Abstract
The fact that the acid-secreting parietal cells undergo continuous renewal has been ignored by many gastroenterologists and cell biologists. In the past, it was thought that these cells were static. However, by using 3H-thymidine radioautography in combination with electron microscopy, it was possible to demonstrate that parietal cells belong to a continuously renewing epithelial cell lineage. In the gastric glands, stem cells anchored in the isthmus region are responsible for the production of parietal cells. The stem cells give rise to three main progenitors: prepit, preneck and preparietal cells. Parietal cells develop either directly from the non-cycling preparietal cells or less commonly via differentiation of the cycling prepit and preneck cell progenitors. The formation of a parietal cell is a sequential process which involves diminishment of glycocalyx, production of cytoplasmic tubulovesicles, an increase in number and length of microvilli, an increase in number and size of mitochondria, and finally, expansion and invagination of the apical membrane with the formation of an intracellular canalicular system. Little is known about the genetic counterparts of these morphological events. However, the time dimension of parietal cell production and the consequences of its alteration on the biological features of the gastric gland are well documented. The production of a new parietal cell takes about 2 d. However, mature parietal cells have a long lifespan during which they migrate bi-directionally while their functional activity for acid secretion gradually diminishes. Following an average lifespan of about 54 d, in mice, old parietal cells undergo degeneration and elimination. Various approaches for genetic alteration of the development of parietal cells have provided evidence in support of their role as governors of the stem/progenitor cell proliferation and differentiation programs. Revealing the dynamic features and the various roles of parietal cells would help in a better understanding of the biological features of the gastric glands and would hopefully help in providing a basis for the development of new strategies for prevention, early detection and/or therapy of various gastric disorders in which parietal cells are involved, such as atrophic gastritis, peptic ulcer disease and gastric cancer.
Keywords: Cell differentiation, Cell dynamics, Cell renewal, Oxyntic gland, Oxyntic mucosa, Parietal cell, Preparietal cell
INTRODUCTION
The gastric parietal cells have attracted great interest in scientists mainly due to their dramatic morphological and biochemical changes which occur during acid secretion. Little attention is paid to the fact that these cells are genetically programmed to have a limited life span and that they undergo perpetual renewal. In this editorial the plan is to focus on the dynamism of the parietal cell population and the tools utilized over the years to study this phenomenon.
Leblond[1] generally defined renewing cell populations by two major criteria: (1) Frequent mitoses which are restricted to a definite subpopulation of cells; and (2) Cell loss which is required to balance cell production and to keep the steady state of the population. In addition, he described 5 successive stages in the life history of renewing cells. They are first present as stem cells which divide and produce new stem cells as well as uncommitted and/or committed progenitor cells which represent the second stage in the renewing cell life. The uncommitted progenitor cells exhibit dual lineage features and eventually become committed progenitor cells with features of one lineage. These progenitor cells are usually capable of undergoing equivalent mitosis and thus amplifying the population before entering the next stage. Transit cells represent the third stage during which cellular specification gradually occurs by synthesizing new gene products. This may be encountered morphologically by gradual changes in cell structure, immunocytochemically by expression of new proteins or sugar residues, and biochemically by changes in enzymatic activities, protein composition and messenger RNA expression. The fourth stage is that of the mature cells which have completed their differentiation and thus become actively functional. In the fifth stage of terminal cells there is a gradual deterioration and eventually death and elimination[1,2].
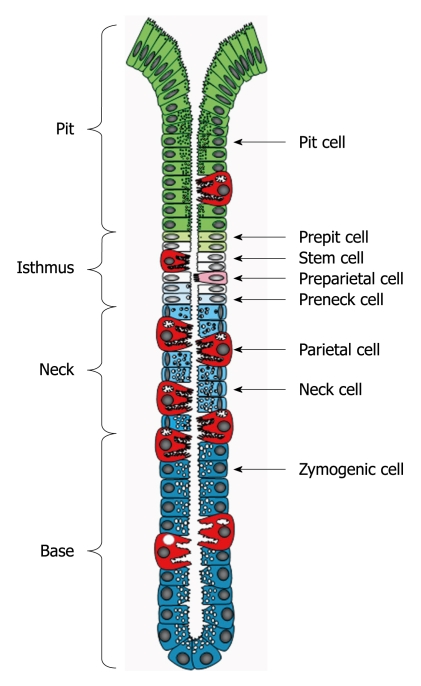
Parietal cells belong to the oxyntic (gastric) epithelium which is composed of one layer of heterogeneous cell populations. These cell populations have a common source of origin, but remarkably vary in their morphological features and functional potentials. The gastric epithelium is organized to form many long tubular units (gastric or oxyntic glands). Based on the allocation of the different cell types, each gastric gland is divided into pit, isthmus, neck and base regions (Figure 1)[3,4].
Figure 1.
Diagrammatic representation of the gastric unit or gland. It is composed of 4 regions: pit, isthmus, neck and base and populated by a heterogeneous population of cell types. Pit, parietal and neck cells originate in the isthmus from stem (granule-free) cells and their immediate descendants (prepit, preparietal and preneck cells). Neck cells are not end cells and therefore, at the neck-base border, they transform into zymogenic cells.
