Abstract
Guard cells must maintain the integrity of the plasma membrane as they undergo large, rapid changes in volume. It has been assumed that changes in volume are accompanied by changes in surface area, but mechanisms for regulating plasma membrane surface area have not been identified in intact guard cells, and the extent to which surface area of the guard cells changes with volume has never been determined. The alternative hypothesis—that surface area remains approximately constant because of changes in shape—has not been investigated. To address these questions, we determined surface area for intact guard cells of Vicia faba as they underwent changes in volume in response to changes in the external osmotic potential. We also estimated membrane internalization for these cells. Epidermal peels were subjected to external solutions of varying osmotic potential to shrink and swell the guard cells. A membrane-specific fluorescent dye was used to identify the plasma membrane, and confocal microscopy was used to acquire a series of optical paradermal sections of the guard cell pair at each osmotic potential. Solid digital objects representing the guard cells were created from the membrane outlines identified in these paradermal sections, and surface area, volume, and various linear dimensions were determined for these solid objects. Surface area decreased by as much as 40% when external osmotic potential was increased from 0 to 1.5 MPa, and surface area varied linearly with volume. Membrane internalization was approximated by determining the amount of the fluorescence in the cell's interior. This value was shown to increase approximately linearly with decreases in the cell's surface area. The changes in surface area, volume, and membrane internalization were reversible when the guard cells were returned to a buffer solution with an osmotic potential of approximately zero. The data show that intact guard cells undergo changes in surface area that are too large to be accommodated by plasma membrane stretching and shrinkage and suggest that membrane is reversibly internalized to maintain cell integrity.
Guard cells regulate stomatal pore size to allow CO2 uptake while controlling water loss. To accomplish this, they respond to environmental factors such as light and CO2 concentration and to chemical signals such as ABA, which may originate in other parts of the plant. Guard cells respond to these factors by regulating ion fluxes across the plasma membrane, and the resulting movement of water causes changes in cell volume, turgor pressure, and shape, leading to changes in the pore aperture. The changes in guard cell turgor pressure and volume caused by these processes can be quite large. In Vicia faba, guard cell turgor pressure has been shown to vary between 1.0 and 4.0 MPa (Franks et al., 1998, 2001) as stomata go from closed to wide open, and volume has been shown to change by as much as 40% (Raschke and Dickerson, 1973; Franks et al., 2001). These changes can occur over a time period of minutes.
An often-unrecognized problem for guard cells is that they must maintain the integrity of the plasma membrane during these large and relatively rapid changes in volume. It is unlikely that simple stretching could be responsible for maintaining membrane integrity because plasma membranes have been shown to have no more than about 5% elasticity (Wolfe and Steponkus, 1983). Thus, guard cells must either: (a) change shape such that plasma membrane surface area remains nearly constant while volume changes, or (b) move membrane between the plasma membrane and internal sources within the cell to accommodate changes in surface area. Although the literature on guard cells assumes the latter solution (Diekmann et al., 1993; Homann, 1998; Blatt, 2000), examples of both solutions can be found in animal cells. For example, mammalian erythrocytes change shape to maintain a constant surface area as volume is changed osmotically, but neurons continuously shuttle membrane between the endomembrane system and the plasma membrane to vary both surface area and volume (Morris and Homann, 2001). Furthermore, it has been shown that the cross-sectional shape of guard cells is nearly circular at high turgor pressures, but becomes more flattened as they lose turgor (Raschke, 1979). This lends credibility to the first solution because flattening could produce changes in volume while maintaining a constant surface area.
The problem of how guard cells maintain the integrity of the plasma membrane during volume changes has been addressed using osmotically induced changes in the volume of guard cell protoplasts (Homann, 1998; Kubitscheck et al., 2000). Those studies showed that vesicles were internalized and retrieved to the plasma membrane as protoplast volume changed. This result suggests that the surface area of the plasma membrane, rather than cell shape, is dynamically adjusted to maintain membrane integrity as the guard cells change volume. However, protoplasts have no turgor pressure, which makes comparison with intact cells problematic, and the existence of vesicle formation in intact, turgid guard cells is not firmly established. There is indirect evidence that guard cells possess the cellular machinery for membrane trafficking (Blatt, 2000), but there is debate about whether endocytosis can occur against turgor pressure (Battey et al., 1999). The prevailing opinion seems to be that endocytosis can occur but that vesicle formation should require energy and that the amount of energy necessary would increase with turgor pressure (Gradmann and Robinson, 1989). Furthermore, the energy requirement for vesicle formation should be less for smaller vesicles (Saxton and Breidenbach, 1988) or for flattened vesicles (Fricke et al., 2000). Despite these theoretical considerations and the data showing reversible membrane internalization in protoplasts, no evidence for endocytosis was observed in intact, turgid guard cells when lucifer yellow was used as an indicator of endocytotic vesicle formation and stomata were closed using osmotically induced changes in guard cell volume (Diekmann et al., 1993).
