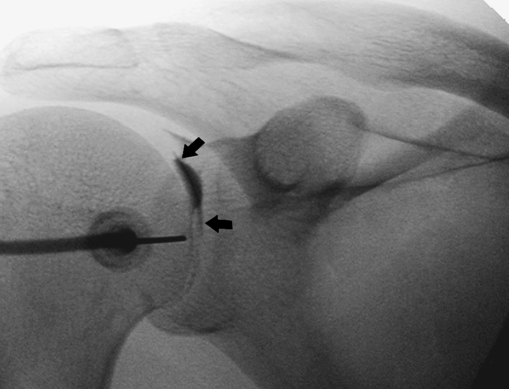
The Fla injections are performed with the patient supine in a straight anteroposterior position with the shoulder slightly externally rotated. The needle is directed vertically under fluoroscopic control at the junction of the middle and lower thirds of the medial part of the humeral head (Figs. 1 and 3).
Fig. 1.
Schematic drawing of the anterior view of the right shoulder showing the puncture sites of the fluoroscopic- (X) and US-guided (●) anterior injection techniques
Fig. 3.
Fluoroscopic-guided anterior approach. X-ray of the anterior view of a right-hand shoulder showing the needle entry site. The needle is inserted vertically at the inferomedial quadrant of the humeral head. The intra-articular position of the needle tip is confirmed by injecting a little iodinated contrast media, which demarcates the hyaline cartilage (black arrows) of the humeral head
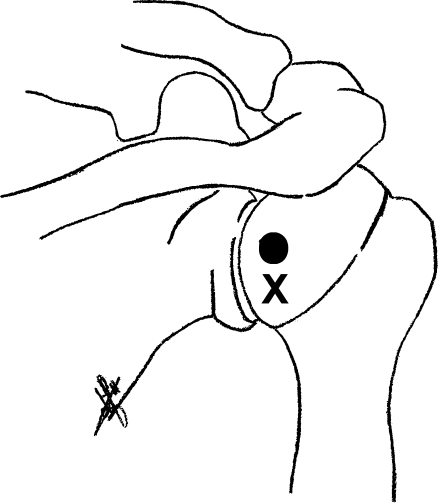
Fig. 2.
Schematic drawing of the posterior view of the right shoulder showing the puncture sites of the fluoroscopic- (X) and US-guided (●) posterior injection techniques
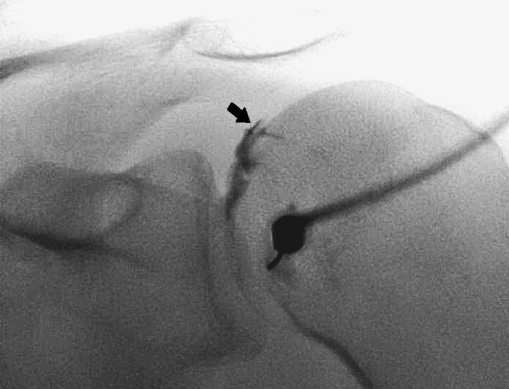
The Flp injections are performed in prone position with the symptomatic shoulder slightly raised until the glenohumeral joint is seen tangentially [9, 10]. The injection site is infiltrated with local anaesthetic [prilocaïnehydrochloride 10 mg/ml (Citanest® 1%), Astra Pharmaceutica, Zoetermeer, The Netherlands] using a 0.8 × 50-mm bevelled needle (21 gauge). With the shoulder in a neutral position or slightly internally rotated, the needle is aimed at the inferomedial quadrant of the humeral head and advanced vertically under fluoroscopic guidance to the cartilage of the humeral head (Figs. 2 and 4) [9, 10].
Fig. 4.
