Abstract
Leachianone G (LG) 2′′-dimethylallyltransferase, a novel prenyl side-chain elongation enzyme, was identified in Sophora flavescens Ait. cultured cells. The enzyme transfers a dimethylallyl group to the 2′′ position of another dimethylallyl group attached at position 8 of LG to form sophoraflavanone G, a branched monoterpenoid-conjugated flavanone characteristic to this plant. This membrane-bound dimethylallyltransferase required Mg2+ (optimum concentration was 10 mm) for the reaction and had an optimum pH of 8.8. It utilized dimethylallyl diphosphate as the sole prenyl donor, and the 2′-hydroxy function in LG was indispensable to the activity. The apparent Km values for dimethylallyl diphosphate and LG were 59 and 2.3 μm, respectively. Subcellular localization of three enzymes that participated in the formation of the lavandulyl group was also investigated by sucrose density gradient centrifugation. Two prenyltransferases, naringenin 8-dimethylallyltransferase and LG 2′′-dimethylallyltransferase, were localized in the plastids, whereas 8-dimethylallylnaringenin 2′-hydroxylase, which catalyzes the crucial step in the lavandulyl-group formation, was associated with the endoplasmic reticulum. These results suggest the close cooperation between the plastids and the endoplasmic reticulum in the formation of lavandulyl groups.
More than 30,000 isoprenoid compounds—the most chemically diverse family of metabolites—are found in nature (Eisenreich et al., 1998). The vast majority of these compounds have “regular” 1′-4 (head-to-tail) linkages between isoprenoid units formed by isoprenyl diphosphate synthases, which are types of prenyltransferase. These enzymes catalyze the prenyl diphosphate elongation reaction that results in the consecutive 1′-4 condensations of isopentenyl diphosphate (IPP), the five-carbon building unit, with allylic isoprenyl diphosphates such as dimethylallyl diphosphate (DMAPP) and geranyl diphosphate (GPP; Ogura and Koyama, 1998; Liang et al., 2002). “Irregular” (non-head-to-tail) isoprenoids are also found, of which the most prominent examples are the 1′-1 (tail-to-tail) condensed isoprenoids such as squalene and phytoene, which are important precursors of sterols and carotenoids, respectively (Poulter, 1990). The biosyntheses of these tail-to-tail condensed terpenoids have been well characterized, but little is known about other non-head-to-tail condensed terpenoids except for chrysanthemyl diphosphate synthase, which catalyzes the condensation of two molecules of DMAPP to produce chrysanthemyl diphosphate, a branched monoterpene with a c1′-2-3 linkage between two isoprenoid units (Poulter, 1990; Rivera et al., 2001). A lavandulyl group, another example of the branched monoterpenoid unit, is found in labiataeous and compositaeous plants as essential oils (de Lampasona et al., 1997; Tsuro et al., 2001; Gunawardena et al., 2002), and in leguminous and moraceous plants as diverse prenylated flavonoids (Barron and Ibrahim, 1996). Epstein and Poulter (1973) suggested very early that chrysanthemyl diphosphate might be an intermediate of lavandulyl monoterpenes. More recently, another mechanism—the direct condensation of two DMAPP molecules to form lavandulyl diphosphate—was postulated by Gunawardena et al. (2002).
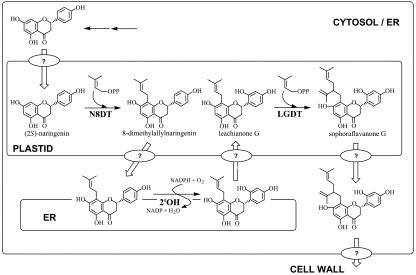
Sophora flavescens, a leguminous plant, produces diverse flavanones with lavandulyl side chain, such as sophoraflavanone G (SFG) and kurarinone (Hatayama and Komatsu, 1971; Wu et al., 1986; Kuroyanagi et al., 1999; Kang et al., 2000). They have the structural characteristic of possessing a lavandulyl group at C-8 or C-6 together with a hydroxyl group at C-2′. Recent pharmaceutical studies showed that the lavandulyl side chain is essential for the antitumor activity and phospholipase-Cγ1-inhibition activity of the flavonoids isolated from this plant (Lee et al., 1997; Ko et al., 2000). We have established cultured cells of this plant, which produce SFG as a major flavonoid (Yamamoto et al., 1991), and demonstrated that the lavandulyl group of SFG was not directly transferred to the flavanone skeleton but is biosynthesized by the discontinuous two-step dimethylallylations between which the 2′-hydroxylation occurred (Fig. 1). That is, the first dimethylallylation occurs on the flavanone nucleus catalyzed by naringenin 8-dimethylallyltransferase (N8DT) that does not accept lavandulyl diphosphate as the prenyl donor to afford 8-dimethylallylnaringenin (Yamamoto et al., 2000). This intermediate is further hydroxylated to form leachianone G (LG) by 8-dimethylallylnaringenin 2′-hydroxylase (2′OH; Yamamoto et al., 2001), and the second dimethylallylation takes place on the prenyl side chain of LG catalyzed by an uncharacterized dimethylallyltransferase to give SFG. More recently, we revealed that two isoprene units in the lavandulyl group of SFG were generated via the 1-deoxy-d-xylulose-5-phosphate (DXP) pathway (Yamamoto et al., 2002), which is an alternative route for IPP biosynthesis in the plastids (Eisenreich et al., 1998), suggesting that lavandulyl group formation involves two discontinuous dimethylallylation steps that take place in the plastids. Nevertheless, no direct evidence has been reported for the subcellular localization of the enzymes responsible for SFG biosynthesis.
Figure 1.