In summary, the mechanisms by which guard cells might dynamically alter plasma membrane surface area in response to rapid changes in volume are not clear, and the alternative hypothesis—that the guard cells change shape to conserve surface area as volume changes—has never been directly tested. There are no measurements of guard cell surface area in the literature of which we are aware. Therefore, the goals of this study were to quantify surface area and to look for membrane internalization in intact, turgid guard cells during osmotically induced volume changes. This was accomplished by labeling guard cells with a membrane-specific fluorescent dye and imaging them with confocal microscopy. The dye allowed visualization of the plasma membrane, and three-dimensional digital models of guard cells, from which surface area and volume could be precisely determined, were constructed from stacks of paradermal confocal images. The dye also allowed internalization of plasma membrane to be detected and quantified on a relative basis.
RESULTS
Stomata opened and remained open for at least 5 h when epidermal peels were floated on buffer and illuminated. When fluorescent dye [N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl) pyridinium dibromide (FM 4-64)] was added to the buffer, it accumulated in the plasma membrane of guard cells but was excluded from the interior of the cell (Fig. 1A). As long as the peels were kept in buffer, exclusion from the interior of the cell was essentially complete, even for control experiments in which peels were maintained in buffer for over 3 h. However, when the osmotic potential of the buffer was increased with mannitol, stomatal aperture decreased, and FM 4-64 moved inside the cell within minutes (Fig. 1B). In some experiments, small points of bright fluorescence were observed inside the cell after the addition of mannitol. These points were usually observed near the cuticular side of the cell and may have been vesicles. However, they were not observed in every experiment, and it was not possible to conclude unequivocally that they originated at the plasma membrane and migrated inwards. Several minutes after the increase in external mannitol concentration, fluorescence became more generalized in the cell's interior and often seemed to localize around the chloroplasts.
Figure 1.
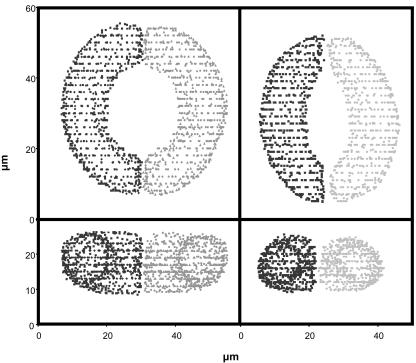
Paradermal (upper) and transverse (lower) confocal images of guard cells before (left) and after (right) the addition of 1.5 MPa mannitol in the external buffer. Scale bar = 20 μm.
To determine if the internalization of FM 4-64 was correlated with loss of membrane surface area, a confocal microscope was used to image guard cells in epidermal peels exposed to a series of increasing concentrations of mannitol. Guard cells were allowed to equilibrate for 10 min at each concentration of mannitol before they were imaged with the confocal microscope (preliminary experiments showed that equilibration was complete at 10 min). To determine cell surface area, stacks of paradermal confocal images were used to create three-dimensional images of the guard cells that could be viewed in either paradermal (x-y) or transverse (x-z) orientations. Digital representations of guard cells at each mannitol concentration were constructed from these three-dimensional images by placing points on the plasma membrane using both paradermal and transverse views. These three-dimensional point clouds were then used to create solid digital objects from which both surface area and volume could be determined (Figs. 2 and 3; as described in “Materials and Methods”).
Figure 2.
Point cloud reconstructions of the cells shown in Figure 1. Image editing software was used to reconstruct the guard cells by placing data points on the membrane creating a “cloud” of triplet data points. Top two panels, paradermal view (x-y plane); bottom two panels, transverse view (x-z plane).
Figure 3.