Fluoroscopic-guided posterior approach. X-ray of the posterior view of a right-hand shoulder showing the needle entry site. The needle is inserted vertically at the inferomedial quadrant of the humeral head. The intra-articular position of the needle tip is confirmed by injecting a little iodinated contrast media, which demarcates the hyaline cartilage (black arrow) of the humeral head
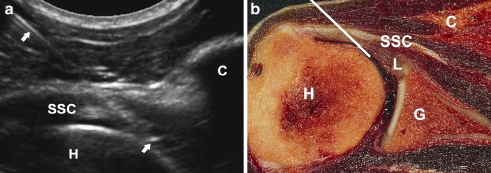
The USa approach was performed according to Valls [3]. Patients are supine with the shoulder slightly externally rotated. A HDI 5000 SonoCT (ATL/Philips, Best, The Netherlands) with a 5–8-MHz curved array transducer was used to visualise the needle (Fig. 5A). After skin and transducer preparation with alcohol 70%, the patient’s shoulder was draped in sterile fashion. The needle is inserted at the level of the coracoid, from lateral to medial, aimed at the medial border of the humeral head (Figs. 1 and 5) [3]. The contrast medium can be seen flowing in the direction of the subscapular recess and joint space.
Fig. 5.
US-guided anterior approach. a Sonogram of a right-hand shoulder showing the needle track (arrows) from lateral to medial with the USa approach. The needle is inserted at the level of the coracoid (C). The tip of the needle is in intra-articular position with the tip underneath the subscapular tendon (SSC) and bordering the humeral head (H). b Corresponding cadaver section showing the optimal needle track (white line)
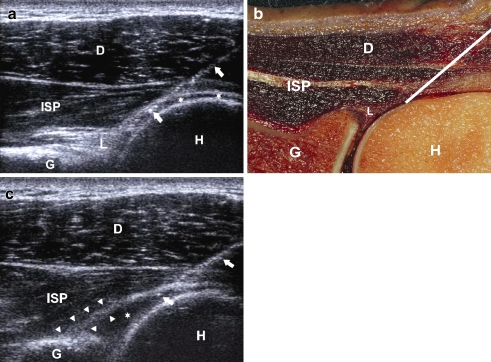
The USp approach, described by Cicak [2] and modified by Zwar [14], was performed using a APLIO US machine (Toshiba Medical Systems Corporation, Tokyo, Japan) with a 7.5–14-MHz linear array transducer (Toshiba PLF-805ST). The patient is either lying obliquely prone on the contralateral shoulder or sitting upright with the back to the radiologist and the ipsilateral hand on the contralateral shoulder. After skin and transducer preparation with alcohol 70%, the patient’s shoulder was draped in sterile fashion. The needle is inserted, from lateral to medial, parallel to the long axis of the transducer and advanced under US control in the joint between the humeral head and the posterior glenoid labrum (Figs. 2 and 6) [14].
Fig. 6.
US-guided posterior approach. a Sonogram of a right-hand shoulder showing the needle track (arrows) from lateral to medial with the USp approach. The needle is inserted at the midlevel of the humeral head (H). The needle is in intra-articular position with the tip underneath the infraspinatus tendon (ISP) and posterior labrum (L) and bordering the hyaline cartilage (asterisks) of the humeral head. G: glenoid. b Corresponding cadaver section showing the optimal needle track (white line). c Sonogram following injection of 15 ml injected gadoterate meglumine (Artirem®) (asterisk). The correct intra-articular position of the needle (arrows) can be visualised real-time during injection, but is also confirmed by the ‘comma’-like configuration of the posterior labrum (arrowheads), which is lifted by the intra-articularly injected fluid (asterisk). G: glenoid, H: humearal head, ISP: infraspinatus tendon
Time assessment
To be able to monitor the time taken for the procedure in four different centres, with different personel, a simple method of scoring how things were done was standardised.
Room time (time between the patient entering and leaving the room), radiologist time (time between entering and leaving the room) and procedure time (time between starting and ending the injection procedure) were recorded. Mean times were assessed, and because of the maximum time of Fla and Flp, we also assessed the median times (Table 3).
Table 3.
