Abstract
Objectives
To assess the cost effectiveness of palivizumab for prevention of severe respiratory syncytial virus (RSV) disease in high-risk infants in Spain, incorporating country-specific observational hospitalisation data.
Methods
An existing decision tree model, designed using data from a large international clinical trial of palivizumab versus no prophylaxis, was updated to include Spanish observational hospitalisation data. The analysis was performed for preterm children born at or before 32 weeks gestational age, who are at high risk of developing severe RSV disease requiring hospitalisation. Data sources included published literature, official price/tariff lists and national population statistics. The primary perspective of the study was that of the Spanish National Health Service in 2006.
Results
The base-case analysis included the direct medical costs associated with palivizumab prophylaxis and hospital care for RSV infections. Use of palivizumab produces an undiscounted incremental cost-effectiveness ratio (ICER) of €6,142 per quality-adjusted life-year (QALY), and a discounted ICER of €12,814/QALY.
Conclusion
Palivizumab provides a cost-effective method of prophylaxis against severe RSV disease requiring hospitalisation among preterm infants in Spain.
Keywords: Cost-effectiveness, Model, Respiratory syncytial virus, Palivizumab, Spain
Background
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract illness in children, the elderly and immunocompromised individuals. Preterm infants and children with heart and lung problems are particularly at risk of hospitalisation due to severe RSV disease [1]. Hospitalisation rates in these high-risk patients may be as high as 20% [2]. A study by Clarke et al. [3] in the United Kingdom assessed the rate of RSV hospitalisation in unprophylaxed children with bronchopulmonary dysplasia (BPD) in Liverpool over two RSV seasons (1998/1999 and 1999/2000) to be 19.7%. Other estimates of RSV hospitalisation rates in at-risk children come from studies of the protective effect of RSV immune globulin intravenous (RSV-IGIV) compared to no prophylaxis. The results of the first multicentre randomised controlled trial with RSV-IGIV, in children less than 48 months of age at study entry who suffered underlying chronic lung disease (CLD) or congenital heart disease or a history of preterm birth (<35 weeks gestation) reported a hospitalisation rate of 20% for the placebo group versus 7% in the children who received prophylaxis [4]. In the PREVENT study of children with CLD and/or a history of preterm birth, the incidence of RSV hospitalisation was 13.5% in the placebo group and 8% in those who received RSV-IGIV [5]. IMpact, a large multinational clinical trial, has reported a significant 55% relative reduction (95% CI: 38–72%) in the overall rate of hospitalisations due to RSV infection in high-risk children given palivizumab compared with those given placebo [6]. Of the palivizimab group, 4.8% experienced RSV hospitalisation, with the figure for the placebo group being 10.6% (P = 0.0004). In Spain it has been estimated that RSV is responsible for 15,000–20,000 emergency visits, 7,000–14,000 hospital admissions, and 70–250 deaths per year [7].
Investigations of the long-term prognosis of patients hospitalised due to RSV infection in infancy have shown measurable respiratory abnormalities that may persist for several years following infection. Among young school children, previous RSV infection may increase the risk of asthma tenfold [8]. In a prospective cohort study, 47 children who had experienced RSV bronchiolitis severe enough to warrant hospitalisation at a mean age of 3.5 months were compared with matched controls. At age 7.5 years, the cumulative prevalence of asthma was 30% in the RSV group and 3% in the control group (P < 0.0001) [8].
In an observational study of 2,415 preterm Canadian infants hospitalised due to RSV and 20,254 matched controls, those in the RSV cohort were at least eight times more likely to be hospitalised for respiratory conditions during the 2-year follow-up period than controls [5]. The overall mortality rate in these patients was also significantly higher than in the control cohort (8.11 vs 1.58%, P = 0.001), showing a significant risk of mortality beyond the initial hospitalisation for RSV [9]. By pooling placebo and intervention subjects from three trials, Joffe et al. [10] have estimated that the probability of death during the acute phase of RSV hospitalisation among high-risk infants is 1.2% (95% confidence interval, 0–2.8%).
As current treatment options for severe RSV infection are limited to supportive care, the focus has shifted to prevention. Palivizumab, a humanised monoclonal antibody given once a month as an intramuscular injection during anticipated periods of risk, is currently the only product approved for use for prevention of severe RSV disease in Spain. Palivizumab is licensed for use in children born at or before 35 weeks gestational age who are less than 6 months old at the onset of the RSV season, in children less than 2 years old who have received treatment for BPD within the proceeding 6 months and in children less than 2 years old with haemodynamically significant congenital heart disease (CHD) [11]. This indication was granted on the basis of two large, multinational, placebo-controlled trials, which reported a 55% reduction in the risk of hospitalisation in preterm/BPD children and a 45% reduction in the risk of hospitalisation in children with CHD, compared to no prophylaxis [6, 12].