Solid digital objects of the guard cells shown in Figure 1. The objects were created from the point clouds shown in Figure 2 by the approach described in “Materials and Methods.”
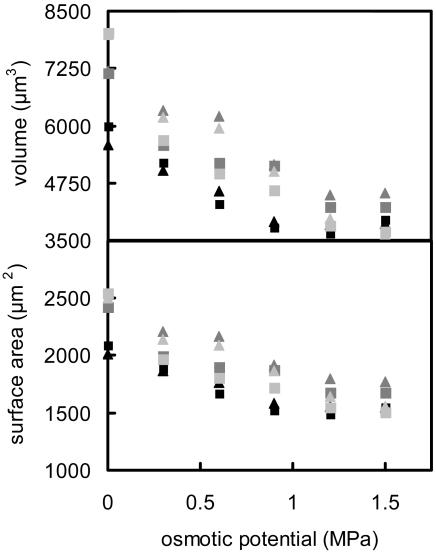
The guard cells chosen for study varied in size such that their maximum volume (i.e. in buffer with no mannitol; osmotic potential = 0 MPa) varied between 5,000 and 8,000 μm3, and their maximum surface area varied between 3,500 and 4,500 μm2 (Fig. 4). Both surface area and volume decreased by approximately 40% as external osmotic pressure was increased with mannitol (Fig. 4).
Figure 4.
Guard cell surface area and volume as affected by increasing the osmotic pressure of the external solution. Data are for six guard cells; each symbol represents a different cell. Approximately 10 min were allowed for equilibration after each change in the osmotic potential of the buffer solution.
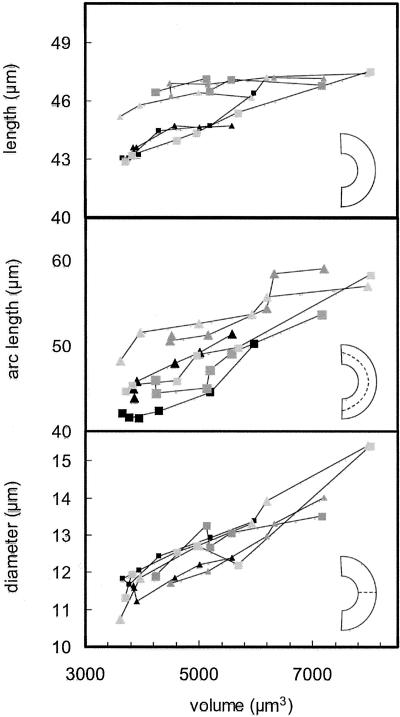
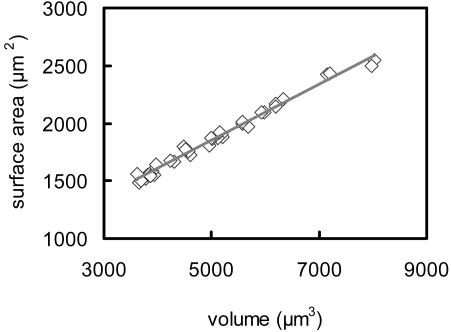
The changes in surface area and volume shown in Figure 4 were related to changes in the linear dimensions of the guard cells by determining three representative dimensions for each guard cell at each volume: (a) the maximum diameter of the cell in the transverse direction, (b) the overall length of the guard cell, and (c) the length of the arc bisecting the guard cell in its long axis (for diagrams identifying these dimensions, see Fig. 5). Figure 5 shows that although overall length of the cells varied by only about 10%, both the diameter and arc length of the cells increased by approximately 40% as volume increased. The relationship between surface area and volume was identical for all six guard cells that were observed (Fig. 6), despite large differences in turgor pressure and cell size.
Figure 5.
Linear dimensions of guard cells as a function of volume. Top, Overall length of the cell; middle, length of an arc bisecting the cell in long direction; bottom, maximum diameter of the cell (always near the middle of the cell). Measurements were made from digital objects as described in “Materials and Methods.”
Figure 6.
Guard cell surface area plotted as a function of volume for six guard cells at six different osmotic potentials. The line shows a linear regression of the data; r2 = 0.988.