Biosynthetic route from naringenin to SFG by two discontinuous dimethylallylations and 2′-hydroxylation and transport of precursors/products in S. flavescens cultured cells. Ovals with question mark indicate postulated transport systems, although simple diffusion mechanisms are also explainable.
To elucidate the mechanism underlying lavandulyl-group formation, in the present study, we identified and characterized the second prenylation enzyme, LG 2′-dimethylallyltransferase (LGDT), from the cultured cells of S. flavescens (Fig. 1). To our knowledge, LGDT is the first example of a prenyltransferase that elongates the conjugated prenyl side chain to form the branched prenyl group. We also investigated subcellular localizations of three membrane-bound enzymes responsible for the lavandulyl group formation, N8DT, 2′OH, and LGDT, and found that two dimethylallyltransferases are localized in the plastids, whereas 2′OH is distributed in the endoplasmic reticulum (ER).
RESULTS
Detection of LGDT Activity in S. flavescens Cultured Cells
When crude cell-free extracts from S. flavescens cells were incubated with 1 mm LG, 2 mm DMAPP, and 10 mm MgCl2 for 30 min at 30°C, the enzymatic formation of a new compound whose retention time and UV absorption pattern were completely identical with those of SFG was observed in HPLC-photodiode array analysis. The reaction product was isolated by preparative HPLC and identified as SFG by comparison of its electron impact mass spectrometry (EIMS) spectrum with that of authentic SFG. Most prenyltransferase activity was recovered from the microsomal fraction prepared by ultracentrifugation, and its specific activity in this fraction was about 11-fold higher than that in crude cell-free extracts, indicating that the enzyme was tightly bound to the membrane fraction of the cells (Table I). The activity was dependent on the presence of LG, DMAPP, Mg2+, and active enzyme (data not shown).
Table I.
Membrane association of LGDT in S. flavescens cultured cells
Preparation of fractions and LGDT assay are described in “Materials and Methods.” Data are the means of duplicate determinations ± se.
| Enzyme Solution | Total Activity | Total Protein | Specific Activity |
|---|---|---|---|
| pkat g cells-1 | mg g cells-1 | pkat mg protein-1 | |
| Crude cell-free extracts | 17.08 ± 0.30 | 7.10 ± 0.49 | 2.41 ± 0.04 |
| 156,000g supernatant | 0.85 ± 0.45 | 5.79 ± 0.48 | 0.15 ± 0.09 |
| Microsomal fraction | 14.23 ± 0.63 | 0.52 ± 0.02 | 27.37 ± 1.21 |
To clarify whether the two dimethylallylations in SFG biosynthesis are catalyzed by the same or by different prenyltransferases, parallel assays on N8DT and LGDT using the microsomal membranes were performed (Table II). If one prenyltransferase catalyzes two different dimethylallylation reactions, the activities of both N8DT and LGDT would be decreased in the coexistence of naringenin and LG compared with those observed with naringenin or LG as the sole prenyl acceptor due to competition between the substrates at the catalytic site. However, when naringenin and LG were co-incubated with microsomal membranes, the activities of N8DT and LGDT were virtually identical to those when the acceptors were used individually, indicating that LGDT is recognized by another prenyltransferase from N8DT.
Table II.
Competition of prenyl acceptors in N8DT and LGDT assays
N8DT and LGDT assay are described in “Materials and Methods.” Data are the means of duplicate determinations ± se.
| Prenyl Acceptor | Specific Activity of N8DT | Specific Activity of LGDT |
|---|---|---|
| pkat mg protein-1 | ||
| Naringenin | 17.32 ± 0.47 | 0 |
| LG | 0 | 4.14 ± 0.01 |
| Naringenin + LG | 15.36 ± 0.32 | 3.92 ± 0.21 |
Biochemical Characterization of LGDT
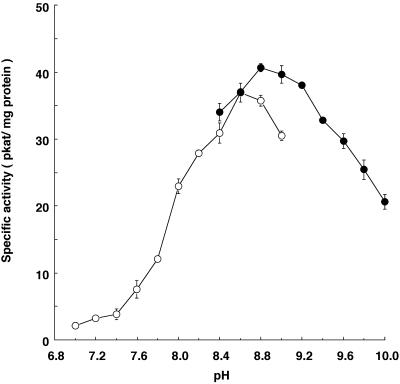
The enzymatic reaction showed a linear dependence on protein amounts between 30 and 120 μg of microsomal protein per assay and was linear up to an incubation time of 30 min. In contrast to previously reported prenyltransferases for which the optimum pH was around 7.5 (Dhillon and Brown, 1976; Schröder et al., 1979; Biggs et al., 1987; Hamerski and Matern, 1988; Welle and Grisebach, 1991; Laflamme et al., 1993; Fellermeier and Zenk, 1998; Mühlenweg et al., 1998; Tholl et al., 2001), LGDT showed an optimum pH of 8.8 in borate buffer, with about 50% of maximum activity at pH 7.9 and 10 (Fig. 2). The activity in Tris-HCl buffer at pH 8.8 was 88% of that observed in borate buffer.
Figure 2.
pH dependency of LGDT. ○, One hundred millimolar Tris-HCl buffer; •, 100 mm borate buffer. Vertical bars, Ranges from two independent enzyme assays.
The LGDT has an absolute requirement for divalent metal ions; the activity was negligible in an assay without any divalent cations. Mg2+ was the most effective among the divalent cations examined, and the saturation for Mg2+ (as chloride salts) was reached at 10 mm. In the presence of Mn2+, the activity was only 15% that of Mg2+. The other divalent metal ions examined also gave low activities: Ca2+ (25%), Zn2+ (10%), Co2+ (5%), Ni2+ (5%), Fe2+ (5%), and Cu2+ (4%).