Required time (minutes) for US- and fluoroscopic-guided glenohumeral injection
| TRoom | TProcedure | TRadiologist | |
|---|---|---|---|
| Min – max (mean/median) | Min – Max (mean/median) | Min – max (mean/median) | |
| FLa | 07:00 – 33:00 (17:03/17:00) | 00:30 – 20:00 (04:09/11:43) | 03:00 – 27:00 (09:50/08:00) |
| FLp | 12:00 – 29:00 (20:05/20:00) | 00:45 – 23:00 (09:48/09:00) | 10:00 – 27:00 (17:58/19:00) |
| USa | 06:00 – 15:00 (09:54/10:00) | 00:30 – 06:00 (01:34/01:00) | 04:00 – 13:15 (06:19/06:00) |
| USp | 05:00 – 16:00 (09:18/09:00) | 00:30 – 07:30 (03:24/03:00) | 01:56 – 12:00 (05:48/05:00) |
| FL(a + p) | 07:00 – 33:00 (18:36/18:00) | 00:30 – 23:00 (06:58/07:00) | 03:00 – 27:00 (13:54/15:00) |
| US(a + p) | 05:00 – 16:00 (09:36/09:00) | 00:30 – 07:30 (02:29/02:00) | 01:56 – 13:15 (06:03/06:00) |
| TUS:TFL | 71%−48% (52%) | 100%−33% (36%) | 64%−49% (44%) |
| Kruskal-Wallis | p < 0.001 | p < 0.001 | p < 0.001 |
TRoom = time that the room is occupied by patient. TProcedure = time required for injection (skin to skin), TRadiologist = overall time radiologist in the US or fluoroscopy room
USa: US-guided anterior injection, USp: US-guided posterior injection, FLa: fluoroscopic-guided anterior injection, FLp: fluoroscopic-guided posterior injection. FL(a + p): fluoroscopic-guided anterior and posterior injection, US(a + p): US-guided anterior and posterior injection, TUS:TFL: percentage of needed room, procedure and radiologist time with US guidance in relation to the time span for fluoroscopic guidance
Evaluation of intra-articular administration of contrast material
The number of attempts needed to enter the joint space was recorded. An attempt to inject a joint was defined as the need for repositioning of the needle after unsuccessful test injection.
The joint space was considered to be entered when the needle contacted the humeral head cartilage, test injection was performed without resistance and the intra-articular position of contrast material was confirmed with fluoroscopy by injection of 1–2 ml iodinated nonionic contrast (Omnipaque 300; Amersham Cygne, Eindhoven, The Netherlands). Confirmation with real time US can be performed without the use of iodinated contrast media by visualising the intra-articular flow of fluid during injection or by visualising the intra-articularly injected fluid underneath the capsule and/or labrum posteriorly and underneath the subscapular tendon anteriorly, or around the long head of the biceps tendon.
The amount of injected contrast material was recorded.
MR imaging was performed within 15 min of the injection with a 1.0-T or a 1.5-T MR system. In all centres a dedicated receive-only shoulder coil was used. Images were used for diagnostic purpose and to assess retrospectively the intra-articular position and contrast leakage by an independent blinded radiologist who did not perform the procedure. Leakage was graded using a five-grade scale (Table 2).
Table 2.