Furthermore, there is evidence that the benefits of prophylaxis with palivizumab persist beyond the initial period of infection resulting from long-term morbidity and mortality. Investigations of the long-term prognosis of patients with RSV disease in infancy have shown measurable respiratory abnormalities immediately or several years following infection. Sampalis [9] investigated the morbidity and mortality after RSV-associated hospitalisations among preterm Canadian infants. This study analysed data from 2,415 preterm infants [32–35 weeks gestational age (GA)] hospitalised for proven or probable RSV and 20,254 matched control infants. The patients in both cohorts were matched with respect to potential confounders. The overall mortality rate in the RSV cohort in the 2 years after the initial hospitalisation was 8.11% compared to 1.58% in the control cohort (P = 0.001), showing a significant risk of mortality beyond the initial hospitalisation for RSV. The odds ratios calculated showed that infants with an RSV hospitalisation were at least eight times more likely to be hospitalised for respiratory conditions during the follow-up period and the overall mortality rate in these patients was also significantly higher compared with the control cohort (8.11 vs 1.58%, P = 0.001) [7].
Another study showed that early RSV infection predisposes children to recurrent wheezing during their early childhood but that airway morbidity is transient and subsides during school age [13]. Among young school children, previous RSV infection may increase the risk of asthma tenfold [14]. In 140 children aged 7.5 year—47 of whom were hospitalized for RSV infection and 93 paired controls—the cumulative prevalence of recurrent wheezing was 30% in the RSV group and 3% in the control group.
A prospective, controlled study carried out in 27 centres in Europe, including Spain, showed that significantly fewer preterm infants in the palivizumab group experienced recurrent wheezing during a 12-month follow up period than those who had not received prophylaxis (6.8 vs 19.1%; P < 0.001), a 64% risk reduction [15]. Children under 36 months of age who had received palivizumab during the previous respiratory season were matched by gestational age (<28 weeks, 28–32 weeks, 33–35 weeks) and age (±3 months) with children who had never received palivizumab; fewer children who received prophylactic treatment with palivizumab required hospitalisation for any kind of respiratory disease (4.2 vs 7%; P = 0.045).
Data were collected over four seasons, comparing RSV hospitalisation rates and risk factors in two Spanish cohorts of at-risk infants. The first cohort comprised 1,583 infants followed during the 1998–1999 and 1999–2000 RSV seasons, before palivizumab was licensed for use in Spain. The second cohort comprised 1,919 infants who received palivizumab prophylaxis for the two subsequent RSV seasons (2000–2001 and 2001–2002). Both cohorts included infants who were preterm (≤32 weeks gestational age) and were less than 6 months old at start of RSV season, in line with Spanish recommendations for the use of palivizumab [7].
The outcomes were validated by comparing data with IMpact. The palivizimab group experienced RSV hospitalisation in 4.8% of cases and the placebo group in 10.6%. The Spanish data showed that the annual mean RSV hospitalisation rate in the palivizumab cohort was 3.95% compared with 13.25% in infants who did not receive prophylaxis [18]. These data show that absolute outcomes for palivizumab and “no prophylaxis” are similar (3.95% vs 4.8% (palivizumab) and 10.6% vs 13.25% (no prophylaxis).
A health economic model has been developed to assess the cost-effectiveness of palivizumab prophylaxis in high-risk groups of children, the structure of which has recently been described for the United Kingdom setting [16]. When cost effectiveness was assessed from the perspective of the Spanish healthcare provider using this model, use of palivizumab produced a discounted incremental cost-effectiveness ratio (ICER) of €13,849 per quality-adjusted life-year (QALY), taking into account the direct medical costs associated with RSV prophylaxis and hospitalisation [17].
While this analysis demonstrates that palivizumab is a cost-effective treatment for the prevention of severe RSV disease in high-risk infants, the model has its limitations. Firstly, the impact of palivizumab use on hospitalisation was limited to a single RSV season, corresponding to data available from the IMpact trial in which children were followed for 150 days from the point of randomisation [6]. However, these 1-year data have limited external validity, as the majority of children who are at risk in any one season will also be at risk in the subsequent season or seasons. Also, in the absence of local data, hospitalisation rates were taken from the international IMpact trial, further limiting the external validity of the results for any one country. This limitation is especially relevant for a prophylaxis model, which is driven by the regional incidence of RSV infection.
Recent publication of multiple season data for Spain by Pedraz et al. [18] on behalf of the Infeccion Respiratoria Infantil por Virus Respiratorio Sincitial (IRIS) Study Group allows customisation of the existing core model to more accurately reflect the impact of palivizumab in the Spanish healthcare setting. Data were collected over four seasons, comparing RSV hospitalisation rates and risk factors in two Spanish cohorts of at-risk infants. The first cohort comprised 1,583 infants followed during the 1998–1999 and 1999–2000 RSV seasons, before palivizumab was licensed for use in Spain. The second cohort comprised 1,919 infants who received palivizumab prophylaxis for the two subsequent RSV seasons (2000–2001 and 2001–2002). Both cohorts included infants who were preterm (≤32 weeks gestational age) and were less than 6 months old at start of RSV season, in line with Spanish recommendations for the use of palivizumab [7].
An overall reduction of 70% in the rate of RSV hospitalisation was observed in the palivizumab group (3.95 vs 13.25%) despite this group’s lower gestational ages, more severe neonatal intensive care unit (ICU) respiratory courses and higher incidence of CLD. Children in the control group had a higher risk for RSV-related hospitalisation than those in the palivizumab group (odds ratio, 3.86; 95% confidence interval, 2.83–5.25) [18]. Data from this study support the effectiveness of palivizumab in significantly reducing RSV-related hospitalisations in high-risk preterm infants over two respiratory seasons [18].