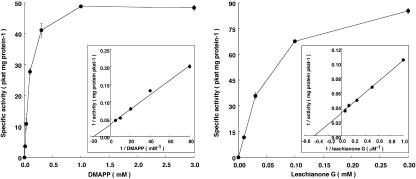
The apparent Km values for DMAPP and LG were calculated as 59 and 2.3 μm, respectively, from the Lineweaver-Burk plot using varying concentrations (12.5–200 μm for DMAPP and 1–16 μm for LG; Fig. 3).
Figure 3.
Dependency of LGDT activity on the concentration of DMAPP and LG measured with a microsomal fraction of S. flavescens cultured cells. Insert, Lineweaver-Burk plot with varying concentrations of DMAPP (12.5–200 μm) and of LG (1–16 μm) to calculate the apparent Km value for DMAPP and LG, respectively. Vertical bars, Ranges from two independent enzyme assays.
Substrate Specificity of LGDT
For the investigation of substrate specificity of LGDT, the microsomal fraction was incubated with prenyl acceptor (0.3 mm), prenyl donor (1 mm), and Mg2+ (10 mm) under the standard assay condition. In each experiment, heat-denatured enzyme-containing assay was used as the control. When the microsomal fraction was incubated with LG, Mg2+ and IPP, or GPP, any additional peaks were not observed in their HPLC profiles, indicating that only DMAPP is utilized by LGDT as the prenyl donor (Table III, left).
Table III.
Substrate specificity of the leachianone G 2″-dimethylallyltransferase activity
| Substrate (Donor) | Relative Activity | Substrate (Acceptor) | Relative Activity |
|---|---|---|---|
| % | % | ||
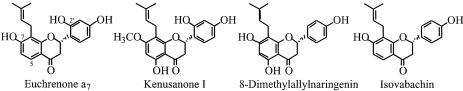
| DMAPP | 100 ± 3.0a | Leachianone G | 100 ± 3.0a |
| IPP | n.d.b | Euchrenone a7 | 54 ± 1.5c |
| GPP | n.d. | Kenusanone I | n.i.d |
| 8-Dimethylallylnaringenin | n.d. | ||
| Isovabachin | n.d. |
The ultimate concentrations of acceptors and donors are 0.3 mM and 1 mM, respectively. Structures of acceptors were shown below (for leachianone G, see Fig. 1). The HPLC assay were performed according to the standard method described in “Materials and Methods”. Data are means of duplicate determinations ± se.
a LGDT activity was 41.1 pkat mg protein-1.
b Compared with heat-denatured control assay, any additional peaks were not detected.
c The prenylated product was identified as lehmannin by comparison with authentic lehmannin using HPLC photodiode-array analysis.
d The production of new compound, the elution profile of which was resemble to that of the expected product was observed (in the present HPLC condition, 8-lavandulyated product was eluted ca. 6 min later than 8-dimethylallylated substrate), though was not identified. Conversion rate of it was 11 ± 0.3 (% of control).
The prenylation activities of LGDT for several 8-dimethylallylated flavanones were also investigated (Table III, right). LGDT did not show absolute specificity for LG. When euchrenone a7 (5-deoxy derivative of LG) was used as the prenyl acceptor, the formation of lehmannin (5-deoxy derivative of SFG) was observed in HPLC-photodiode array analysis. Its prenylation activity was 54% of the rate of LG. Incubation with kenusanone I, 7-methoxy derivative of LG, also afforded a new more lipophyllic compound, although its structure could not be identified in the present study. LGDT did not prenylate the 2′-hydroxyl group lacking 8-dimethylallylflavanones such as 8-dimethylallylnaringenin and isovabachin.
Subcellular Localization of the Lavandulyl Group-Forming Enzymes
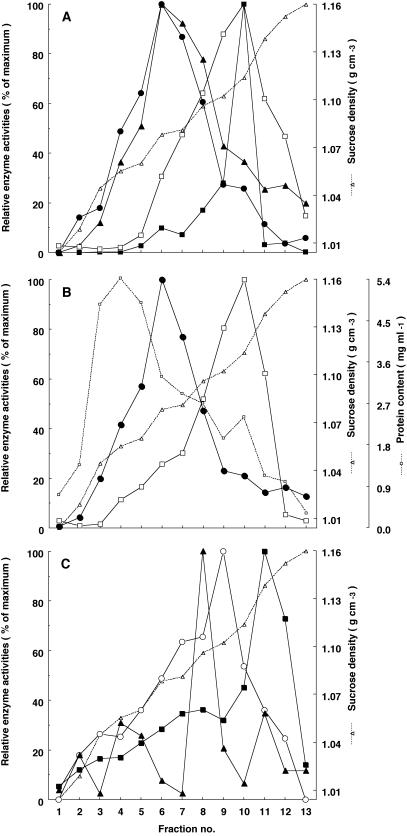
As mentioned above, three membrane-bound enzymes participate in the formation of the lavandulyl group: two prenyltransferases, N8DT and LGDT, and a cytochrome P450 monooxygenase, 2′OH. Our recent [1-13C]Glc-feeding experiment revealed that two isoprene units in the lavandulyl group of SFG originated from a DXP pathway (Yamamoto et al., 2002), suggesting that two dimethylallylation steps were carried out in the plastids, whereas 2′OH, a cytochrome P450 monooxygenase, was thought to be localized in the ER. To confirm the subcellular localization of these enzymes, microsomal membranes were separated by Suc density gradient centrifugation. As shown in Figure 4A, both N8DT and LGDT activities were in a fraction with a density of ρ = 1.07–1.08 g cm–3 (fraction 6), coinciding with UDP-Gal:diacylglycerol galactosyltransferase activity, a well-established marker of the plastids (Douce et al., 1984), indicating that two prenyltransferases were associated with the membrane of the plastids.
Figure 4.