To explore the possibility that internalization of plasma membrane was responsible for the decreases in surface area shown in Figure 4, we examined changes in fluorescence intensity inside of the cells as external osmotic potential was varied. As noted above, guard cells in buffer only (with no mannitol) showed essentially no internalization of FM 4-64 even after 3 h. However, when the osmotic pressure of the buffer solution was increased, the fluorescence inside the cell began to increase within minutes. An estimate of the amount of FM 4-64 inside the guard cells was obtained by quantifying average pixel intensity inside the guard cells. This average pixel intensity showed a positive and approximately linear correlation with loss of plasma membrane surface area (Fig. 7).
Figure 7.
Internalization of plasma membrane quantified by increases in fluorescence within the guard cell plotted as a function of the decrease in surface area. Surface area decrease and membrane internalization were caused by decreases in volume associated with increasing the osmotic potential of the buffer solution around the cells. Fluorescence within the guard cell was estimated by calculating the average pixel intensity for the entire cytosol in the medial section of the paradermal image stack.
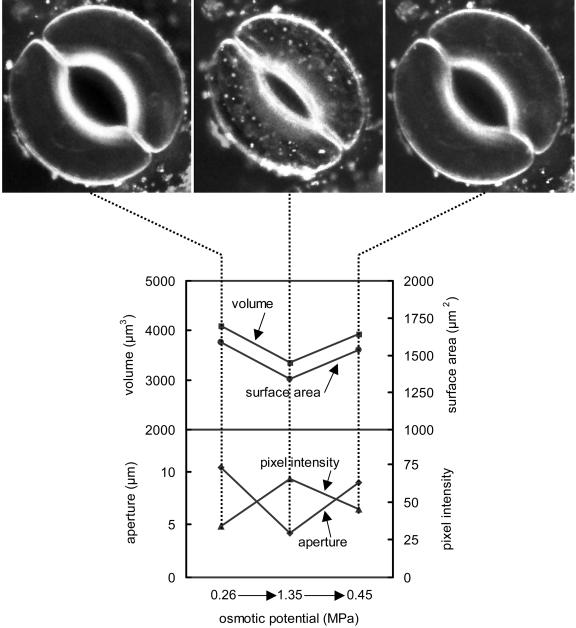
The effects of external osmotic potential on surface area, volume, and membrane internalization were found to be completely reversible. When the mannitol in the external buffer was diluted to approximately the original concentration, aperture, volume, and surface area, all increased to approximately their starting values, and the amount of FM 4-64 inside the cell decreased to a very low level (Fig. 8). All of these changes were complete in approximately 10 min.
Figure 8.
Effects of increasing and then decreasing the osmotic potential of the external buffer on surface area, volume, and membrane internalization. Approximately 10 min were allowed for equilibration after each change in the osmotic potential of the buffer solution. Volume and surface area are shown on the upper plot; aperture and fluorescence intensity inside the cell are shown on the lower plot; the pictures correspond to the time points indicated by the dotted lines.
DISCUSSION
Guard cells were represented as solid digital objects to test directly two hypothetical mechanisms by which plasma membrane integrity is maintained when volume changes: (a) that changes in cell shape conserve surface area, versus (b) that reversible membrane internalization allows surface area to change. Our data refute the first hypothesis and support the second hypothesis: Surface area varied linearly with volume (Fig. 6), and membrane-specific dye was internalized (Figs. 7 and 8) as volume was manipulated via external osmotic pressure.
The guard cells used in this study varied considerably in size, but the measured volumes are consistent with those reported in previous studies of V. faba guard cells (Raschke and Dickerson, 1973; Willmer and Fricker, 1996; Franks et al., 2001). Given this variation in size, it is remarkable that all the guard cells used in this study fit a single, approximately linear relationship between surface area and volume (Fig. 6). Because volume usually changes as the cube of the linear dimensions and surface area as the square, the relationships underlying this observation are not immediately obvious.