Accuracy, number of attempts to gain access to the glenohumeral joint, injected volume and leakage of contrast media with four different image-guided approaches
| Overall | FLa | FLp | USa | USp | FL(a + p) | US(a + p) | |
|---|---|---|---|---|---|---|---|
| Number of patients (N) | 100 | 25 | 25 | 25 | 25 | 50 | 50 |
| Intra-articular injection | 100 (100%) | 25 (100%) | 25 (100%) | 25 (100%) | 25 (100%) | 50 (100%) | 50 (100%) |
| 1st attempt | 83 (83%) | 19 (76%) | 17 (68%) | 24 (96%) | 23 (92%) | 36 (72%) | 47 (94%) |
| 2nd attempt | 14 (14%) | 5 (20%) | 6 (24%) | 1 (4%) | 2 (8%) | 11 (22%) | 3 (6%) |
| 3rd attempt | 3 (3%) | 1 (4%) | 2 (8%) | 0 (0%) | 0 (%) | 3 (6%) | 0 (0%) |
| No leakage | 49 (49%) | 8 (32%) | 13 (52%) | 16 (64%) | 12 (48%) | 21 (42%) | 28 (56%) |
| Minimal leakage noncompromising | 24 (24%) | 12 (48%) | 3 (12%) | 4 (16%) | 5 (20%) | 15 (30%) | 9 (18%) |
| Massive leakage noncompromising | 27 (27%) | 5 (20%) | 9 (36%) | 5 (20%) | 8 (32%) | 14 (28%) | 13 (26%) |
| Minimal leakage compromising | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Massive leakage compromising | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Minimal injected volume (ml) | 5.0 | 10.0 | 9.0 | 5.0 | 10.0 | 9.0 | 5.0 |
| Maximal injected volume (ml) | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Mean injected volume (ml) | 15.6 | 14.5 | 14.9 | 14.6 | 18.6 | 14.7 | 16.6 |
| Local anaesthetics | – | No | Yes | No | No | – | – |
| Needle size (gauge) | – | 20 | 18 | 21 | 21 | – | – |
USa: US-guided anterior injection, USp: US-guided posterior injection, FLa: fluoroscopic-guided anterior injection, FLp: fluoroscopic-guided posterior injection, FL (a + p): fluoroscopic-guided anterior and posterior approach, US (a + p): US-guided anterior and posterior approach
Visual analogue scale
Before and after injection, patients graded their anticipated and experienced level of pain. A visual analogue scale (VAS) was used, with a scale from 0 (“no pain”) to 100 (“unbearable pain”). Complications were recorded.
Statistical analysis
For descriptive purposes, mean and standard deviations are reported for room time, radiologist time, total procedure time and VAS scores. Because the variables were non-normally distributed, nonparametric Kruskal-Wallis tests were used to test for statistical differences. Statistical significance was accepted if the P value was less than 0.05. The comparisons between the techniques were not adjusted for the patients’ sex, age or right or left shoulder. The distribution of all categorical variables was presented in frequencies. Differences in these distributions were tested for statistical significance using chi-square tests.
All statistical analyses were performed using SPSS, version 14.0.2 for Windows (Chicago, IL) by an experienced statistician (L.K.).
Results
Patient groups
As illustrated in Table 1, the patient groups involved in this study were quite similar in sex, age distribution and sidedness of the affected shoulder. There was a slight predominance in right shoulders, as one might expect, as most people are right-handed. Patients’ gender and left or right shoulder were not significantly related to room time, radiologist time, total procedure time and difference between the pre- and post-procedure VAS scores. The age of the patients was only weakly (r2 < 0.04) correlated with the three time variables and not at all correlated with the VAS score difference. Therefore, the analyses were not adjusted for age, sex and laterality.
Accuracy
Injection of the glenohumeral joint was successfully performed in all 100 patients enrolled in this study (Table 2). Overall access to the joint was gained on the first attempt in 83 (83%) patients, on the second attempt in 14 (14%) patients and on the third attempt in 3 (3%) patients. For fluoroscopic or US guidance the first attempt scores were 72% and 94%, respectively. With fluoroscopy a second and third attempt was needed in 22% and 6%, respectively, whereas with US guidance a second attempt in only 6%, and a third attempt was not needed in any of the cases (Table 2).
The differences between the techniques in the frequency of more than one attempt needed were statistically significant (chi-square test df = 3: p = 0.026).
No complications of the vital structures, such as vessels, nerves and tendons, were encountered clinically and with MR imaging. Four (4%) patients had a vasovagal reaction, two patients in the USp group and two patients in the FLa group. No further complications were reported in any of the groups as, for example, bleeding at the injection site, swelling or compromise of movements.