Location of the activities of LGDT, N8DT, 2′OH, cinnamate 4-hydroxylase (C4H), and marker enzymes in S. flavescens membranes fractionated by Suc density gradient centrifugation. Marker enzyme activities and LGDT, N8DT, 2′OH, and C4H activities were estimated in each fraction as described in “Materials and Methods.” Fraction 1, Top of the gradient. Data are from a typical experiment—similar results were obtained from four other independent experiments. Activities are expressed as the percentage of the highest activity obtained in the peak fraction. A: •, LGDT; ▴, N8DT; ▪, 2′OH; □, C4H. B: •, UDP-Gal:diacylgylcerol galactosyltransferase (plastids marker); □, antimycin A-insensitive NADH cytochrome c reductase (ER marker); □ (with dashed line), protein content of separated membranes. C: ○, NADPH cytochrome c oxidase (mitochondria marker); ▴, KNO3-sensitive ATPase (tonoplast marker); ▪, Na3VO4-sensitive ATPase (plasma membrane marker).
On the other hand, the activities of 2′OH and cinnamate 4-hydroxylase (C4H), another cytochrome P450 monooxygenase known to exist in the ER (Ro et al., 2001), were observed in fraction 10 (Fig. 4A). The density of this fraction (ρ = 1.11–1.12 g cm–3) was in agreement with that of the ER (Robinson et al., 1994; Martinec et al., 2000). Moreover, the activity of antimycin A-insensitive NADH cytochrome c reductase, a marker of ER, was also found in this fraction. These results indicate that 2′OH is localized in the ER membranes, as it is in most cytochrome P450s in animals and plants.
Distribution patterns of other membranes in Suc density gradient (Fig. 4C) were similar to those from Beta vulgaris (Bennett et al., 1984) and Chenopoium rubrum (Martinec et al., 2000), although Robinson et al. (1994) reported that the density of tonoplast varies with plant species and tissues.
DISCUSSION
LGDT Is a Novel Prenyl Side-Chain Elongation Enzyme
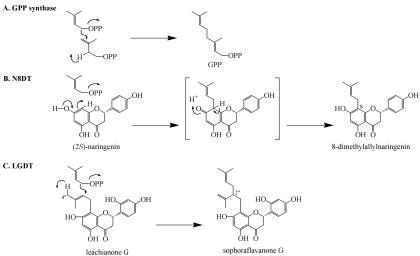
It is well known that many prenyl side-chain-conjugated compounds with important biological functions, such as aromatics and proteins, are widely distributed in organisms. In animals, prenylated proteins act as the modulator of cell cycle progression (Tamanoi et al., 2001), and prenylated phenolics from plants exhibit many biological activities against other organisms (Barron and Ibrahim, 1996). In these prenyl side-chain conjugation reactions, the adequate length prenyl diphosphate biosynthesized before-hand by prenyl diphosphate elongation enzymes were thought to be directly transferred to the prenyl acceptor (Fellermeier and Zenk, 1998; Mühlenweg et al., 1998; Liang et al., 2002). In the present study, we first identified a novel prenyl side-chain elongation enzyme, LGDT, from cultured cells of S. flavescens. In contrast to previously reported prenyl diphosphate elongation enzymes that transfer dimethylallyl group to prenyl diphosphate to form C5-elongated prenyl ones (Ogura and Koyama, 1998; Liang et al., 2002), LGDT transfers a dimethylallyl group to the dimethylallyl side chain already bound to 2′-hydroxyflavanones to afford a branched prenyl side chain. LGDT only accepted DMAPP as the prenyl donor (IPP or GPP were not transferred to the flavanone; Table III), suggesting that LGDT strictly recognizes the chain length and the position of the double bond in the prenyl donor and catalyzes the single 1′-2 condensation. The possible reaction mechanisms of LGDT and other known prenyltransferases are illustrated in Figure 5. The reaction mechanism of LGDT (Fig. 5C) seems to resemble that of the 1′-4 chain elongation prenyltransferases; for example, GPP synthase (Fig. 5A), rather than that of the prenyltransferases, which transfer the prenyl group to the aromatic compounds, such as N8DT (Fig. 5B). The reaction is started by the ionization of DMAPP, as in the case of the 1′-4 chain elongation reaction. Different from 1′-4 condensation, we suppose the deprotonation at C-4′′ and the activation of C-2′′ of LG forms a carbanion at C-2′′ that can be attacked by the DMAPP cation, resulting in the formation of the lavandulyl group in SFG. The results on substrate specificity for several 8-dimethylallylated flavanones indicated that the 2′-hydroxy function of substrates is indispensable to this chain elongation reaction (Table III). This is consistent with the fact that the 2′-hydroxy function commonly exists in most of lavandulylated flavonoid metabolites in leguminous and moraceous plants (Barron and Ibrahim, 1996).
Figure 5.
Possible prenylation mechanisms of GPP synthase (A), N8DT (B), and LGDT (C).
It is noteworthy that LGDT, which is definitively a type of chain elongation prenyltransferase, is tightly bound to the membrane, similar to most prenyltransferases using aromatics as prenyl acceptor (Dhillon and Brown, 1976; Schröder et al., 1979; Biggs et al., 1987; Welle and Grisebach, 1991; Laflamme et al., 1993; Yamamoto et al., 1997, 2000; Mühlenweg et al., 1998), with an exception being olivetolate geranyltransferase (Fellermeier and Zenk, 1998), whereas previously reported prenyl diphosphate elongation enzymes are soluble proteins (Ogura and Koyama, 1998; Liang et al., 2002). Molecular biological investigation of LGDT having two unique features—a catalyzing prenyl side-chain elongation reaction and a membrane-bound chain elongation enzyme—would contribute to knowledge of the structural and functional properties of this novel prenyltransferase and provide some clues to the synthesis of novel bioactive branched prenyl side-chain-conjugated compounds.