To explore the relationship between surface area and volume, we considered two general shapes as approximations of a guard cell: a cylinder and a prolate spheroid (a three-dimensional ellipse that is elongate like an egg but bilaterally symmetrical). The formulas for surface area and volume for these two shapes are given in Table I. For both of these shapes, increases in length with no increase in radius produce a linear relationship between surface area and volume. On the other hand, increases in the radius without increases in length cause volume to increase as the square of surface area. Therefore, the apparent linearity of the data in Figure 5 would suggest that most of the increase in volume is caused by the increase in guard cell length (arc) as has been suggested previously (Raschke, 1979). However, this conclusion is not supported by the data in Figure 6, which show that both radius and length increase by approximately equal proportions. Numerical simulations (data not shown) of surface area and volume for both a cylinder and a prolate spheroid indicate that for the ranges of guard cell dimensions in this study, the relationship between surface area and volume is very close to linear (r2 for a linear regression is greater than 0.999) when both radius and length vary equally. This occurs because changes in length produce a linear relationship, and the increases in radius are not enough to produce a marked nonlinearity. Thus, although the relationship between surface area and volume is probably not strictly linear for guard cells, it can be approximated by a linear relationship with a high degree of accuracy. Furthermore, the similarity in overall shape and size of the guard cells in this study makes them appear to fit a single linear relationship.
Table I.
Formulas for calculating surface area and volume of two approximations of a guard cell
| Shape | Volume | Surface Area |
|---|---|---|
| Prolate spheroid | 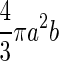 |
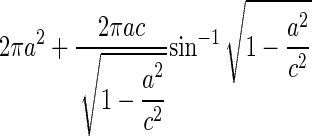 |
| Cylinder | 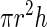 |
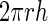 |
The data in this study support the hypothesis that membrane internalization occurs in intact, turgid guard cells in response to osmotically induced decreases in guard cell volume. FM 4-64 and related dyes have been well characterized as markers for membrane internalization by endocytosis (Vida and Emr, 1995), and our data clearly show that that FM 4-64 was excluded from the cell except when volume was reduced osmotically. Based on estimates and measurements of guard cell turgor pressure in V. faba (Franks et al., 1998, 2001), even the largest osmotic potentials of the external solutions used in this study should not have been sufficient to reduce guard cell turgor pressure to zero, and comparison of our data with pressure probe measurements of the relationship between stomatal aperture and guard cell turgor in V. faba (Franks et al., 1995, 2001) suggests that the maximum turgor pressure of our cells was between 3.0 and 4.0 MPa. Because the largest osmotic potential used in this study was 1.5 MPa, and membrane internalization and loss of surface area were evident for increases in osmotic potential as small as 0.3 MPa, it seems almost certain that membrane internalization occurred in the presence of substantial turgor pressure in these experiments. In addition, there was no evidence of plasmolysis in the experiments, whereas in other experiments with larger osmotic potentials, plasmolysis was clearly visible (data not shown).
The data in Figure 7 are consistent with the idea that membrane internalization was responsible for the decreases in surface area that occurred as the guard cells lost volume. Although it is possible that the fluorescence intensity inside the cell was not linearly related to the amount of membrane that had been internalized, it seems likely that these two parameters are at least monotonically related. Therefore, it is significant that the fluorescence intensity inside the cell rose approximately linearly as surface area decreased. Even more significant is that the fluorescence intensity inside the cell returned to its original very low level when the osmoticum was removed from the guard cells, and they rapidly swelled back to near their original volume and surface area in less than 10 min. These results show that plasma membrane integrity was not lost during the experiment and that the cells were capable of reincorporating at least some of the internalized membrane into the plasma membrane as volume and surface area increased. The data do not reveal the exact proportion of internalized membrane that was returned to the plasma membrane, nor do they imply that all internalized components were returned. Nevertheless, it is notable that a substantial fraction of the internalized membrane-specific dye was apparently returned to the plasma membrane, which would suggest that the internalized membrane was not rapidly diluted into a large internal membrane pool. It should be noted that the data shown are for one shrink-swell cycle and that the cells were kept at the smaller volume for less than 20 min. Multiple shrinkswell cycles or longer periods of time might result in a greater turnover of membrane material or a greater mixing of the internalized membrane with existing internal membrane.
Previous work with guard cell protoplasts using a membrane-specific fluorescent dye similar to the one used in this study showed internalization of plasma membrane and vesicle formation in response to osmotic solutions (Kubitscheck et al., 2000). The lack of turgor pressure in protoplasts makes it difficult to extrapolate those findings from protoplasts to intact guard cells, and there are several notable differences between our results and those with protoplasts. In the study on protoplasts, some membrane internalization was observed to occur at constant osmotic potential, and this was taken as evidence of a continuous low rate of plasma membrane turnover. However, in our study, membrane internalization was not visible unless the osmotic potential of the external solution was raised, which would suggest that there is not a continuous low rate of membrane turnover in intact guard cells. Also, in the protoplast study, vesicles were clearly observed, whereas in our study vesicles may have been present but could not be unequivocally identified. An earlier study using intact guard cells (Diekmann et al., 1993) found no evidence of vesicle formation during osmotically induced decreases in volume. In that study, the fluorescent dye, lucifer yellow, which is water soluble and generally assumed not to cross the plasma membrane, was used as an indicator for vesicle formation, so membrane internalization that was not accompanied by vesicle formation would not have been visible.