The objective of the current analysis was to incorporate the new multiple season data from Pedraz into the existing core cost-effectiveness model, in order to increase the validity of the results for the Spanish healthcare setting.
Methods
The model considers the clinical and economic impact of palivizumab versus no prophylaxis in a hypothetical cohort of high-risk children, corresponding with those in the IRIS studies: preterm children born at or before 32 weeks gestational age who were less than 6 months old at the onset of RSV season. In order to accurately reflect the Spanish healthcare setting in 2006, the existing decision tree model was adjusted using hospitalisation data from the IRIS Study Group, other published literature, official Spanish price/tariff lists and national population statistics. Clinical events and utilities, which are not country-specific, were derived from international studies where no Spanish clinical data are available [19]. For economic measures and information on therapeutic choices, country-specific data sources have been used [19]. The primary perspective was that of the national health financer, the base-case analysis including the direct costs associated with use of palivizumab and those resulting from hospitalisation of children with RSV infection. The costs of managing possible sequelae caused by severe RSV infection (recurrent wheezing) were also considered, as were potential indirect costs due to lost productivity following the death of a child (societal perspective).
The base-case analysis assumed a lifetime follow-up period in order to capture the impact of palivizumab on long-term morbidity (recurrent wheezing) and mortality resulting from an RSV infection. The reduction in RSV hospitalisation resulting from use of palivizumab was extended in this analysis to 2 years, using observational data from the IRIS studies. The number of RSV hospitalisations from the IRIS studies was extrapolated to provide an estimate of life-years gained (LYG) and utilities incorporated to calculate QALYs by the impact of RSV hospitalisation on morbidity (recurrent wheezing) and mortality. The life time impact is also required to include the costs beyond the RSV hospitalisation period, which include the medical costs for management of wheezing and future lost productivity of a child resulting from mortality.
A discount rate of 3% was applied to both costs and clinical outcomes in the base-case analysis, as recommended by Spanish guidelines for pharmacoeconomic analyses [20]. This rate was varied between 0 and 6% in a sensitivity analysis.
Design
Probabilities and life expectancy
Figure 1 shows the structure of the model. This is identical to the core decision tree model developed for the UK [16] and the original Spanish analysis, but the probabilities have been modified using observational data for Spain [18; Table 1]. Children may develop an RSV infection leading to hospitalisation. The majority of these children will be managed in a paediatric ward, but a proportion will require transfer to a neonatal or paediatric intensive care unit (NICU or PICU) [21]. A small proportion of the children may die. Among the surviving children, a significant proportion may develop long-term sequelae (e.g. wheezing, asthma). The analysis was based on a comparison of prophylaxis with palivizumab versus no prophylaxis. Details of the model are also provided in the published study for the United Kingdom, which was the template (basis) for the model in Spain [16].
Fig. 1.
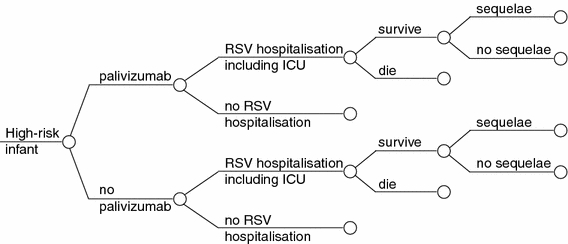
Decision tree model for infants at high risk of respiratory syncytial virus (RSV) infection. ICU Intensive care unit
Table 1.
Clinical probabilities
| Preterm (≤32 weeks) | Probability | P value | Reference | |
|---|---|---|---|---|
| Palivizumab (%) | No prophylaxis (%) | |||
| Hospitalisation | ||||
| 1-year | 13.40 | 3.90 | <0.001 | Pedraz et al. [18] |
| 2-year | 13.10 | 3.95 | <0.001 | |
| 1 and 2 year: cumulative | 26.50 | 7.90 | 0.001 | |
| 1 and 2 year: annual mean | ||||
| Subpopulation | ||||
| ≤ 28 weeks | 5.4 | 13.0 | <0.001 | |
| 29–32 weeks | 2.5 | 9.9 | <0.001 | |
| 1 and 2 year: cumulative | 5.5 | 19.7 | <0.007 | |
| 1 and 2 year: annual mean | ||||
| Probability | P value | Impact trial | ||
|---|---|---|---|---|
| Palivizumab (%) | No prophylaxis (%) | |||
| Hospitalisation | ||||
| Overall | 4.8 | 10.6 | <0.001 | |
| Preterm, excluding BPD | 1.8 | 8.1 | <0.001 | |
| BPD | 7.9 | 12.8 | 0.038 | |
| Probability | P value | Impact trial | ||
|---|---|---|---|---|
| RSV hosp. (%) | Control (%) | |||
| Mortality | ||||
| Observational study | 8.12 | 1.58 | 0.001 | Sampalis [9] |
| Observational study Spain | 0.31 | 1.39 | NS | Pedraz et al. [18] |
| Life expectancy (years) | 78.05 | Official life tables on Spanish statistics | ||
BPD Bronchopulmonary dysplasia
Hospitalisation
For both the palivizumab group and infants not receiving prophylaxis, the rate of hospitalisation in the base-case analysis was derived from the IRIS studies. The annual mean RSV hospitalisation rate in the palivizumab cohort was 3.95% (3.9% in the first season and 3.95% in the second), compared with 13.25% (13.4% in the first season and 13.1% in the second) in infants who did not receive prophylaxis [18].