LGDT Is Localized in the Plastids
The plastids have long been accepted as a major subcellular site of isoprenoid metabolism, a fact underscored by the recent discovery that these organelles possess a DXP pathway for the production of the universal isoprenoid precursor IPP (Eisenreich et al., 1998). In the case of prenylated aromatics, it has been reported that the dimethylallyl group of several aromatic compounds (Goese et al., 1999; Stanjek et al., 1999; Asada et al., 2000) and the geranyl group of cannabinoids (Fellermeier et al., 2001) are derived from a DXP pathway and that the prenylations of isoflavonoids in French bean (Phaseolus vulgaris) and soybean (Glycine max; Biggs et al., 1990) and furanocoumarins in Ruta graveolens (Dhillon and Brown, 1976) occurred in the plastids. In contrast, in Lithospermum erythrorhizon cultured cells, GPP supplied from the mevalonate pathway (Li et al., 1998) in the ER/cytosol (Sommer et al., 1995) was utilized for the prenylation of p-hydroxybenzoic acid, which was associated with the ER (Yamaga et al., 1993).
The present subcellular localization studies clearly demonstrated that both activities of LGDT and N8PT coincided with that of UDP-Gal:diacylglycerol galactosyltransferase, the marker enzyme for the plastids (Douce et al., 1984), indicating that both prenyltransferases were localized in these organelles, whereas 2′OH, a characterized P450 monooxygenase between N8DT and LGDT in the biosynthetic pathway of SFG, is localized to the ER (Fig. 4). Because these two prenyltransferases only utilized DMAPP originated from DXP pathway (Yamamoto et al., 2002), they should be faced to inside of the plastids and not be located in the cytosolic side where the DMAPP supplied from mevalonate pathway could be used. These data indicate that a close cooperation between the plastids and the ER, which were sites of two dimethylallylations and 2′-hydroxylations, respectively, is absolute necessary for the formation of a lavandulyl group in SFG biosynthesis and may be important in regulating the production of SFG. Moreover, this result also implies that flavanone intermediates move between the membranes of these two organelles.
Figure 1 shows a model summarizing our current understanding on the formation of the lavandulyl group in SFG biosynthesis. In that, we postulate some intermediate/product transport mechanisms for the efficient production and accumulation of SFG, although simple diffusion may be also explainable. Naringenin synthesized in the cytosol/ER is transferred to the plastids for the first dimethylallylation, subsequently returned to the ER for the 2′-hydroxylation, and then transferred to the plastids again for the formation of a lavandulyl group by the second dimethylallylation. Finally, SFG as the final product accumulated in the cell wall of cultured cells (Yamamoto et al., 1996) or cork tissue of root system in intact plants (Yamamoto et al., 1992). The abovedescribed connection between the mechanism of transport and targeting specificity is of great interest because a similar movement of intermediates/products between the plastids and the ER was also presumed in the biosynthesis of phytoalexin glyceollin and phaseollin in soybean and bean, respectively (Biggs et al., 1990).
MATERIALS AND METHODS
Chemicals
DMAPP was synthesized according to the method of Cornforth and Popjak (1969). IPP and GPP were a kind gift from Prof. Kazufumi Yazaki (Wood Research Institute, Kyoto University). LG, 8-dimethylallylnaringenin, euchrenone a7, kenusanone I, and isovabachin were isolated from Sophora flavescens Ait. cells cocultured with cork tissues (Zhao et al., 2003). UDP-[14C]Gal and antimycin A were purchased from Sigma-Aldrich (Tokyo), and cytochrome c was purchased from Nacalai Tesque (Kyoto). All reagents and solvents used were of analytical grade.
Cell Cultures
The origin and subculturing of callus cultures and the establishment of cell-suspension cultures of S. flavescens were performed as described by Yamamoto et al. (1991, 1996, respectively).
zEnzyme Preparation
S. flavescens cells were harvested by suction filtration after 6 to 8 d of cultivation in Murashige and Skoog medium (Murashige and Skoog, 1962) containing 1 μm 2,4-D and 1 μm kinetin. All further steps were performed at 4°C. The cells (5 g fresh weight) were ground in a mortar and pestle with 5 mL of 100 mm K-phosphate buffer (pH 6.5) containing 10 mm dithiothreitol (DTT), 0.5 g of polyvinylpolypyrrolidone, and sea sand. The homogenate was centrifuged at 12,000g for 20 min to remove cell debris, and the resulting supernatant was centrifuged at 156,000g for 40 min. The microsomal pellet was resuspended twice in 100 mm borate buffer (pH 8.8) containing 10 mm DTT, recentrifuged (156,000g for 40 min) and finally resuspended in 0.5 mL of the same buffer and used for enzyme assays.
Crude cell-free extracts were obtained by passing the 12,000g supernatant through a Sephadex G-25 column (PD-10, Amersham Biosciences, Tokyo) equilibrated with 100 mm borate buffer (pH 8.8) containing 10 mm DTT. The soluble fraction was also prepared by PD-10 using the 156,000g supernatant.
Assay of LGDT by HPLC
The standard assay mixture contained in a total volume of 200 μL of 100 mm borate buffer (pH 8.8), 60 nmol LG (in 10 μL of ethanol), 200 nmol DMAPP, 2 μmol MgCl2, and 50 μL of the microsomal fraction (approximately 60 μg of microsomal protein). In a control experiment, DMAPP was not added to the assay mixture. The reaction was initiated by the addition of LG to the mixture, and after the incubation for 30 min at 30°C, it was terminated by the addition of 50 μL of 6 n HCl. The reaction mixture was extracted with 200 μL of ethyl acetate containing 50 nmol 1-naphthaleneacetic acid as an internal standard. The amount of SFG in the ethyl acetate extract was analyzed by HPLC using a CAPCELL PAK C18 column (5 μm, 4.6 × 250 mm, Shiseido, Tokyo) in an oven at 40°C, with a methanol/water linear gradient solvent system containing 1% (v/v) acetic acid, from 54% to 90% (v/v) methanol in 20 min, at a flow rate of 0.9 mL min–1, by monitoring the absorption at 294 nm. The quantities were calculated from the peak area at 294 nm recorded by a Chromatopac C-R4A (Shimadzu, Kyoto).