It is notable that the changes in surface area and volume in this study were very rapid (less than 10 min), even for guard cells, and that the changes in volume were entirely passive on the part of the guard cells; i.e. the changes in volume were not caused by metabolic changes in the guard cells. This places some limits on the types of mechanisms that could be proposed for maintaining the integrity of the plasma membrane. Mechanisms for regulation of surface area based on membrane tension have been proposed for animal and plant cells (Morris and Homann, 2001). Such a mechanism could account for the changes in surface area observed in this study, but more work is necessary to clarify the processes involved in membrane internalization and retrieval in guard cells.
MATERIALS AND METHODS
Vicia faba was grown in a greenhouse in 1-L pots containing a mixture of perlite:vermiculite:peat moss (1:1:1 [v/v]). Plants were watered to excess twice daily with a modified Peters 20-10-20 fertilizer (Grace Sierra Horticultural Products, Milpitas, CA; 9.1 mm nitrogen, 1.8 mm phosphorus, 2.7 mm potassium, and 11 μm chelated iron). Day length was extended to 16 h when necessary using pressure sodium lights that provided a photon flux density of approximately 500 μmol m–2 s–1.
Epidermal strips were isolated from the abaxial surface of fully expanded V. faba leaves. Strips were floated on a buffer solution consisting of 10 mm MES-KOH (pH 6.15), 50 mm KCl, 500 μm CaCl, and then placed under light (photon flux density = 750 μmol m–2 s–1) for 120 min to allow stomata to open. A membrane-selective fluorophore, FM 4-64 (Molecular Probes, Eugene, OR), which was kept as a 5 mm stock solution in DMSO, was then added to the buffer solution to a final a concentration of 500 nm, and the epidermal strips were placed back under the lights for an additional 60 min.
After the 60-min period in the presence of FM 4-64, the epidermal strips were transferred to petri dishes containing buffer in the absence of FM 4-64 until use. Epidermal strips were anchored to a coverslip with the mesophyll (inner) surface of the strip facing away from the coverslip. The coverslip was then sealed with silicone grease to a small glass plate that had an 8-mm hole drilled through it, thus providing a small sealed chamber where the epidermal strip was immersed in buffer solution. Epidermal strips were allowed to equilibrate on the stage of the confocal microscope for 10 min before imaging. To increase the osmotic potential of the buffer solution around the epidermal strips, the buffer solution in the chamber was briefly drawn into a pipette tip containing a known volume of 1.5 m mannitol solution in buffer. The resulting mixture was then returned to the chamber, and 10 min were allowed for the guard cells to equilibrate. The procedure was repeated to produce further increases in the concentration of mannitol. For experiments demonstrating the ability of the guard cells to reopen, the osmotic potential was increased by the addition of mannitol as described above, and the cell was imaged after the 10-min equilibrium period. The osmotic potential was then decreased by quickly withdrawing the mannitol solution and replacing it with buffer, and 10 min were allowed for equilibrium before the cell was imaged again.
A Bio-Rad MRC 1024 confocal microscope (Bio-Rad Laboratories, Hercules, CA) in the Keller position attached to a Nikon TE-200 (Nikon, Melville, NY) was used to collect images of the guard cells. Images were acquired using a 60× PLAN APO (1.4 numerical aperture) oil immersion objective (Nikon, Melville, NY). The FM 4-64 was excited using the 488-nm wavelength produced by a krypton/argon laser, and emitted light was collected through a 598/40 bandpass emission filter. Each guard cell pair was imaged as a stack of paradermal images at 2-μm spacing using Bio-Rad LaserSharp collection software and saved as PIC files. Each individual layer in the stack was a 512 × 512-pixel, 8-bit, grayscale image with a pixel size of 0.123 μm, which was saved as a PIC file. The three-dimensional stacks of images were manipulated with AutoVisualize 3-D rendering software (AutoQuant, Watervilet, NY). Images were first reoriented so that the major axis of the stoma was on the y axis. The pixel dimensions in the z direction were then corrected for distortion arising from the fact that materials with differing refractive indices were in the path of the emitted light (a correction factor of 0.7 was applied; see White et al., 1996; Franks et al., 2001). The individual processed images were then saved as a series of x-y (paradermal) images. A second reorientation was then applied by rotating the images 90° on the x axis, and the resulting images were saved as a series of x-z (transverse) images (Fig. 1).