Contrast material leakage
The various injection techniques were qualitatively comparable with regard to extra-articular leakage of contrast material. In 51% of all procedures minimal (24%) and massive (27%) leakage occurred, which did not hamper in any way the evaluation of the MR images. Minimal leakage was most frequent in the FLa approach (48%) and massive leakage in both posterior approaches (FLp: 36% and USp: 32%) (Table 2).
The differences in non-compromising leakage were statistically significant (chi-square test, df = 6: p = 0.045).
Time assessment
Fluoroscopy and US room times, overall procedure times and radiologist’s times are summarised in Table 3 as minimum - maximum times (mean).
The mean room times for US guidance were significantly shorter when compared to the time needed using fluoroscopic guidance (Table 3). The shortest mean procedure time required was noted for the USa approach (1:34 min). The longest mean procedure time noted was that using the FLp approach (9:48 min). Also noteworthy is the difference in mean procedure time between the FLa (4:09 min) and FLp approach (9:48 min). The minimum procedure times were similar among all four approaches; however, the maximum procedure times were significantly shorter using US guidance (Table 3).
On average the procedure under US guidance was shorter than under fluoroscopic guidance, using significantly less (>50%) room time and radiologist time (Table 3).
Discomfort
The VAS score obtained before and after shoulder injection is summarised in Table 4. The VAS scores before the procedure varied among the four different procedures, but not significantly so (Kruskal-Wallis p = 0.108). The variation in the VAS scores post-procedure was a little bit larger and statistically significant (Kruskal-Wallis p = 0.023). In three of the four groups there was a general tendency for patients to indicate that the procedure was less painful than they had expected. In 44 patients the VAS score decreased following injection, whereas in 28 patients it increased, and in 28 patients the VAS score measured pre- and post-procedure remained equal. The mean VAS score of the FLp, USa and USp injections decreased 14, 11 and 11 points following injection, respectively, while it increased with 9 points in the FLa group (Kruskal-Wallis p = 0.001). In fact, the post-procedure VAS score of the FLa procedure increased in 60% of the patients. There was a higher average pain score after FLa injection as compared to FLp. Patients who underwent US-guided procedures reported less pain than those who underwent the fluoroscopic-guided procedures. The USa approach was rated with the lowest pre- and post-injection VAS scores.
Table 4.
VAS pain score for US- and fluoroscopy-guided glenohumeral injections
| Pre-procedure | Post-procedure | VAS difference | |||
|---|---|---|---|---|---|
| Min–max (mean) | Min–max (mean) | Mean | Decrease | Increase | Equal |
| No. of patients | No. of patients | No. of patients | |||
| FLa 0 – 90 (30) | 0 – 100 (39) | +9 | 3 (12%) | 15 (60%) | 7 (28%) |
| FLp 3 – 100 (42) | 0 – 100 (28) | −14 | 15 (60%) | 4 (16%) | 6 (24%) |
| USa 0 – 75 (27) | 0 – 70 (16) | −11 | 13 (52%) | 4 (16%) | 8 (32%) |
| USp 0 – 80 (38) | 0 – 80 (27) | −11 | 13 (52%) | 5 (20%) | 7 (28%) |
USa: US-guided anterior injection, USp: US-guided posterior injection, FLa: fluoroscopic-guided anterior injection, FLp: fluoroscopic-guided posterior injection, VAS-difference: VAS score post-procedure - VAS score pre-procedure
Discussion
The main advantage of image-guided glenohumeral joint injection over blind injection is that the needle position can be confirmed and injection of contrast medium can be controlled. This is especially advantageous in teaching hospitals (when training residents) and when the number of MR arthrographies are limited.
The current study describes four different image-guided approaches. Intra-articular deposition of contrast material was successful in all patients, which is comparable with reported results in the literature [2, 3, 10, 13]. The success rate of the first attempt with US guidance (97%) was significantly better compared to fluoroscopic guidance (72%).