Identification of the Reaction Product
The microsomal fraction obtained from 20 g of fresh cells was incubated with 2.4 μmol LG, 8 μmol DMAPP, and 80 μmol MgCl2 in 100 mm borate buffer (pH 8.8, total volume of 8 mL) at 30°C for 2 h. The reaction was terminated by the addition of 2 mL of 6 n HCl, and the products were extracted with ethyl acetate (8 mL × 3). The organic layers were combined and concentrated in vacuo. The residue was dissolved in methanol and purified by preparative HPLC under the following conditions: column, same as above; solvent, methanol/water linear gradient solvent system containing 1% (v/v) acetic acid, from 54% to 74% (v/v) methanol in 40 min; flow rate, 0.9 mL min–1; oven temperature, 40°C; and detection at 294 nm. The fraction around a retention time of 33 min was collected, evaporated in vacuo, and analyzed by an HPLC photodiode array system (MD-910, JASCO International, Hachioji, Japan) and EIMS (JMS DX-303, JEOL, Akishima, Japan).
SFG: UV λmax nm (log ε, in methanol): 294 (4.5), 340sh (3.9); EIMS, m/z: 424 (Mr), 406, 301, 283, 209, 165, 136.
Suc Density Gradient Centrifugation
For the separation of microsomal membranes, 10 mm DTT, 10 mm KCl, 1 mm MgCl2, and 1 mm EDTA were added to the homogenization, resuspension, and centrifugation buffers and to the Suc gradient solution. The microsomal fractions prepared from 50 g of fresh cells were washed twice using 10 mm Tris-HCl buffer (pH 7.5, 8.6 g/100 mL Suc), resuspended in 1 mL of 10 mm Tris-HCl buffer (pH 7.5, 8.6 g/100 mL Suc), placed on the top of a Suc gradient solution (9 mL; 1.5 mL of 15%, 20%, 25%, 32%, 43%, and 50% [w/w] Suc in 10 mm Tris-HCl buffer [pH 7.5]) in a tube, and centrifuged for 90 min at 30,000 rpm in a swing-out rotor (SW40, Beckman, Japan). Equivalent fractions (0.75 mL) were removed from the top to the bottom of the gradient and analyzed for enzyme activity, Suc density, and protein content, respectively.
Assays of LGDT, N8DT, 2′ OH, and C4H by HPLC
LGDT activity was assayed as described above by using 50 μL of each gradient fraction, whereas the activities of N8DT and 2′OH were measured as described by Yamamoto et al. (2000, 2001). The C4H assay was initiated by adding NADPH at a final concentration of 1 mm to a reaction mixture (150 μL) containing 100 mm Tris-HCl buffer (pH 7.5), 1 mm trans-cinnamic acid, and 100 μL of each gradient fraction. After incubation for 1 h at 30°C, the reaction was stopped by adding 50 μL of 6 n HCl. The reaction mixture was extracted with 200 μL of ethyl acetate containing 20 μg of caffeic acid as an internal standard, and then the ethyl acetate extract was subjected to HPLC analysis.
The quantification of each enzyme assay was performed under the same HPLC conditions as in the LGDT assay except for the following modifications: N8PT, 1% (v/v) acetic acid containing acetonitrile/water linear gradient system from 40% to 70% (v/v) acetonitrile within 40 min; 2′OH, 1% (v/v) acetic acid containing acetonitrile/water gradient system from 20% to 70% (v/v) acetonitrile within 40 min; and C4H, 1% (v/v) acetic acid containing acetonitrile/water gradient system from 15% to 75% (v/v) acetonitrile within 40 min. Eluting substances were monitored spectrophotometrically at 294 nm (for N8PT and 2′OH assays) or 280 nm (for C4H assay).
Marker Enzyme Assays
To ensure that subcellular organelle membrane fractions were successfully separated by Suc density gradient centrifugation, the following marker enzymes were assayed: plastids, UDP-Gal:diacylglycerol galactosyltransferase (Douce and Joyard, 1980); ER, antimycin A-insensitive NADH cytochrome c reductase (Yoshida, 1979); mitochondria, NADPH cytochrome c oxidase (Yoshida, 1979); tonoplast, KNO3-sensitive ATPase (Uemura and Yoshida, 1986); and plasma membrane, Na3VO4-sensitive ATPase (Uemura and Yoshida, 1986).
Protein Quantification
Protein contents were quantified using the Bradford assay (Bradford, 1976) with bovine serum albumin as the standard.
Acknowledgments
We thank Prof. Kazufumi Yazaki (Wood Research Institute, Kyoto University) for the generous gift of IPP and GPP and for his critical reading of this manuscript. We are also thankful to Mr. Noriaki Yamaguchi (Nagasaki University, Japan) for mass spectroscopy measurements.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.025213.