To create digital objects of the guard cells, point clouds representing the plasma membrane were created from the three-dimensional image stacks. The stacks were analyzed as a series of individual paradermal images, and points were placed on the outline of the plasma membrane using Image-Pro 4.0 software (Media Cybernetics, Silver Spring, MD). The procedure was repeated for the stacks of transverse images that were created as described above. The triplet data (x, y, and z coordinates) from both the paradermal and transverse images were combined to create a point cloud that represented the three-dimensional shape of the cell as defined by the plasma membrane (Fig. 2). Approximately 300 points were used to characterize each guard cell.
A solid digital object was created from the point clouds using Geomagic Studio 4 software (Raindrop Geomagic, Research Triangle, NC). Each point cloud was fitted with a closed surface, spikes were removed, and a cleaning algorithm was applied to create a smooth solid object (Fig. 3). The surface area, volume, and dimensions of these objects were measured using Solid-View software (Solid Concepts, Valencia, CA). This procedure was used to determine the surface area and volume of a sphere of known dimensions and was found to be accurate to within 2%.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027698.
This work was supported by the National Science Foundation (grant no. IBN–0222378).
References
- Battey NH, James NC, Greenland AJ, Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11: 643–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Diekmann W, Hedrich R, Raschke K, Robinson DG (1993) Osmocytosis and vacuolar fragmentation in guard cell protoplasts: their relevance to osmotically-induced volume changes in guard cells. J Exp Bot 44: 1569–1577 [Google Scholar]
- Franks PJ, Buckley TN, Shope JC, Mott KA (2001) Guard cell volume and pressure measured concurrently by confocal microscopy and the cell pressure probe. Plant Physiol 125: 1577–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21: 94–100 [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farquhar GD (1995) Guard cell pressure/aperture characteristics measured with the pressure probe. Plant Cell Environ 18: 795–800 [Google Scholar]
- Fricke W, Jarvis MC, Brett CT (2000) Turgor pressure, membrane tension and the control of exocytosis in higher plants. Plant Cell Environ 23: 999–1003 [Google Scholar]
- Gradmann D, Robinson DG (1989) Does turgor prevent endocytosis in plant cells? Plant Cell Environ 12: 151–154 [Google Scholar]
- Homann U (1998) Fusion and fission of plasma-membrane material accommodates for osmotically induced changes in the surface area of guard-cell protoplasts. Planta 206: 329–333 [Google Scholar]
- Kubitscheck U, Homann U, Thiel G (2000) Osmotically evoked shrinking of guard-cell protoplasts causes vesicular retrieval of plasma membrane into the cytoplasm. Planta 210: 423–431 [DOI] [PubMed] [Google Scholar]
- Morris CE, Homann U (2001) Cell surface area regulation and membrane tension. J Membr Biol 179: 79–102 [DOI] [PubMed] [Google Scholar]
- Raschke K (1979) Movements of stomata. In W Haupt, ME Feinleib, eds, Encyclopedia of Plant Physiology. Springer-Verlag, Berlin, pp 381–441
- Raschke K, Dickerson M (1973) Changes in shape and volume of guard cells during stomatal movement. Plant Res 1972: 149–153 [Google Scholar]
- Saxton MJ, Breidenbach RW (1988) Receptor-mediated endocytosis in plants is energetically feasible. Plant Physiol 86: 993–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD (1995) A new vial stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 128: 779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Errington RJ, Fricker MD, Wood JL (1996) Aberration control in quantitative imaging of botanical specimens by multidimensional fluorescence microscopy. J Microsc 181: 99–116 [Google Scholar]
- Willmer C, Fricker M (1996) Stomata. Chapman & Hall, London
- Wolfe J, Steponkus PL (1983) Mechanical properties of the plasma membrane of isolated protoplasts. Plant Physiol 71: 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]