Studying the dynamism of parietal cells is important for several reasons: (1) The gastric epithelium is highly vulnerable and the turnover of its cells is one of the effective defense mechanisms against damaging agents (e.g. alcohol and drugs); (2) Analysis of the normal production and loss of parietal cells provides the basis for understanding the pathogenesis of several diseases that affect parietal cell mass (e.g. Zollinger-Ellison syndrome and chronic atrophic gastritis); (3) The common gastric pathogen, Helicobacter pylori, is believed to play an important role in the pathogenesis of gastritis and peptic ulcer disease and may have an effect on parietal cell dynamics; (4) Studying gastric adenocarcinoma and “parietal cell carcinoma”[5] requires better understanding of the normal process of parietal cell production and loss; and (5) Identifying the origin of parietal cells, their immature forms and the factors controlling their differentiation may lead to the design of new drugs for the treatment of peptic ulcer disease, chronic atrophic gastritis and gastric tumors.
METHODS USED TO STUDY PARIETAL CELL DYNAMISM
Over the years, four main methods have been used to study the dynamic features of gastric epithelial parietal cells.
Search for mitotic figures
The microscopic search for mitotic figures was the method used by pioneers in this field who first discovered mitotic cells at the bottom of gastric pits[6]. Some of these mitotic figures were thought to be parietal cells[7]. The area of mitotic activity described by Bizzozero[6] was later called the “isthmus” by Stevens et al[8]. Bizzozero[6] correlated the presence of mitotic cells with the gradual increase in the secretion content of the pit lining cells from the bottom of the foveola to the free surface. He then concluded that the cells at the bottom of the pits were poorly differentiated, multiply by mitosis and migrate outward to the free surface while completing their differentiation. Deep in the mucosa, cells of the long tubular glands (including parietal cells) were thought to be stable permanent structures. Bizozzero’s conclusion was then confirmed by Benseley[9] who also observed degenerated cells from the pit within the gastric lumen. Benseley[9] then proposed that, upon reaching the free surface, pit cells were desquamated in response to damage by food. Later this food damaging idea was considered a rapid replacement of the surface epithelium following damage induced by dilute acid or alcohol. This replacement was generally considered as a regenerative process in response to damage.
A disadvantage of this mitosis search method is that no measurement of time is involved, and thus the rate at which cells are replaced cannot be calculated. The mitotic index is the parameter that can be estimated by this method. This index would only be valuable when relative measurements are needed in comparing various agents on the renewal of a cell population. However, in that case, it should be assumed that the agents have no effect on the duration of mitosis.
Colchicine method
In the 1950s, the colchicine method of arresting and accumulating mitotic cells at metaphase was applied in the gastric epithelium by Stevens et al[8]. The site of mitotic activity, the isthmus region, became apparent. Moreover, by examining fasting and normally-fed rats sacrificed at different times of the day 4 h after colchicine injection, it was found that the mitotic activity and desquamation of cells were not dependent on ingested food. Thus, it was concluded that mitosis is not just a response to damage, but is a sign of the normal constant “renewal” phenomenon. Moreover, Stevens et al[8] found that the mucous cells lining the surface and pit turnover very quickly and have a turnover time of about 3 d. Deep in the glandular epithelium, the turnover time of mucus-secreting neck cells was estimated to be about 1 wk.
3H-thymidine radioautography
Mitosis occurs rapidly, so unless mitotic figures are frequent they may not be observed even with the colchicine method. A third method was thus developed which took advantage of the long duration of S-phase, the preparatory stage for mitosis. In mice, while the combined duration of metaphase and anaphase is about 0.4 h, the period of DNA synthesis (S-phase) extends over 7.3 h[10,11], therefore the chances of detecting S-phase cells are high. If a radioactive DNA-precursor is injected into an animal, it will be incorporated into the DNA of dividing cells and will be retained throughout the entire life span of their daughter cells. Thus, by using radioautography not only would sites of mitotic activity be visualized, but also the post-mitotic differentiation and migration pathway of the daughter cells may be traced[12]. Based on these facts, the third method of radio-thymidine labeling of dividing cells was developed and used to study cell proliferation and migration in the gastric epithelium.
In 1960, Messier et al[13] demonstrated in radioautographs taken at different time intervals, evidence of the migration of mucus-secreting pit cells in rats. Labeled cells were initially present in the isthmus; within a few days they were found at the free surface. This observation was later confirmed in the mouse[14], hamster[15], and man[16,17].
To analyze the dynamic parameters of a renewing cell population, 3H-thymidine may be administered either by single injection, by multiple injections or by continuous infusion. It should be assumed that the population is in a steady state; i.e. cell production balances cell loss. Then, any of the three methods may be used to determine the rate at which the cells turn over (turnover rate) and the time taken for cells to be replaced (turnover time). The turnover rate may be defined as the fraction of the cell population which is replaced per unit time, whereas the turnover time is the interval taken for the replacement of the total number of cells in the population.