Mortality
Infant mortality is an important consideration from a clinical and societal perspective as a small reduction in the rate of mortality in children can lead to a large increase in life expectancy. The base-case analysis was based on the overall mortality rates observed in the IRIS studies: six patients in the palivizumab cohort died (0.31%) compared with 22 in the no prophylaxis group (1.39%) [18]. Two scenario analyses were performed. The first assumed that there was no difference in mortality between groups, while the second scenario was based on mortality rates in the 2 years following hospitalisation due to RSV, observed by Sampalis [9]. The overall mortality rate in the RSV cohort in the 2 years after the initial hospitalisation was 8.11% compared to 1.58% in the control cohort (P = 0.001).
Life expectancy
There are no clinical data to suggest a reduced life expectancy due to childhood RSV infection. It was therefore assumed that life expectancy after surviving an RSV episode is similar to life expectancy in the general population of cohort children at 1 year of age, derived from official population statistics for Spain [22]. Life expectancy at birth in Spain is 79.29 years. Consequently, life expectancy at age 1 is approximately 78.29 years.
Utilities
The incorporation of utilities in this model was based on the study by Greenough et al. which was also used in the published study for the UK [16]. This study by Greenough et al. [23] reported utilities in children with a history of RSV hospitalisation (Table 2). The median health utility index (HUI) two multi-attribute utility function (HUI2) was 0.88 (range 0.16–1.00) in the RSV proven children, which was significantly lower (P = 0.0088) than that in the non-RSV children (median 0.95, range 0.03–1.00). The median HUI three multi-attribute score (HUI3) of the two groups did not differ significantly (0.93, range 20.05–1.00 vs 0.97, range 20.32–1.00). The following assumptions were made for the model base-case analysis:
High-risk children who have not been hospitalised for RSV will not have perfect health, but will have a utility corresponding to the median HUI2 score for children without RSV (u = 0.95).
High-risk children who have been hospitalised for RSV will have a utility corresponding to the median HUI2 score for children with RSV (u = 0.88).
This model assumes that, beyond 16 years of age, there will be no difference in utility between the different high-risk groups, regardless of whether or not they develop RSV or long-term respiratory morbidity after an RSV hospitalisation. All patients older than 16 are assumed to have perfect health (u = 1).
The median multi-attribute score on the HUI3 did not differ significantly between the two groups. Thus, a scenario analysis was performed based on a utility of 0.93 (the utility measured in RSV children in this study) for all children up to 16 years of age.
Table 2.
Utilities for health states
| Preterm infants with BPD | |||
|---|---|---|---|
| Literature | RSV proven | Non-RSV proven | P value |
| HUI 2 | 0.88 (0.16–1.00) | 0.95 (0.03–1.00) | 0.0088 |
| HUI 3 | 0.93 (–0.05–1.00) | 0.97 (–0.32–1.00) | NS |
| Model input | RSV hospitalisation | No RSV hospitalisation | >16 years |
|---|---|---|---|
| Base-case analysis (uses HUI 2 data) | 0.88 (0.16–1.00) | 0.95 (0.03–1.00) | 1 |
| Scenario analysis (uses HUI 3 data) | 0.93 (–0.05–1.00) | 0.93 (–0.05–1.00) | 1 |
HUI Health utilities index
Resource use and costs
A range of costs were included in the analysis: costs associated with the acquisition and administration of palivizumab; costs for RSV hospitalisation and costs for treating the longer-term consequences of severe RSV infection, such as recurrent wheezing. All costs were adjusted for inflation to correspond to the year 2006.
Palivizumab
Palivizumab is available in 50 mg and 100 mg vials with a mean acquisition cost, provided by Abbott Laboratories in Spain, of €8.78/mg. The recommended dosage is 15 mg/kg body weight administered by intramuscular injection once a month for 4–5 months. It was assumed that a 100 mg vial would be used in 95% of administrations, whereas a 50 mg vial would be used for the remaining 5%, based on an estimation provided by the Spanish Society of Neonatology [24]. The mean number of injections per child in the IRIS studies was 4.1, giving a total acquisition cost of €3,880 per RSV season. Sensitivity analyses were conducted to assess the impact on cost-effectiveness of varying vial use.
It was assumed that each complete administration of palivizumab would take approximately 20 min of a nurse’s time. The base salary of a nurse who works in the Spanish National Health Service was obtained from official sources [25] and inflated to take account of salary increases for length of service and corresponding social costs (social security, etc.), providing a cost of € 5.33 for each administration [17].
Days of RSV hospitalisation
From the IRIS studies, the length of hospital stay due to RSV was significantly lower for the palivizumab group than for those who did not receive prophylaxis (6 days vs 8 days; P < 0.01) [18]. Length of stay (LOS) in the ICU was derived from another study conducted by the IRIS Study Group, in which the median length of time spent in the ICU by preterm children who had not received prophylaxis was 6 days [26]. In both IRIS cohorts, a considerable proportion of the children required admission to the ICU, although the difference between groups was not statistically significant: 13% of those who had received palivizumab and 20% of controls [18]. Therefore, it was assumed that the average child would spend 1.2 days in the ICU (20% of 6 days) and that the remainder of the stay would be spent on a general paediatric ward: 4.8 days for the palivizumab group and 6.8 days for those who did not receive prophylaxis. Length of hospital stay was varied by ±10% in a sensitivity analysis.