In the literature the accuracy of “blind” glenohumeral injections varies from 26-100% [15–19]. Catalano et al. [19]] showed that with a palpation-directed posterior approach, the glenohumeral joint could be entered successfully on respectively the first, second and third attempt in 85%, 13% and 2% of the cases [19].
In this study the US procedure was less time consuming than the fluoroscopic-guided procedure (2.30 min versus 7 min, i.e., only 36% of the time needed with fluoroscopic guidance). This may be due to the received pre-procedural local anaesthetics and the injection with iodinated contrast to confirm the intra-articular position. The reported time of blind injection by Catalano was 3 min [19]. The occupancy of the US room is only 52% of the fluoroscopy room (Table 3).
The figures of leakages are summarised in Table 2. The number is high compared to those reported by Catalano et al. [19]. This may be due to strict criteria. Every small amount of contrast material along the needle tract was graded minimal leakage. The frequent leakage did not cause interpretative difficulties. All patients were clinically suspected of having anterior labral problems, and therefore posterior leakage did not hamper interpretation. Contrast material anteriorly did not compromise diagnostic quality, because the contrast medium was usually depicted in close relationship to the needle tract. In six cases massive leakage was obviously due to capsular disruption (n = 3), pathology of the labrum (n = 2) and a full-thickness rotator cuff tear (n = 1).
Patients who undergo arthrography in general experience less pain than expected [23]. In our study 44% of the patients considered discomfort less than expected and 28% more than expected. However, in the subgroup of patients who underwent the FLa approach (Table 4), 60% experienced more discomfort than expected. This can be explained by the fact that no local anaesthetics and a thicker needle (20 gauge) were used and the fact that manipulation of the stylet and syringe causes unintended movements of the needle.
In this study four minor complications, e.g., vasovagal reactions, were reported, which resolved without medical intervention: two in the USp group and two in the FLa group. Other minor complications included pain, urticaria and headaches [25]. All four procedures were difficult due to adipositas (n = 3) and postoperative status (n = 1). Hugo [25] showed in a multi-institutional survey a total complication rate of 3.6%. Serious complications account for 0.02% and consist mainly of infections and rarely of anaphylaxis and vascular complications [25].
In our study there were no advantages comparing the anterior approach with the posterior approach. Some authors [2, 9, 10, 14, 19] advocate the posterior approach, because most pathologies of the stabilising structures of the glenohumeral joint are encountered on the anterior aspect of the joint. Chung [9] shows that if a standard anterior fluoroscopic approach is used, the needle may traverse the primary stabilisers. To overcome this drawback a modified anterior approach through the rotator interval can be performed [13]. This is comparable with the US-guided anterior approach. With this approach the needle entry site is at the level between the coracoid process and the superior medial quadrant of the humeral head, which is cranial to the stabilising structures of the shoulder. This limits the risk of compromising diagnostic quality because of leakage of contrast material.
This study was not a strict comparative study between US and fluoroscopic techniques because all radiologists kept their own routine, introducing variables like needle size and the use of local anaesthetics. Also the differences in injection skills may play a role. Nevertheless, an important observation of this study is the wide variation in delivered patient care. Although this study is too small to analyse the contribution of different factors to this variation, it is important for physicians to be aware of these phenomena.
The results suggest that US-guided glenohumeral joint injection is preferable to fluoroscopic-guided glenohumeral joint injection. To confirm the findings a prospective study without variables such as needle size and local anaesthetics is needed. Furthermore, the number of operators should be extended to minimise the effect of differences in operator skills.
Conclusion
The four image-guided injection techniques are successful in injection of contrast material into the glenohumeral joint. Although the effect of other variables, such as size of the injection needle and the use of local anaesthetics, needs to be investigated, we encourage US-guided injections and especially the anterior approach, because in this study it was significantly less time consuming, more successful on the first attempt, can be performed with less patient discomfort and obviates the need for radiation and iodine contrast.