Single injection method: The use of the single injection method is based on the premise that some cells in S-phase will incorporate the label during the period between injection and sacrifice (30 min). These cells will eventually divide and yield all the cells in the population. With this single injection method, it is possible to determine the percentage of cells in the population which are synthesizing DNA and, thereby, preparing to divide. Animals, therefore, are sacrificed 30 min after a single injection of 3H-thymidine, and the labeling index would be the ratio of the number of labeled cells to the total number of cells (labeled plus unlabeled cells). Because of diurnal variation, it is recommended that the average of labeling indices measured at different times throughout a 24-h period be used. The calculation of the number of new cells produced or the labeling index depends on the length of time each dividing cell spends in the S phase. Therefore, in order to calculate the turnover rate, the duration of S phase must be known. It may be obtained by plotting the percentages of labeled mitoses at different times (hours) following a single 3H-thymidine injection. The distance between the ascending and descending portions of the resulting curve measured at the 50% labeling level provides the mean duration of the S phase in hours. In the mouse pyloric antrum, the S duration is 7.3 h[11]. The turnover rate is then calculated by dividing the labeling index by the S phase duration and is expressed as the percentage of cells formed per hour. The turnover time is the inverse of the turnover rate, i.e. 100/turnover rate = turnover time expressed in hours.
The single injection method has two disadvantages: the results depend on the time of day the experiment was carried out (diurnal variation), and the cells labeled after a single injection are not all necessarily committed to a single cell line.
Multiple injection method: The multiple injection method is based on accumulating a labeled fraction of the cell population within a certain period of time, and then following the disappearance of these labeled cells at different time intervals after the last injection. Thus, the percentage of labeled cells would decrease steadily with time along a regression line. The rate of labeled cell loss is obtained from the decreasing slope of the line. Assuming a steady state, the rate of cell loss would be equal to the turnover rate.
The proliferative capacity of certain types of cells can be determined not only from the 30-min labeling experiment (as mentioned above) but also from the multiple injection experiment. This can be done by counting the number of silver grains over cell nuclei at different time intervals following the last injection. The percentages of cells with a given grain count are then plotted against grain count. Similar curves are prepared at the other time intervals. If the labeled cells have divided, the grains would be equally distributed to daughter cells and thus, a shift in the grain count curves with time toward a lower number of grains should occur.
A disadvantage of this method is the stress induced with the multiple injections over several days. Thus the following method is usually preferred.
Continuous infusion method: The continuous infusion method is based on the radio-labeling of newly produced cells in the population for different periods of infusion time. This technique minimizes the disadvantages of the single (diurnal variation) and the multiple (stress) injection methods. The percentage of labeled cells would increase steadily with time along a regression line. The rate of cell production, which under steady state is equal to the turnover rate, is the slope of the line. The “production rate” of a certain cell type can be estimated by simply multiplying the turnover rate by the number of these cells in the population. Assuming steady state, the “exit rate” of these cells would be equal to their production rate.
Genetic manipulation
The use of genetic engineering technology for studying cell specification and differentiation has become a powerful tool for unraveling the role of the parietal cell population in maintaining homeostasis of the gastric glands. Promoters of parietal cell-specific genes are available to deliver biologically interesting foreign gene products exclusively to a parietal cell lineage throughout the lifespan of the organism[18]. These promoters can be used to design parietal cell-specific gain-of-function experiments as well as loss-of-function experiments. Thus, the mouse has become the model system for chimeric, transgenic, chimeric-transgenic, knock-out, and knock-in technology. These models will help answer many questions of parietal cell dynamics, including factors regulating their production and maintaining their organization along the pit-gland unit.
SIZE OF THE PARIETAL CELL POPULATION
Parietal cells are scattered along the gastric glands and occupy much space due to their large size. Helander et al[19,20] estimated that in gastric mucosal tissue sections, the parietal cell number comprises 16%-21% of all epithelial cells, in rats[19] and 12% in humans[20].
When some individual gastric glands of adult mice were followed in serial cross sections starting from the bottom of their base regions all the way up to opening of their pits, the parietal cell number was found to comprise about 13% of the total cell population, representing an average of 26 parietal cells out of the 194 epithelial cells present per gland[3]. An average of 4 parietal cells was found in the pit; 6 in the isthmus; 5 in the neck and 11 in the base region.
Preparietal cells are the least numerous cell type found in the gastric gland. They comprise about 0.3% of all epithelial cells, representing 0-3 cells per gland[3].
PARIETAL CELL PROGENITORS AND THEIR SPECIFICATION
Over the years, several theories have been raised regarding the source of parietal cells. The first idea was that parietal cells were able to divide[7] and to reproduce themselves. This idea was later refuted by studies using the colchicine arrest technique[8], 3H-thymidine radioautography[17,21,22], and the method of time-grain count curve[23]. Then, various investigators[24,25] re-supported the self renewal theory. It was Hunt et al[21] who first demonstrated radio-labeled parietal cells in paraffin sections of the rat stomach only 2 d after a pulse of 3H-thymidine injection. No labeled parietal cells were found earlier than that. Hunt et al[21] suggested that parietal cells are not able to divide and that they originate from the mucus-secreting neck cells which were labeled at earlier time intervals. On the other hand, studies using electron microscopy[26] and in regenerating mouse gastric mucosa[27] have led to the proposal that parietal cells come from immature mucus-secreting pit cells.