Direct medical costs
Table 3 shows a summary of the unit costs used in the model for each type of resource. Costs per day in the paediatric ward, the paediatric ICU, and general ward were estimated based on information from the previous Spanish analysis [17]. Costs of outpatient and hospital paediatric consultations were derived from price lists for public health services in the Autonomous Community of Madrid [27]. The cost of hospitalisation was varied in a sensitivity analysis between that of a general paediatric ward (€495/day) and that of intensive care (€871/day).
Table 3.
Unit costs
| Item | Cost/unit (€) | Reference | |
|---|---|---|---|
| Hospitalisation | ICU ward | 871 | [17] |
| Paediatric ward | 495 | ||
| General ward | 495 | ||
| Other | Outpatient visit (hospital) | 154 | [27] |
| Primary care visit | 49 | ||
| Primary care visit for respiratory disease | 49 |
ICU Intensive care unit
Long-term sequelae
Among surviving children, a significant proportion may develop long-term sequelae (e.g. recurrent wheezing, asthma) [9, 28]. Data on healthcare resource use related to hospitalisation for RSV infection were taken from a United Kingdom study (Table 4) [29]. Neonates born at less than 32 weeks gestational age with a proven RSV admission made greater use of hospital and community healthcare resources in the first 2 years of life than a control group who had been admitted to hospital for non-respiratory reasons or had not been hospitalised at all.
Table 4.
Resource use relating to hospitalisation for RSV infection [29]
| RSV provena | Controlb | Difference | |
|---|---|---|---|
| Days in ICU | 2.9 | 0 | 2.9 |
| Days in paediatric ward | 30.7 | 1.6 | 29.1 |
| Outpatient visits | 11.9 | 8.6 | 3.3 |
| GP contacts | 16.3 | 14.6 | 1.7 |
| Community care contacts | 28.2 | 18.7 | 9.5 |
| Consultations with GP for respiratory illness | 8.3 | 5.6 | 2.7 |
GP General practitioner
aNeonates born at < 32 weeks gestational age with at least one proven RSV hospital admissionbNeonates born at < 32 weeks gestational age who had been admitted to hospital for non-respiratory reasons or had not been hospitalised at all
Indirect costs
Indirect costs arise from loss of productive employment due to premature death. To estimate indirect costs it was assumed that the proportion of children reaching working age who are employed, unemployed or not in the labour market will be similar to the current situation in Spain. Thus, the proportion of the gross domestic product (GDP; € 744,754 million) corresponding to the Spanish population of both sexes between 16 and 64 years of age (29,370,800 inhabitants) was calculated [30]. This translates to an average GDP for a person aged 16–64 years of € 25,356, which was adjusted to € 19,600 after correction for employment rate (77.3%). which is the value assumed by the model for each year of life lost [17]. Since it is assumed that a year in the future is worth less than a year in the present, the model applies an annual 3% discount rate to these costs.
Results
Base-case analysis
Costs and clinical outcomes are presented in Table 5. When the direct costs associated with RSV prophylaxis and hospitalisation are taken into account, use of palivizumab results in an additional cost of €6,321 per child. Inclusion of the costs associated with the long-term consequences of severe RSV disease (sequelae disease like asthma and recurrent wheezing) reduces the incremental cost of palivizumab to €3,205. From the societal perspective, including both direct and indirect costs, use of palivizumab results in overall cost savings of €396.
Table 5.
Costs and clinical outcomes (base-case analysis)
| Palivizumab | No prophylaxis | Difference | |
|---|---|---|---|
| Costs | |||
| Direct medical costs (€) | 7,490 | 1,169 | 6,321 |
| Direct medical costs + sequelae (€) | 8,813 | 5,608 | 3,205 |
| Indirect costs (€) | 1,034 | 4,634 | −3,601 |
| Total costs (direct + indirect) (€) | 9,846 | 10,242 | −396 |
| Outcomes | |||
| Undiscounted | |||
| Life-years lost | 0.24 | 1.09 | −0.85 |
| QALYs gained | 77.22 | 76.19 | 1.03 |
| Discounted | |||
| Life-years lost | 0.10 | 0.43 | −0.33 |
| QALYs gained | 30.20 | 29.70 | 0.49 |
QALY Quality-adjusted life-year
With regard to clinical outcomes, 0.85 fewer undiscounted life-years are lost with the use of palivizumab than with no prophylaxis, and 0.33 fewer life-years are lost after discounting. The inclusion of utilities produces a gain of 0.49 and 1.03 quality-adjusted life-years (QALYs) with palivizumab, with and without discounting, respectively.
Table 6 shows the cost-effectiveness results. In the base-case analysis (direct medical costs excluding sequelae diseases, the use of palivizumab produces an undiscounted incremental cost-effectiveness ratio (ICER) of €6,142/QALY, which becomes €12,814/QALY after discounting. The cost per life-year gained (LYG) is somewhat higher: €7,475 undiscounted and €18,872 discounted. Inclusion of the cost of treating sequelae diseases decreases the undiscounted ICER to €3,114/QALY and the discounted ICER to €6,498/QALY. When indirect costs are included, palivizumab becomes the dominant strategy from the societal perspective, as its use results in both clinical benefits and cost savings versus no prophylaxis.