References
- Asada Y, Li W, Yoshikawa T (2000) Biosynthesis of the dimethylallyl moiety of glabrol in Glycyrrhiza glabra hairy root cultures via a non-mevalonate pathway. Phytochemistry 55 323–326 [DOI] [PubMed] [Google Scholar]
- Barron D, Ibrahim RK (1996) Isoprenylated flavonoid: a survey. Phytochemistry 43 921–982 [Google Scholar]
- Bennett AB, O'Neill SD, Spanswick RM (1984) H+-ATPase activity from storage tissue of Beta vulgaris. Plant Physiol 74 538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs DR, Welle R, Grisebach H (1990) Intracellular localization of prenyltransferases of isoflavonoid phytoalexin biosynthesis in bean and soybean. Planta 181 244–248 [DOI] [PubMed] [Google Scholar]
- Biggs DR, Welle R, Visser FR, Grisebach H (1987) Dimethylallylpyrophosphate:3,9-dihydroxypterocarpan 10-dimethylallyl transferase from Phaseolus vulgaris: identification of the reaction product and properties of the enzyme. FEBS Lett 220 223–226 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Cornforth RH, Popjak G (1969) Chemical synthesis of substrates of sterol biosynthesis. Methods Enzymol 15 359–390 [Google Scholar]
- de Lampasona MEP, Catalan CAN, Gedris TE, Herz W (1997) Benzofurans, benzofuran dimmers and other constituents from Ophryosporus charua. Phytochemistry 46 1077–1080 [Google Scholar]
- Dhillon DS, Brown SA (1976) Localization, purification, and characterization of dimethylallylpyrophosphate:umbelliferone dimethylallyltransferase from Ruta graveolens. Arch Biochem Biophys 177 74–83 [DOI] [PubMed] [Google Scholar]
- Douce R, Block MA, Dorne AJ, Joyard J (1984) The plastid envelope membranes: their structure, composition, and role in chloroplast biogenesis. In DB Roodyn, ed, Subcellular Biochemistry, Vol 10. Plenum Press, New York, pp 1–84 [DOI] [PubMed] [Google Scholar]
- Douce R, Joyard J (1980) Chloroplast envelope lipids: detection and biosynthesis. Methods Enzymol 69 290–301 [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A (1998) The deoxyxylylose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol 5 R221–R233 [DOI] [PubMed] [Google Scholar]
- Epstein WW, Poulter CD (1973) A survey of some irregular monoterpenes and their biogenetic analogies to presqualene alcohol. Phytochemistry 12 737–747 [Google Scholar]
- Fellermeier M, Eisenreich W, Bacher A, Zenk MH (2001) Biosynthesis of cannabinoids. Incorporation experiments with 13C-labeled glucoses. Eur J Biochem 268 1596–1604 [DOI] [PubMed] [Google Scholar]
- Fellermeier M, Zenk MH (1998) Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett 427 283–285 [DOI] [PubMed] [Google Scholar]
- Goese M, Kammhuber K, Bacher A, Zenk MH, Eisenreich W (1999) Biosynthesis of bitter acid in hops. A 13C-NMR and 2H-NMR study on the building blocks of humulone. Eur J Biochem 263 447–454 [DOI] [PubMed] [Google Scholar]
- Gunawardena K, Rivera SB, Epstein WW (2002) The monoterpenes of Artemisia tridentata ssp. vaseyana, Artemisia cana ssp. viscidula and Artemisia tridentata ssp. spiciformis. Phytochemistry 59 197–203 [DOI] [PubMed] [Google Scholar]
- Hamerski D, Matern U (1988) Elicitor-induced biosynthesis of psoralens in Ammi majus L. suspension cultures: microsomal conversion of demethylsuberosin into (+)-marmesin and psoralen. Eur J Biochem 171 369–375 [DOI] [PubMed] [Google Scholar]
- Hatayama K, Komatsu M (1971) Studies on the constituents of Sophora species: V. Constituents of the root of Sophora angustifolia Sieb Et Zucc (2). Chem Pharmacol Bull 19 2126–2131 [Google Scholar]
- Kang TH, Jeong SJ, Ko WG, Kim NY, Lee BH, Inagaki M, Miyamoto T, Higuchi R, Kim YC (2000) Cytotoxic lavandulyl flavanones from Sophora flavescens. J Nat Prod 63 680–681 [DOI] [PubMed] [Google Scholar]
- Ko WG, Kang TH, Kim NY, Lee SJ, Kim YC, Ko GI, Ryu SY, Lee BH (2000) Lavandulylflavonoids: a new class of in vitro apoptogenic agents from Sophora flavescens. Toxicol In Vitro 14 429–433 [DOI] [PubMed] [Google Scholar]
- Kuroyanagi M, Arakawa T, Hirayama Y, Hayashi T (1999) Antibacterial and antiandrogen flavonoids from Sophora flavescens. J Nat Prod 62 1595–1599 [DOI] [PubMed] [Google Scholar]
- Laflamme P, Khouri H, Gulick P, Ibrahim R (1993) Enzymatic prenylation of isoflavones in white lupin. Phytochemistry 34 147–151 [Google Scholar]
- Lee HS, Ko HR, Ryu SY, Oh WK, Kim BY, Ahn SC, Mheen TI, Ahn JS (1997) Inhibition of phospholipase Cγ1 by the prenylated flavonoids from Sophora flavescens. Planta Medica 63 266–268 [DOI] [PubMed] [Google Scholar]
- Li SM, Hennig S, Heide L (1998) Shikonin: a geranyl diphosphate-derived plant hemiterpenoid formed via the mevalonate pathway. Tetrahedron Lett 39 2721–2724 [Google Scholar]
- Liang PH, Ko TP, Wang AH (2002) Structure, mechanism and function of prenyltransferases. Eur J Biochem 269 3339–3354 [DOI] [PubMed] [Google Scholar]