In the 1960s, undifferentiated cells were identified in the oxyntic epithelium of the immature hamster[28] and bullfrog tadpole[29]. It was suggested that these cells are epithelial stem cells which are the source of parietal cells. The idea of the gastric epithelial stem cell was supported by Hattori[30] but questioned and even denied by Tamura et al[25]. Kataoka[14] first reported the existence of these stem cells which could be the source of parietal cells in the mouse. Later Kataoka et al[31], observed some parietal cells which contained a few mucous granules and proposed that they came from the mucus-secreting neck cells.
In a systematic electron microscopic study, all cell types lining the epithelial unit of the mouse stomach were defined and quantified in serial cross sections. The tissue sections included the whole thickness of the gastric units starting from the bottom of the base region next to the muscularis mucosae, up to the orifice of the pit where mucus-secreting surface cells are found. In addition to the well recognized mature cell types of the gastric epithelium (pit, neck, parietal and zymogenic cells), four different poorly differentiated cell types were identified in a narrow region along the gastric gland (Figure 1)[3]. This is the isthmus region observed earlier by Stevens et al[8], which could be delimited between the first pit cell inward and the first neck cell outward (Figure 1)[3]. While one of these isthmal cells did not show any sign of differentiation, each of the three others exhibited one sign of early commitment. Thus, the first cell type was named granule-free (stem) cell and the three others, prepit, preneck and preparietal cells, reflecting their early commitment to three different cell lineages[3].
In 30-min 3H-thymidine-labeling experiments, three of the isthmal cells incorporated the radio-marker and thus were proliferative[32]. The preparietal cells, however, were never seen in mitosis and did not incorporate the label early after injection of the isotope. Thus, it was concluded that preparietal cells cannot divide and may have come from other cells which are capable of dividing and differentiating. In the isthmus, granule-free cells are similar to many embryonic cells. These cells have significant free ribosomes and dispersed chromatin, and large nucleoli, but other organelles are fewer and smaller. In addition, these are the most proliferative cell type within the gastric epithelium. All these criteria made granule-free cells good candidates as the source, not only for parietal cell lineage, but also for all other cell lineages (Figures 1 and 2).
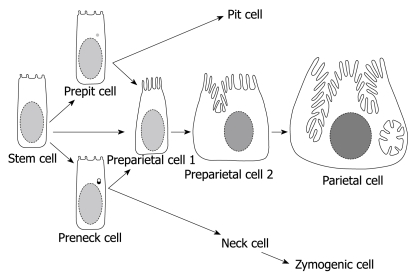
Figure 2.
Diagram summarizing the origin and differentiation program of parietal cells. The stem cell either directly or indirectly (via prepit or preneck cells) give rise to the committed progenitors of parietal cells (preparietal cells). Preparietal cells evolve in 4 successive stages (2 are depicted here) to become mature parietal cells. Preparietal cell 1 is characterized by long microvilli and preparietal cell 2 by an incipient canaliculus which expands around the nucleus in the mature parietal cell.
The search for signs of cell lineage specification in the Golgi apparatus of granule-free (stem) cells revealed that they constitute three different subtypes[32]: (1) Cells with primitive Golgi having no sign of secretory activity and, hence, named “undifferentiated granule-free cells”; (2) Cells with trans-Golgi face including prosecretory vesicles with homogeneous dense material similar to those in the pit cell lineage and, hence, considered to be “prepit cell precursors”; and (3) Cells with trans-Golgi face exhibiting prosecretory vesicles with irregular dense material in the center and light periphery similar to those of the neck cell lineage, hence, named “preneck cell precursors”.
Three different forms of preparietal cells have also been identified based on (1) the absence of secretory granules (as in granule-free cells) or (2) the presence of dense granules (as those of the pit cell lineage) or (3) the presence of cored-granules (as those of the neck cell lineage). The expression of pit- or neck-like mucous granules in a preparietal cell reflected the diversity in parietal cell origin and the phenotypic plasticity of the derivatives of granule-free cells. Thus, each subtype of granule-free cells provided a slightly different phenotype of preparietal cells which would then all pool into a single phenotype (parietal cells). Whether or not this phenotypic plasticity is unidirectional (from mucous to parietal), or bidirectional is not known. However, the occasional existence of a few mucous granules in mature parietal cells and the absolute absence of any parietal cell features (long microvilli or canaliculus, or H,K-ATPase expression) in mature mucous (pit or neck) cells suggest that it is unidirectional and only from prepit or preneck cell precursors to preparietal cells.
The origin of parietal cells was also demonstrated by radioautography. A pulse of 3H-thymidine given to a group of mice first appeared in granule-free cells after 30 min. After 1 d, label started to appear in preparietal cells. At least 2 d later, some labeled mature parietal cells appeared, reflecting the short time required for their differentiation[33].