Table 6.
Incremental cost-effectiveness ratios (ICER)
| Undiscounted ICER | Discounted ICER | ||
|---|---|---|---|
| Base-case analysis | LYG | €7,475 | €18,872 |
| QALY | €6,142 | €12,814 | |
| Direct costs + sequelae diseases | LYG | €3,791 | €9,570 |
| QALY | €3,114 | €6,498 | |
| Total costs | LYG | Dominant | Dominant |
| QALY | Dominant | Dominant |
LYG life-year gained
Scenario analyses
A scenario analysis was performed assuming that there was no difference in mortality between palivizumab-treated children and those receiving no prophylaxis, based on the no prophylaxis rate of mortality from the IRIS studies (1.39%). The results indicate the high impact of mortality on the cost-effectiveness outcomes for palivizumab: the ICERs increase to €32,819/QALY (undiscounted) and €38,050/QALY (discounted) for the base-case analysis.
Table 7 shows the results of the scenario analysis based on the 2-year mortality data from the Sampalis study [9]. As in the base-case analysis, the use of palivizumab results in an additional medical cost of €6,321, which reduces to €3,205 when the costs associated with sequelae diseases are included. However, changes in the assumed rate of mortality have a significant impact on indirect costs. In this scenario, use of palivizumab leads to cost savings of €1,828 from a societal perspective. Clinical benefits also increase relative to the base-case analysis: use of palivizumab results in 1.18 fewer life-years lost (undiscounted) than with no prophylaxis; 0.47 fewer life-years are lost after discounting. When utilities are included, the incremental gain achieved with palivizumab prophylaxis is 0.62 and 1.36 QALYs, with and without discounting, respectively. The ICER decreases from €6,142 to €5,108 (no discounting) and it decreases from €12,814 to €12,279 (discounting).
Table 7.
Scenario analysis based on alternative 2-year mortality data
| Palivizumab | No prophylaxis | Difference | |
|---|---|---|---|
| Costs | |||
| Direct medical costs (€) | 7,490 | 1,169 | 6,321 |
| Direct medical costs + sequelae (€) | 8,813 | 5,608 | 3,205 |
| Indirect costs (€) | 2,138 | 7,171 | −5,033 |
| Total costs (direct + indirect) (€) | 10,950 | 12,778 | −1,828 |
| Outcomes | |||
| No discounting | |||
| Life-years lost | 0.50 | 1.68 | −1.18 |
| QALYs gained | 76.96 | 75.60 | 1.36 |
| Discounting | |||
| Life-years lost | 0.20 | 0.67 | −0.47 |
| QALYs gained | 30.10 | 29.48 | 0.62 |
The median multi-attribute score on the HUI3 did not differ significantly between the two groups. Thus, a scenario analysis was performed based on a utility of 0.93 (the utility measured in RSV children in this study) for all children up to 16 years of age (Table 8).
Table 8.
Scenario analysis based on alternative 3-year mortality data
| Palivizumab | No prophylaxis | Difference | |
|---|---|---|---|
| Costs | |||
| Direct medical costs (€) | 7,490 | 1,169 | 6,321 |
| Direct medical costs + sequelae (€) | 8,813 | 5,608 | 3,205 |
| Indirect costs (€) | 2,138 | 7,171 | −5,033 |
| Total costs (direct + indirect) (€) | 10,950 | 12,778 | −1,828 |
| Outcomes | |||
| No discounting | |||
| Life-years lost | 0.24 | 1.09 | −0.85 |
| QALYs gained | 77.00 | 76.17 | 0.83 |
| Discounting | |||
| Life-years lost | 0.10 | 0.43 | −0.33 |
| QALYs gained | 30.01 | 29.69 | 0.33 |
In this scenario, the life-years lost and economic outcomes do not change. The use of palivizumab results in incremental gain of 0.33 QALYs and 0.83 QALYs, with and without discounting, respectively. The ICER increases from €6,142 to €7,616 (no discounting) and it increases from €12,814 to €19,155 (discounting). This scenario also captures the impact of long-term sequelae, because there is no long-term difference in utility for patients with and without long-term sequelae.
Sensitivity analyses
Figure 2 shows the results of univariate sensitivity analyses, which were performed on discounting, vials, hospitalisation costs, LOS. These results show that the cost-effectiveness outcome for palivizumab is sensitive to the rate of discounting applied to effectiveness measures. When discounting is applied only to costs, variations in the rate used have no effect as only short-term costs are included in the base-case analysis. However, model outcomes are very sensitive to changes in the discount rate when this is also applied to effectiveness. The model outcomes are also sensitive to assumptions regarding vial use. In the base-case analysis, it was assumed that a 100 mg vial would be used for the majority (95%) of administrations of palivizumab. When it is assumed that only 50 mg vials are used, the ICER falls from €12,814/QALY to €6,768/QALY.
Fig. 2.