Acknowledgment
The authors gratefully thank Stephan Muurling for his dedicated contribution in preparing the drawings in this article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Matthieu J. C. M. Rutten, Phone: +31-73-6992000, FAX: +31-73-6992601, Email: m.rutten@jbz.nl
James M. P. Collins, Phone: +31-58-2866666, FAX: +31-58-2866142, Email: j.collins@znb.nl
Bas J. Maresch, Phone: +31-318-434343, FAX: +31-318-613914, Email: mareschb@zgv.nl
Jacques H. J. M. Smeets, Phone: +31-314-329911, FAX: +31-314-329150, Email: jsmeets@planet.nl
Caroline M. M. Janssen, Phone: +31-73-6992000, FAX: +31-73-6992601, Email: c.janssen@jbz.nl
Lambertus A. L. M. Kiemeney, Phone: +31-24-3613745, FAX: +31-24-3613505, Email: b.kiemeney@ebh.umcn.nl
Gerrit J. Jager, Phone: +31-73-6992000, FAX: +31-73-6992601, Email: g.jager@jbz.nl
References
- 1.Jbara M, Chen Q, Marten P, Morcos M, Beltran J. Shoulder MR arthrography: how, why, when. Radiol Clin North Am. 2005;43:683–692. doi: 10.1016/j.rcl.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Cicak N, Matasovic T, Bajraktarevic T. Ultrasonographic guidance of needle placement for shoulder arthrography. J Ultrasound Med. 1992;11:135–137. doi: 10.7863/jum.1992.11.4.135. [DOI] [PubMed] [Google Scholar]
- 3.Valls R, Melloni P. Sonographic guidance of needle position for MR arthrography of the shoulder. AJR. 1997;169:845–847. doi: 10.2214/ajr.169.3.9275909. [DOI] [PubMed] [Google Scholar]
- 4.DeMouy EH, Menendez CV, Jr, Bodin CJ. Palpation-directed (non-fluoroscopically guided) saline-enhanced MR arthrography of the shoulder. AJR. 1997;169:229–231. doi: 10.2214/ajr.169.1.9207530. [DOI] [PubMed] [Google Scholar]
- 5.Lewin JS, Petersilge CA, Hatem SF, et al. Interactive MR imaging-guided biopsy and aspiration with a modified clinical C-arm system. AJR. 1998;170:1593–1601. doi: 10.2214/ajr.170.6.9609180. [DOI] [PubMed] [Google Scholar]
- 6.Trattnig S, Breitenseher M, Rand T, et al. MR imaging-guided MR arthrography of the shoulder: clinical experience on a conventional closed high-field system. AJR. 1999;172:1572–1574. doi: 10.2214/ajr.172.6.10350291. [DOI] [PubMed] [Google Scholar]
- 7.Hilfiker PR, Weishaupt D, Schmid M, Dubno B, Hodler J, Debatin JF. Real-time MR-guided joint puncture and arthrography. Eur Radiol. 1999;9:201–204. doi: 10.1007/s003300050655. [DOI] [PubMed] [Google Scholar]
- 8.Miller TT. MR arthrography of the shoulder and hip after fluoroscopic landmarking. Skeletal Radiol. 2000;29:81–84. doi: 10.1007/s002560050014. [DOI] [PubMed] [Google Scholar]
- 9.Chung CB, Dwek JR, Feng S, Resnick D. MR arthrography of the glenohumeral joint: A tailored approach. AJR. 2001;177:217–219. doi: 10.2214/ajr.177.1.1770217. [DOI] [PubMed] [Google Scholar]
- 10.Farmer KD, Hughes PM. MR arthrography of the shoulder: fluoroscopically guided technique using a posterior approach. AJR. 2002;178:433–434. doi: 10.2214/ajr.178.2.1780433. [DOI] [PubMed] [Google Scholar]