The occasional presence of bi-nucleated parietal cells might give the impression that parietal cell mitosis could occur. However, during the years of our studies we have never visualized a mitotic parietal or even a preparietal cell, or observed an immuno-labeled (using antibodies for the nuclear CC-3 phosphoprotein or the proliferating cell nuclear antigen or by using the bromodeoxyuridine method) or a 3H-thymidine-labeled cell. Our interpretation for these bi-nucleated parietal cells is that their early ancestors (prepit cell precursors, preneck cell precursors or undifferentiated granule-free cells) underwent an incomplete mitosis i.e. during telophase, karyokinesis occurred without cytokinesis and then the cell differentiated and matured as a bi-nucleated parietal cell[33].
DIFFERENTIATION COMMITMENT OF PARIETAL CELLS
Once a precursor cell enters the pathway to become a parietal cell, the gradual acquisition of specific features for acid secretion means differentiation commitment. It was Arnold[28] who first reported the presence of immature parietal cells in the hamster embryo. Similarly, differentiating oxyntic cells were identified in the metamorphosing bullfrog tadpole[29]. In the adult mouse stomach, such growing cells were also identified[31] and named “preparietal cells”[3]. These cells are also found in the human stomach[4]. They gradually lose features of their precursors (large nucleoli, many free ribosomes, and relatively few organelles) and simultaneously acquire features of acid-secreting cells (long apical microvilli, intracellular canaliculi, and large numerous mitochondria).
There are four successive stages in the maturation of preparietal cells (two of them are diagrammatically presented in Figure 2)[33]. The first stage was revealed only when preparietal cells were amplified in a transgenic mouse model[34]. This stage is characterized by an increased number of apical microvilli and diminishment of their glycocalyx. The second stage (similar to that described by Arnold[28] and Forte et al[29]) is characterized by elongation of apical microvilli to reach the same size and shape as those of mature parietal cells (Figure 3A)[4,33]. This stage is diagrammatically represented in Figure 2 as preparietal cell 1. In the third stage, gradual invagination of the apical cytoplasm leads to the formation of an intracellular incipient canaliculus on one side of the nucleus. In addition, a few tubules and vesicles appear in the apical cytoplasm as an early indication of the formation of the tubulovesicular system (preparietal cell 2 in Figures 2 and 3B)[4,33]. In the fourth stage, the canaliculus expands and appears at both sides of the nucleus. The four stages are characterized by abundant free ribosomes and a relatively large nucleolus. With maturation of the cell from the first to the fourth stage, there is a gradual increase in the cell size and the size and number of mitochondria are gradually increased (Figure 3)[4,33].
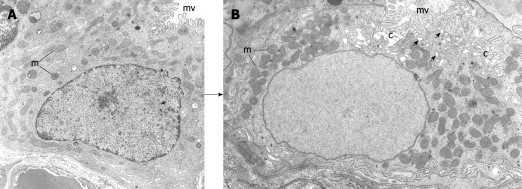
Figure 3.
Electron micrographs depicting 2 stages of the differentiating preparietal cells as seen in the human stomach. Original magnification, × 12 000. A: Preparietal cell showing relatively long numerous microvilli (mv). The mitochondria (m) are relatively few and small; B: Preparietal cell showing an incipient canaliculus (c) and relatively numerous mitochondria (m) which appear larger than those in A. The apical cytoplasm shows few tubulovesicles (small arrows) and long numerous microvilli (mv). A is reproduced from Karam et al[4] with permission from Wiley-Blackwell/AlphaMed press.
PARIETAL CELL MIGRATION AND FORMATION OF TWO PHYSIOLOGICALLY DISTINCT POPULATIONS
In the mouse, the central position of preparietal cells in the isthmus and the distribution of mature parietal cells throughout the four unit regions is the first indication of the bi-directional migration of parietal cells. In man, rat and rabbit, parietal cells are also scattered in the four regions, but are fewer in the pit[4,35].
Continuous 3H-thymidine-infusion in mice for different time intervals varying from 1 up to 52 d shows that labeled parietal cells start to appear in the isthmus after 3 d of infusion. With time, labeled parietal cells increase in the isthmus and then gradually appear in the pit region and, with time, ascend the pit. Simultaneously, labeled parietal cells make their appearance in the neck region and descend, eventually crossing the neck-base border and continuing their migration toward the bottom of the gland. An estimation of the turnover rate of parietal cells in the four unit regions indicated that it was 2.84, 3.20, 1.86, and 0.53, respectively in the pit, isthmus, neck and base. Since the average number of parietal cells in each region is known, simple calculations revealed that, every month, 6 parietal cells are produced in one isthmus. Within the same period, 3 cells appear in the pit and 3 in the neck. Thus, every month the newly produced parietal cells in the isthmus equal the sum of those appearing in both the pit and neck (6 cells). This indicates that there is a steady state in the parietal cell population.
It is likely that parietal cells located in the pit are those which originated from prepit cell precursors which were already assigned to migrate upward. The same might apply to basal parietal cells which might have originated from preneck cell precursors and are committed for inward migration.