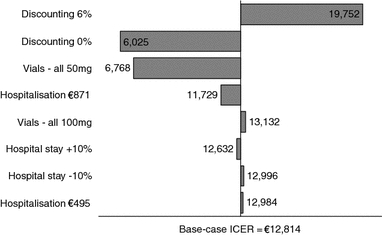
Sensitivity analyses. ICER Incremental cost-effectiveness ratio
Figure 3 shows the cost-effectiveness acceptability curves for palivizumab versus no prophylaxis. This figure was based on a probabilistic sensitivity analysis with effectiveness discounted, using beta distributions for hospitalisation probabilities, mortality and vial use, and a normal distribution for hospital LOS and resource use for recurrent wheezing, respectively. The cost-effectiveness acceptability curve illustrates that there is close to 100% probability that the ICER of palivizumab will be less than €30,000/QALY—the threshold below which interventions are generally considered to be cost effective [31].
Fig. 3.
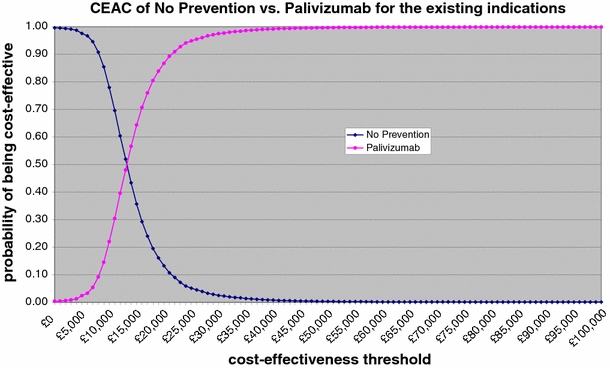
Incremental cost/utility curve
Discussion and conclusions
The objective of the current analysis was to confirm the cost-effectiveness of palivizumab prophylaxis in Spain, originally calculated based on international trial data, through incorporation of country-specific observational data. The original core cost-effectiveness model calculated a discounted ICER of €13,849/QALY, taking into account the direct medical costs associated with RSV prophylaxis and hospitalisation [17]. Even in the least favourable scenario, the ICER was lower than the €30,000/QALY threshold. The comparable ICER produced by the new model, incorporating data from the IRIS Study Group, was €12,814/QALY. These results show that the use of Spanish observational data not only increases the external validity of the results, but also yields a slightly more favourable cost-effectiveness outcome than the use of international clinical trial data.
Improvements in the cost-effectiveness outcome may be explained by differences in the rates of hospitalisation used in the two models. The original cost-effectiveness model was based on data from the international IMpact trial, which was conducted in 139 centres in the United States, United Kingdom and Canada. The average rate of hospitalisation in the palivizumab arm was 4.8% compared with 10.6% in the no prophylaxis arm: a 55% reduction in relative risk [6]. The revised model is based on Spanish hospitalisation rates from the IRIS studies. Use of palivizumab resulted in a 70% overall reduction in the rate of RSV hospitalisation in Spain, from 13.25% in the control cohort to 3.95% in those infants receiving palivizumab [18]. The 13.25% RSV hospitalisation in the control cohort also corresponds with the incidence of RSV hospitalisation, which was 13.5% in the placebo group in the PREVENT Study [5].
The current cost-effectiveness model eliminated some of the limitations of the previous cost-effectiveness model. The impact of palivizumab was extended beyond a single RSV season and the hospitalisation rates were based on local Spanish data corresponding with treatment practise in Spain, whereas the previous model was based on hospitalisation rates derived from an international clinical trial (IMpact). The results of the current model show that the outcomes of the model are rather robust, as the ICER of the current model (€12,814/QALY) is close to the initial ICER of €13,849/QALY. Therefore the current analysis is a validation of the initial cost-effectiveness results from the previous model.
The analysis also shows that the cost-effectiveness outcomes are sensitive to the assumed rate of mortality. If it is assumed that palivizumab prophylaxis has no impact on the rate of mortality, as in the scenario analysis in which the no prophylaxis rate of mortality was employed for both cohorts of at-risk infants, cost-effectiveness outcomes become significantly less favourable than in the base-case analysis (€38,050/QALY versus €12,814, respectively). Conversely, incorporation of the 2-year mortality data from the Sampalis study results in more favourable outcomes for palivizumab compared with the base-case, due to improved clinical outcomes and greater indirect cost savings.
The results presented here confirm that palivizumab provides a cost-effective method of prophylaxis against severe RSV disease among preterm infants in Spain. Use of Spanish observational data further increases the validity of the results for the Spanish healthcare setting in 2006 and confirms the conclusion of the previous cost-effectiveness study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Mark J. Nuijten, Phone: +31-756422455, FAX: +31-756422456 Email: nuijten@bmg.eur.nl
Wolfgang Wittenberg, Phone: +49-6215893127, FAX: +49-6215891423 Email: Wolfgang.wittenberg@abbott.com.