- 11.Binkert CA, Verdun FR, Zanetti M, Pfirrmann CW, Hodler J. CT arthrography of the glenohumeral joint: CT fluoroscopy versus conventional CT and fluoroscopy. Comparison of image-guidance techniques. Radiology. 2003;229:153–158. doi: 10.1148/radiol.2291020314. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson JA, Lin J, Jamadar DA, Hayes CW. Aids to successful shoulder arthrography performed with a fluoroscopically guided anterior approach. Radiographics. 2003;23:373–379. doi: 10.1148/rg.232025706. [DOI] [PubMed] [Google Scholar]
- 13.Dépelteau H, Bureau NJ, Cardinal E, Aubin B, Brassard P. Arthrography of the shoulder: a simple fluoroscopically guided approach for targeting the rotator cuff interval. AJR. 2004;182:329–332. doi: 10.2214/ajr.182.2.1820329. [DOI] [PubMed] [Google Scholar]
- 14.Zwar RB, Read JW, Noakes JB. Sonographically guided glenohumeral joint injection. AJR. 2004;183:48–50. doi: 10.2214/ajr.183.1.1830048. [DOI] [PubMed] [Google Scholar]
- 15.Sehti PM, Kingston S, Elattrache N. Accuracy of anterior intra-articular injection of the glenohumeral joint. Arthroscopy. 2005;21:77–80. doi: 10.1016/j.arthro.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Berna-Serna JD, Redondo MV, Martinez F, et al. A simple technique for shoulder arthrography. Acta Radiol. 2006;47:725–729. doi: 10.1080/02841850600774050. [DOI] [PubMed] [Google Scholar]
- 17.Sehti PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29:149–152. doi: 10.3928/01477447-20060201-01. [DOI] [PubMed] [Google Scholar]
- 18.Hanchard N, Shanahan DHT, Thompsom J, Goodchild L. Accuracy and dispersal of subacromial and glenohumeral injections in cadavers. J Rheumatol. 2006;33:1143–1146. [PubMed] [Google Scholar]
- 19.Catalano OA, Manfredi R, Vanzulli A, et al. MR arthrography of the glenohumeral joint: modified posterior approach without imaging guidance. Radiology. 2007;242:550–554. doi: 10.1148/radiol.2422051964. [DOI] [PubMed] [Google Scholar]
- 20.Petersilge CA, Lewin JS, Duerk JL, Hatem SF. MR arthrography of the shoulder: rethinking traditional imaging procedures to meet the technical requirements of MR imaging guidance. AJR. 1997;169:1453–1457. doi: 10.2214/ajr.169.5.9353479. [DOI] [PubMed] [Google Scholar]
- 21.Oberholzer J. Die Arthropneumoradiographie bei Habitueller Shulterluxation. Röntgenpraxis. 1933;5:589–590. [Google Scholar]
- 22.Schneider R, Ghelman B, Kaye JJ. A simplified injection technique for shoulder arthrography. Radiology. 1975;114:738–739. doi: 10.1148/114.3.738b. [DOI] [PubMed] [Google Scholar]
- 23.Robbins MI, Anzilotti KFJ, Katz LD, Lange RC. Patient perception of magnetic resonance arthrography. Skeletal Radiol. 2000;29:265–269. doi: 10.1007/s002560050605. [DOI] [PubMed] [Google Scholar]
- 24.Binkert CA, Zanetti M, Hodler J. Patient’s assessment of discomfort during MR arthrography of the shoulder. Radiology. 2001;221:775–778. doi: 10.1148/radiol.2213010277. [DOI] [PubMed] [Google Scholar]
- 25.Hugo PC, Newberg AH, Newman JS, Wetzner SM. Complications of Arthrography. Semin Musculoskelet Radiol. 1998;2:345–348. doi: 10.1055/s-2008-1080115. [DOI] [PubMed] [Google Scholar]