The bidirectional migration of parietal cells along the pit-gland axis is associated with a gradual loss in their functional activity. Thus, young parietal cells present in the isthmus and neck would be expected to be structurally and functionally different from the old cells found in the pit and base regions. Evidence for this comes from at least five groups of studies on the heterogeneity of parietal cells. (1) It was Lawn[36] who first noticed that parietal cells in the neck region have narrow and less extensive canaliculi than basal ones. Helander[37] then reported that neck parietal cells have fewer tubulovesicles and more elaborate secretory canaliculi than base parietal cells. This was later confirmed by Jacobs et al[38] who, in a morphometric study, found that parietal cells in the neck region have higher canalicular volume and rER surface area than those located in the base region. Electron microscopic studies on the mouse stomach revealed that, while almost all isthmus and neck parietal cells are morphologically normal, 25% of those in the pit and those in the base show signs of degeneration[33]. Similar but less pronounced degeneration of old parietal cells was also found in the rabbit[35] and this was enhanced by the omeprazole-induced inhibition of acid secretion; (2) After a 24-h-fasting, Coulton et al[39] were able to actively stimulate neck parietal cells with food but base parietal cells remained inactive. They proposed that basal parietal cells are either switched off or have a function other than acid secretion; (3) In a histochemical study, Firth et al[40] found that the H,K-ATPase activity is prominent in neck parietal cells, but absent in the basal cells; (4) In gastric glands isolated from rabbits, measurements of the morphological responsiveness of parietal cells to acid inhibitors and secretagogues revealed that lumenal parietal cells respond much more efficiently than basal parietal cells[41]; and (5) The detection of H,K-ATPase β-subunit mRNA using a digoxigenin-labeled cRNA probe revealed a differential pattern of expression. Parietal cells located in the isthmus-neck region have high level of mRNA, while the level was low in the senescent pit and base parietal cells[41].
The senescence-related decrease in functional and synthetic activity of parietal cells could be a sign of an alteration either in the membrane receptors, or in an intracellular mediator of the cAMP activation pathway, or in the effector proteins (proton pumps) themselves.
ROLE OF PARIETAL CELLS AS GOVERNORS OF PROLIFERATION AND DIFFERENTIATION OF STEM/PROGENITOR CELLS
Several genetic manipulation studies have revealed different lines of evidence confirming the important role that parietal cells play as governors of both progenitor/stem cell proliferation and their differentiation into other cell lineages in the oxyntic glands. (1) In the first approach, forced expression of Simian virus 40 large T antigen gene in preparietal cells to induce their proliferation and thus block parietal cell production was also associated with a block of zymogenic cell differentiation and a progressive increase in the number of progenitor cells starting from embryonic day 18[34,42]. With age, the situation in these mice became more dramatic due to development of dysplastic changes leading to invasive gastric adenocarcinoma with distant metastasis[43]; (2) Genetically engineered ablation of parietal cells using the attenuated Diphtheria toxin gene in transgenic mice was also associated with a block in terminal differentiation of zymogenic cells and a massive increase in the proliferation of the multipotent stem cells and committed progenitors[44]; (3) Genetic enhancement of parietal cell apoptosis leading to a reduction in their population is also associated with a blockade in zymogenic cell differentiation and glandular hyperplasia[45]; and (4) Deletion mutants of the potassium channel, KCNQ1 in parietal cells of both mice and rats showed altered zymogenic cell lineage differentiation and hyperproliferation of glandular progenitor cells[46].
PHYSIOLOGICAL VS INDUCED DEATH OF PARIETAL CELLS
In physiologically renewing cell populations, before the life of a cell ends, it enters a “terminal stage” where its functional activity deteriorates and it gradually degenerates until death[1]. An occasional degeneration of parietal cells has been reported in the mouse by Kataoka et al[31]. When parietal cells degenerate they either show signs of necrosis or apoptosis[33]. Dead parietal cells are immediately eliminated from the epithelium by different mechanisms. Necrotic cells are usually extruded into the gland lumen, whereas, apoptotic ones are phagocytosed by neighboring pit cells, zymogenic cells or connective tissue macrophages invading through a break in the basement membrane[33,35].
In addition to their physiological loss, death of parietal cells has been induced by various compounds. (1) Omeprazole is known to bind to the catalytic α subunit of H,K-ATPase and hence, inhibit acid secretion. This covalent binding was found to be associated with a reduction in the amount of H,K-ATPase due to induced degeneration of parietal cells in rabbits[35]; (2) H2 receptor antagonists (ranitidine and cimetidine) which inhibit acid secretion have also been shown to enhance parietal cell degeneration and loss in mice[47]; and (3) The orally active, cell-permeant neutrophil elastase inhibitor DMP-777 induces a rapid degeneration and loss of parietal cells in rodents[48]. This induced loss of parietal cells is associated with enhanced progenitor cell proliferation and, hence, hyperplasia develops.
CONCLUSION
Revealing the renewal concept of parietal cells and analyzing their dynamic features have improved our understanding of the biology and pathophysiology of the gastric glands. For example: (1) The fact that parietal cells are not static provides an explanation for their functional heterogeneity. Therefore, young parietal cells in the isthmus and neck regions are more active in acid secretion than old parietal cells which have migrated to the glandular base; (2) Maturation of parietal cells in the isthmus region seems to be necessary to maintain the normal proliferation and differentiation program of gastric epithelial stem cells and their immediate descendents; (3) Migration of parietal cells to the base region seems to be a prerequisite for the transformation of mucous neck cells into pepsinogen-secreting zymogenic cells; and (4) Regeneration of parietal cells explains the recurrence of symptoms following therapy aimed at inhibiting acid secretion and explains the possibility of hyperplastic changes and carcinoids.