- Martinec J, Feltl T, Scanlon CH, Lumsden PJ, Machackova I (2000) Subcellular localization of a high affinity binding site for d-myo-inositol 1, 4, 5-trisphosphate from Chenopodium rubrum. Plant Physiol 124 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenweg A, Melzer M, Li SM, Heide L (1998) 4-Hydroxybenzoate 3-geranyltransferase from Lithospermum erythrorhizon: purification of a plant membrane-bound prenyltransferase. Planta 205 407–413 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Ogura K, Koyama T (1998) Enzymatic aspects of isoprenoid chain elongation. Chem Rev 98 1263–1276 [DOI] [PubMed] [Google Scholar]
- Poulter CD (1990) Biosynthesis of non-head-to-tail terpenes. Formation of 1′-1 and 1′-3 linkages. Acc Chem Res 23 70–7716518460 [Google Scholar]
- Rivera SB, Swedlund BD, King GJ, Bell RN, Hussey Jr CE, Shattuck-Eidens DM, Wrobel WM, Peiser GD, Poulter CD (2001) Chrysanthemyl diphosphate synthase: Isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc Natl Acad Sci USA 98 4373–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro DK, Mah N, Ellis BE, Douglas CJ (2001) Functional characterization and subcellular localization of poplar (Populus trichocarpa × Populus deltoides) cinnamate 4-hydroxylase. Plant Physiol 126 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Hinz G, Oberbeck K (1994) Isolation of endo- and plasma membranes. In N Harris, KJ Oparka, eds, Plant Cell Biology. Oxford University Press, Oxford, pp 245–272
- Schröder G, Zähringer U, Heller W, Ebel J, Grisebach H (1979) Biosynthesis of antifungal isoflavonoids in Lupinus albus: enzymatic prenylation of genistein and 2′-hydroxygenistein. Arch Biochem Biophys 194 635–636 [DOI] [PubMed] [Google Scholar]
- Sommer S, Severin K, Camara B, Heide L (1995) Intracellular localization of geranylpyrophosphate synthase from cell cultures of Lithospermum erythrorhizon. Phytochemistry 38 623–627 [Google Scholar]
- Stanjek V, Piel J, Boland W (1999) Biosynthesis of furanocoumarins: mevalonate-independent prenylation of umbelliferone in Apium graveolens (Apiaceae). Phytochemistry 50 1141–1145 [Google Scholar]
- Tamanoi F, Kato-Stankiewicz J, Jiang C, Machado I, Thapar N (2001) Farnesylated proteins and cell cycle progression. J Cell Biochem Suppl 37 64–70 [DOI] [PubMed] [Google Scholar]
- Tholl D, Croteau R, Gershenzon J (2001) Partial purification and characterization of the short-chain prenyltransferases, geranyl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (Grand Fri). Arch Biochem Biophys 386 233–242 [DOI] [PubMed] [Google Scholar]
- Tsuro M, Inoue M, Kameoka H (2001) Variation in essential oil components in regenerated lavender (Lavandula vera DC) plants. Sci Hortic 88 309–317 [Google Scholar]
- Umemura M, Yoshida S (1986) Studies on freezing injury in plant cells. Plant Physiol 80 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle R, Grisebach H (1991) Properties and solubilization of the prenyltransferase of isoflavonoid phytoalexin biosynthesis in soybean. Phytochemistry 30 479–484 [Google Scholar]
- Wu LJ, Miyase T, Ueno A, Kuroyanagi M, Noro T, Fukushima S, Sasaki S (1986) Studies on the constituents of Sophora flavescens Ait. V. Yakugaku Zasshi 106 22–26 [Google Scholar]
- Yamaga Y, Nakanishi K, Fukui H, Tabata M (1993) Intracellular localization of p-hydroxybenoate geranyltransferase, a key enzyme involved in shikonin biosynthesis. Phytochemistry 32 633–636 [Google Scholar]
- Yamamoto H, Ichimura M, Ishikawa N, Tanaka T, Iinuma M, Mizuno M (1992) Localization of prenylated flavonoids in Sophora flavescens var. angustifolia plants. Z Naturforsch 47c 535–539 [Google Scholar]
- Yamamoto H, Kawai S, Mayumi J, Tanaka T, Iinuma M, Mizuno M (1991) Prenylated flavanone production in callus cultures of Sophora flavescens var. angustifolia. Z Naturforsch 46c 172–176 [Google Scholar]
- Yamamoto H, Kimata J, Senda M, Inoue K (1997) Dimethylallyl diphosphate:kaempferol 8-dimethylallyltransferase in Epimedium diphyllum cell suspension cultures. Phytochemistry 44 23–28 [Google Scholar]
- Yamamoto H, Senda M, Inoue K (2000) Flavanone 8-dimethylallyltransferase in Sophora flavescens cell suspension cultures. Phytochemistry 54 649–655 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamaguchi M, Inoue K (1996) Absorption and increase in the production of prenylated flavanones in Sophora flavescens cell suspension cultures by cork pieces. Phytochemistry 43 603–608 [Google Scholar]
- Yamamoto H, Yatou A, Inoue K (2001) 8-Dimethylallylnaringenin 2′-hydroxylase, the crucial cytochrome P450 mono-oxygenase for lavandulylated flavanone formation in Sophora flavescens cultured cells. Phytochemistry 58 671–676 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Zhao P, Inoue K (2002) Origin of two isoprenoid units in a lavandulyl moiety of sophoraflavanone G from Sophora flavescens cultured cells. Phytochemistry 60 263–267 [DOI] [PubMed] [Google Scholar]
- Yoshida S (1979) Freezing injury and phospholipid degradation in vivo in woody plant cells. Plant Physiol 64 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Inoue K, Yamamoto H (2003) Efficient production and capture of 8-prenylnaringenin and leachianone G-biosynthetic intermediates of sophoraflavanone G-by the addition of cork tissue to cell suspension cultures of Sophora flavescens. Phytochemistry 62 1093–1099 [DOI] [PubMed] [Google Scholar]