References
- 1.Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354(9181):847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 2.Canfield SD, Simoes EA. Prevention of respiratory syncytial virus (RSV) infection: RSV immune globulin intravenous and palivizumab. Pediatr. Ann. 1999;28(8):507–514. doi: 10.3928/0090-4481-19990801-08. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Beresford M, Subhedar N, Shaw N. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch. Dis. Child. 2000;83:313–316. doi: 10.1136/adc.83.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N. Engl. J. Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 5.The PREVENT Study Group Reduction of RSV hospitalisation among preterm infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 6.The IMpact Study Group Palivizumab, a humanised respiratory syncytial virus monoclonal antibody, reduces hospitalisation from respiratory syncytial virus infection in high risk infants. Pediatrics. 1998;102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 7.Carbonell Estrany X, Quero Jimenez J. Comité de Estándares de la Sociedad Española de Neonatología, y Junta Directiva de la Sociedad Española de Neonatología. Recomendaciones para la prevención de la infección por virus respiratorio sincitial. An Esp Pediatr. 2000;52(4):372–374. [PubMed] [Google Scholar]
- 8.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 9.Sampalis JS. Morbidity and mortality after RSV-associated hospitalisations among premature Canadian infants. J. Pediatr. 2003;143(5 Suppl):S150–S156. doi: 10.1067/s0022-3476(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 10.Joffe S, Ray GT, Escobar GJ, Black SB, Lieu TA. Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics. 1999;104(3 Pt 1):419–427. doi: 10.1542/peds.104.3.419. [DOI] [PubMed] [Google Scholar]
- 11.The European Medicines Agency: http://www.emea.eu.int/index/indexh1.htm, accessed December 2007
- 12.Feltes TF, Cabalka AK, Meissner HC, et al. Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalisation due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J. Pediatr. 2003;143:532–540. doi: 10.1067/S0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 13.Bont L, van Aalderen W, Kimpen J. Long term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr. Respir. Rev. 2000;1:221–227. doi: 10.1053/prrv.2000.0052. [DOI] [PubMed] [Google Scholar]
- 14.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 15.Simoes EAF, Carbonell-Estrany X, Kimpen J, Rieger CHL, Morris DD, Pollack PF, Groothuis JR. Palavizumab use decreases risk of recurrent wheezing in preterm children. Eur. Respir. J. 2004;24(Suppl 48):212s. [Google Scholar]
- 16.Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: a UK analysis. Pharmacoeconomics. 2007;25(1):55–71. doi: 10.2165/00019053-200725010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lázaro y de Mercado P, Figueras Aloy J, Domenech Martinez E, et al. The efficiency (cost-effectiveness) of palivizumab as prophylaxis against respiratory syncytial virus infection in premature infants with a gestational age of 32–35 weeks in Spain. An Pediatr (Barc) 2006;65(4):316–324. doi: 10.1157/13092505. [DOI] [PubMed] [Google Scholar]
- 18.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J, IRIS Study Group Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalisations in premature infants. Pediatr. Infect. Dis. J. 2003;22(9):823–827. doi: 10.1097/01.inf.0000086403.50417.7c. [DOI] [PubMed] [Google Scholar]
- 19.Nuijten MJC. Data management in modelling studies: the selection of data sources. Pharmacoeconomics. 1998;3(3):305–316. doi: 10.2165/00019053-199813030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Pinto-Prades JL, Ortún-Rubio V, Puig-Junoy J. El análisis coste-efectividad en sanidad. Aten Primaria. 2001;27:275–278. doi: 10.1016/S0212-6567(01)78808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson, S., Burls, A.: A systematic review of the effectiveness and cost-effectiveness of palivizumab (Synagis®) in the prevention of respiratory syncytial virus (RSV) infection in infants at high risk of infection. A West Midlands Development and Evaluation Service Report Department of Public Health & Epidemiology. University of Birmingham Edgbaston, Birmingham B15 2TT. ISBN No. 0704423219 Copyright, West Midlands Health Technology Assessment Group Department of Public Health and Epidemiology. University of Birmingham (2001)
- 22.Instituto Nacional de Estadística (INE).: Datos demográficos básicos, esperanza de vida al nacimiento. Disponible en: http://www.ine.es/(consultado en septiembre de 2005)
- 23.Greenough A, Alexander J, Burgess S, et al. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch. Dis. Child. 2004;89:673–678. doi: 10.1136/adc.2003.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Personal communication with Dr P Lázaro y de Mercado: Técnicas Avanzadas de Invetigatión en Servicios de Salud, April 2007
- 25.Retribuciones del personal sanitario.: Generalitat de Valencia. Available at http://www.dival.es. (Accessed August 2005)
- 26.Carbonell-Estrany X, Quero J, Bustos G, Cotero A, Domenech E, Figueras-Aloy J, Fraga JM, Garcia LG, Garcia-Alix A, Del Rio MG, Krauel X, Sastre JB, Narbona E, Roques V, Hernandez SS, Zapatero M. Rehospitalisation because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr. Infect. Dis. J. 2000;19(7):592–597. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Orden 234/2005, de 23 de febrero, del Consejero de Sanidad y Consumo, por la que sefijan los precios públicos por la prestación de los servicios y actividades de naturaleza sanitaria de la Red de Centros de la Comunidad de Madrid. Boletín Oficial de la Comunidad de Madrid (BOCM) núm. 56, 8 de Marzo de, 2005
- 28.Bont L, Aalderen WM, Kimpen JL. Long-term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr. Respir. Rev. 2000;1(3):221–227. doi: 10.1053/prrv.2000.0052. [DOI] [PubMed] [Google Scholar]
- 29.Greenough A, Cox S, Alexander J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch. Dis. Child. 2004;85(6):463–468. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Instituto Nacional de Estadística (INE).: Contabilidad Regional de España. Available at http://ine.es. (Consulted in January 2006)
- 31.Laufer F. Thresholds in cost-effectiveness analysis—more of the story. Value Health. 2005;8(1):86–87. doi: 10.1111/j.1524-4733.2005.08103.x. [DOI] [PubMed] [Google Scholar]