Acknowledgments
The author is grateful to Dr. Gerald Buzzell for the critical comments and discussions.
Footnotes
Supported by Terry Fox Fund for Cancer Research and UAE University
Peer reviewers: Vittorio Ricci, MD, PhD, Department of Physiology, Human Physiology Section, University of Pavia Medical School, Via Forlanini 6, Pavia, 27100, Italy; Bronislaw L Slomiany, PhD, Professor, Research Center, C-875, UMDNJ-NJ Dental School, 110 Bergen Street, PO Box 1709, Newark, NJ 07103-2400, United States
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1.Leblond CP. The life history of cells in renewing systems. Am J Anat. 1981;160:114–158. doi: 10.1002/aja.1001600202. [DOI] [PubMed] [Google Scholar]
- 2.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–D298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 3.Karam SM, Leblond CP. Identifying and counting epithelial cell types in the "corpus" of the mouse stomach. Anat Rec. 1992;232:231–246. doi: 10.1002/ar.1092320208. [DOI] [PubMed] [Google Scholar]
- 4.Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 5.Rychterova V, Hägerstrand I. Parietal cell carcinoma of the stomach. APMIS. 1991;99:1008–1012. doi: 10.1111/j.1699-0463.1991.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 6.Bizzozero G. Ueber die schlauchformigen Drusen des magendarmkanals und die beziehungen ihres Epithels zu dem Oberflachenepithel der Schleimhaut. Arch Mikr Anat. 1893;42:82–152. [Google Scholar]
- 7.Harms W. Replacement of the gastric chief and parietal cells in the mouse. Anat Embryol (Berl) 1910;41:392–398. [Google Scholar]
- 8.Stevens CE, Leblond CP. Renewal of the mucous cells in the gastric mucosa of the rat. Anat Rec. 1953;115:231–245. doi: 10.1002/ar.1091150206. [DOI] [PubMed] [Google Scholar]
- 9.Benseley RR. The gastric glands. In: Cowdry EV, editor. Special Cytology. vol 1. New York: P.B. Hoeber; 1932. pp. 198–230. [Google Scholar]
- 10.el-Alfy M, Leblond CP. Long duration of mitosis and consequences for the cell cycle concept, as seen in the isthmal cells of the mouse pyloric antrum. II. Duration of mitotic phases and cycle stages, and their relation to one another. Cell Tissue Kinet. 1987;20:215–226. doi: 10.1111/j.1365-2184.1987.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 11.el-Alfy M, Leblond CP. Visualization of chromosome assembly during the S and G2 stages of the cycle and chromosome disassembly during the G1 stage in semithin sections of mouse duodenal crypt cells and other cells. Am J Anat. 1988;183:45–56. doi: 10.1002/aja.1001830103. [DOI] [PubMed] [Google Scholar]
- 12.Leblond CP, Messier B, Kopriwa B. Thymidine-H3 as a tool for the investigation of the renewal of cell populations. Lab Invest. 1959;8:296–306; discussion 306-308. [PubMed] [Google Scholar]
- 13.Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka K. Electron microscopic observations on cell proliferation and differentiation in the gastric mucosa of the mouse. Arch Histol Jpn. 1970;32:251–273. doi: 10.1679/aohc1950.32.251. [DOI] [PubMed] [Google Scholar]
- 15.Hattori T, Fujita S. Tritiated thymidine autoradiographic study on cellular migration in the gastric gland of the golden hamster. Cell Tissue Res. 1976;172:171–184. doi: 10.1007/BF00226025. [DOI] [PubMed] [Google Scholar]
- 16.Lipkin M, Sherlock P, Bell B. Cell proliferation kinetics in the gastrointestinal tract of man. II. Cell renewal in stomach, ileum, colon, and rectum. Gastroenterology. 1963;45:721–729. [PubMed] [Google Scholar]
- 17.MacDonald WC, Trier JS, Everet NB. Cell proliferation and migration in the stomach, duodenum, and rectum of man: radioautographic studies. Gastroenterology. 1964;46:405–417. [PubMed] [Google Scholar]
- 18.Lorenz RG, Gordon JI. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993;268:26559–26570. [PubMed] [Google Scholar]
- 19.Helander HF, Poorkhalkali N. Parietal cell density during gastric ulcer healing in the rat. Scand J Gastroenterol. 2004;39:20–26. doi: 10.1080/00365520310006900. [DOI] [PubMed] [Google Scholar]
- 20.Helander HF, Leth R, Olbe L. Stereological investigations on human gastric mucosa: I. Normal oxyntic mucosa. Anat Rec. 1986;216:373–380. doi: 10.1002/ar.1092160306. [DOI] [PubMed] [Google Scholar]
- 21.Hunt TE, Hunt EA. Radioautographic study of proliferation in the stomach of the rat using thymidine-H3 and compound 48/80. Anat Rec. 1962;142:505–517. doi: 10.1002/ar.1091420408. [DOI] [PubMed] [Google Scholar]
- 22.Willems G, Galand P, Vansteenkiste Y, Zeitoun P. Cell population kinetics of zymogen and parietal cells in the stomach of mice. Z Zellforsch Mikrosk Anat. 1972;134:505–518. doi: 10.1007/BF00307670. [DOI] [PubMed] [Google Scholar]
- 23.Ragins H, Wincze F, Liu SM, Dittbrenner M. Theorigin and survival of gastric parietal cells in the mouse. Anat Rec. 1968;162:99–110. doi: 10.1002/ar.1091620109. [DOI] [PubMed] [Google Scholar]
- 24.Chen KY, Withers HR. Proliferative capability of parietal and zymogen cells. J Anat. 1975;120:421–432. [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura S, Fujita H. Fine structural aspects on the renewal and development of surface mucous cells and glandular cells of the gastric body of the adult golden hamster. Arch Histol Jpn. 1983;46:501–521. doi: 10.1679/aohc.46.501. [DOI] [PubMed] [Google Scholar]
- 26.Corpron RE. The ultrastructure of the gastric mucosa in normal and hypophysectomized rats. Am J Anat. 1966;118:53–90. doi: 10.1002/aja.1001180105. [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama M, Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970;169:385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- 28.Arnold M. Functional development of gastric mucosa in the golden hamster. I. Drusenmagen. Z Zellforsch Mikrosk Anat. 1966;71:69–93. [PubMed] [Google Scholar]
- 29.Forte GM, Limlomwongse L, Forte JG. The development of intracellular membranes concomitant with the appearance of HCl secretion in oxyntic cells of the metamorphosing bullfrog tadpole. J Cell Sci. 1969;4:709–727. doi: 10.1242/jcs.4.3.709. [DOI] [PubMed] [Google Scholar]
- 30.Hattori T. On cell proliferation and differentiation of the fundic mucosa of the golden hamster. Fractographic study combined with microscopy and 3H-thymidine autoradiography. Cell Tissue Res. 1974;148:213–226. doi: 10.1007/BF00224583. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka K, Sakano Y. Panoramic observation of the mouse gastric mucosa by superwide-field electron microscopy. Arch Histol Jpn. 1984;47:209–221. doi: 10.1679/aohc.47.209. [DOI] [PubMed] [Google Scholar]
- 32.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 33.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 34.Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol. 1997;272:G1209–G1220. doi: 10.1152/ajpgi.1997.272.5.G1209. [DOI] [PubMed] [Google Scholar]
- 35.Karam SM, Forte JG. Inhibiting gastric H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am J Physiol. 1994;266:G745–G758. doi: 10.1152/ajpgi.1994.266.4.G745. [DOI] [PubMed] [Google Scholar]
- 36.Lawn AM. Observations on the fine structure of the gastric parietal cell of the rat. J Biophys Biochem Cytol. 1960;7:161–166. doi: 10.1083/jcb.7.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helander HF. Stereological changes in rat parietal cells after vagotomy and antrectomy. Gastroenterology. 1976;71:1010–1018. [PubMed] [Google Scholar]
- 38.Jacobs DM, Sturtevant RP. Circadian ultrastructural changes in rat gastric parietal cells under altered feeding regimens: a morphometric study. Anat Rec. 1982;203:101–113. doi: 10.1002/ar.1092030110. [DOI] [PubMed] [Google Scholar]
- 39.Coulton GR, Firth JA. Effects of starvation, feeding, and time of day on the activity of proton transport adenosine triphosphatase in the parietal cells of the mouse gastric glands. Anat Rec. 1988;222:42–48. doi: 10.1002/ar.1092220108. [DOI] [PubMed] [Google Scholar]
- 40.Firth JA, Stranks GJ. Gastric proton pump localization. Application of triphosphatase and monophosphatase techniques. J Histochem Cytochem. 1981;29:344–350. doi: 10.1177/29.3.6263967. [DOI] [PubMed] [Google Scholar]
- 41.Karam SM, Yao X, Forte JG. Functional heterogeneity of parietal cells along the pit-gland axis. Am J Physiol. 1997;272:G161–G171. doi: 10.1152/ajpgi.1997.272.1.G161. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Karam SM, Gordon JI. Simian virus 40 T antigen-induced amplification of pre-parietal cells in transgenic mice. Effects on other gastric epithelial cell lineages and evidence for a p53-independent apoptotic mechanism that operates in a committed progenitor. J Biol Chem. 1995;270:15777–15788. doi: 10.1074/jbc.270.26.15777. [DOI] [PubMed] [Google Scholar]
- 43.Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, Gordon JI. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci USA. 2004;101:4471–4476. doi: 10.1073/pnas.0307983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 45.Jain RN, Al-Menhali AA, Keeley TM, Ren J, El-Zaatari M, Chen X, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest. 2008;118:2459–2470. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 2007;22:335–341. doi: 10.1152/physiol.00016.2007. [DOI] [PubMed] [Google Scholar]
- 47.Karam SM, Alexander G. Blocking of histamine H2 receptors enhances parietal cell degeneration in the mouse stomach. Histol Histopathol. 2001;16:469–480. doi: 10.14670/HH-16.469. [DOI] [PubMed] [Google Scholar]
- 48.Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]