Abstract
We evaluated the efficacy and tolerability of mebeverine, a musculotropic antispasmodic agent, in irritable bowel syndrome (IBS) and compared its usual dosages by meta-analysis. Medical databases and all relevant literature were searched from 1965 to June 2009 for any placebo-controlled clinical trials of mebeverine, using search terms such as mebeverine, clinical trials, and IBS. Eight randomized trials met our criteria, including six trials that compared mebeverine with placebo and two that compared mebeverine tablets with capsules. These eight trials included 555 patients randomized to receive either mebeverine or placebo with 352 (63%) women and 203 (37%) men in all subtypes of IBS. The pooled relative risk (RR) for clinical improvement of mebeverine was 1.13 (95% CI: 0.59-2.16, P = 0.7056) and 1.33 (95% CI: 0.92-1.93, P = 0.129) for relief of abdominal pain. The efficacy of mebeverine 200 mg compared to mebeverine 135 mg indicated RRs of 1.12 (95% CI: 0.96-1.3, P = 0.168) for clinical or global improvement and 1.08 (95% CI: 0.87-1.34, P = 0.463) for relief of abdominal pain. Thus, mebeverine is mostly well tolerated with no significant adverse effects; however, its efficacy in global improvement of IBS is not statistically significant.
Keywords: Clinical trial, Meta-analysis, Mebeverine, Placebo, Irritable bowel syndrome, Systematic review
INTRODUCTION
Irritable bowel syndrome (IBS) is a complex and widely-encountered syndrome. It is a condition characterized by abdominal pain associated with disordered defecation in the absence of any demonstrable abnormality. Despite recent advances in the treatment of IBS[1-3] the exact pathophysiology of IBS is still incompletely understood[4]. Alteration in neurohumoral mechanisms and psychological factors, bacterial overgrowth, genetic factors, gut motility, visceral hypersensitivity, and immune system factors are currently believed to influence the pathogenesis of IBS[4,5]. There are three IBS subgroups: those with constipation, those with diarrhea, and those with alternating constipation or diarrhea[6]. The treatment of IBS is targeted at the management of constipation, diarrhea, and abdominal pain and usually includes pharmacotherapy with alosetron and other 5-HT(3)-receptor antagonists[7].
Mebeverine is an antispasmodic that has been successfully used in the management of IBS for many years. Mebeverine is a musculotropic agent that has antispasmodic activity and regulatory effects on the bowel function[8]. During oral administration at doses of 135-270 mg tid, it shows no typical anticholinergic side effects, such as dry mouth, blurred vision, and impaired micturition. The incidence of side effects caused by mebeverine has not been demonstrated to be higher than that of a placebo[9]. This agent is now sold in approximately 56 countries, and its efficacy and tolerability have been demonstrated in 10 controlled studies and in many open clinical trials[9-19]. Although several clinical trials on the utility of mebeverine in patients with IBS exist, no statistical meta-analysis has been done regarding its efficacy and safety. In the present work, we systematically reviewed all the available data to examine the dose level efficacy and tolerability of mebeverine in IBS by a meta-analysis technique.
DATA SOURCES AND META-ANALYSIS
PubMed, Embase, Scopus, Cochrane, and Google were searched from 1965 to June 2009 for clinical trials on the efficacy of mebeverine vs placebo. The search terms were mebeverine, clinical trial, and IBS. No language restriction was applied. The reference list from retrieved articles was also reviewed for additional applicable studies.
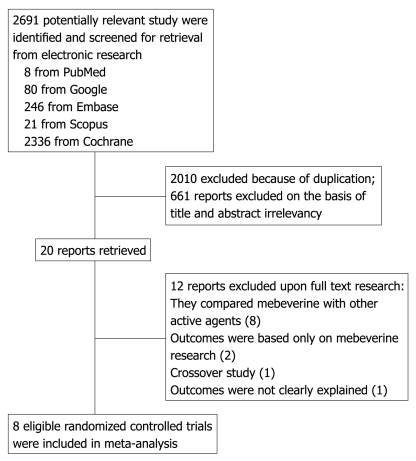
A total of 2691 results were examined and studies that were duplicates, case studies, and uncontrolled trials were eliminated. A high fiber diet or fiber supplementation with mebeverine was not considered a source of exclusion. Trials were disqualified if they compared mebeverine with other active agents, had not used a placebo, had used a combination of drugs, were crossover studies, and their outcomes did not relate to efficacy. Included studies used at least one clinical end point of “global assessment of symptoms by the patient or physician” or “abdominal pain and distention”. The definition of global response varied widely among studies. Some trials recorded improvement vs no improvement, whereas others evaluated the subject’s global assessment of relief. Responders in the included studies were patients who showed a global response according to the study’s definition. In studies lacking a global response definition, patients who showed global improvement in symptoms were included. Two reviewers independently extracted data on patients’ characteristics, therapeutic regimens, dosage, trial duration, and outcome measures. Disagreements, if any, were resolved by consensus. Among eight included studies, two compared mebeverine 135 mg with mebeverine 200 mg, and the remaining studies compared mebeverine with placebo (Figure 1).
Figure 1.
Flow diagram of the study selection process.
The methodological quality of included trials was assessed using the Jadad score, which judges the descriptions of randomization, blinding, and dropouts (withdrawals) in the trials[20] (Table 1). This is summarized as follow: (1) whether randomized or not (yes = 1 point, No = 0); (2) whether randomization was described appropriately or not (yes = 1 point, No = 0); (3) double blind (yes = 1 point, No = 0); (4) was the double blinding described appropriately (yes = 1 point, No = 0); and (5) whether withdrawals and dropouts described or not (yes = 1 point, No = 0). The quality scale ranges from 0 to 5 points with a low quality report of score 2 or less and a high quality report of score at least 3.
Table 1.
Jadad quality score of randomized, controlled trials included in the meta-analysis
| Study | Total score | Withdrawals and dropouts | Blinding | Randomization |
| Kruis et al[21] 1986 | 4 | 0 | 2 | 2 |
| Connell[13] 1965 | 5 | 1 | 2 | 2 |
| Tasman-Jones[22] 1973 | 4 | 0 | 2 | 2 |
| Berthelot et al[11] 1981 | 4 | 0 | 2 | 2 |
| Secco et al[19] 1983 | 4 | 0 | 2 | 2 |
| Enck et al[23] 2005 | 5 | 1 | 2 | 2 |
| Gilbody et al[24] 2000 | 4 | 1 | 2 | 1 |
| Inauen et al[25] 1994 | 3 | 1 | 0 | 2 |
Data from selected studies were extracted in the form of 2 × 2 tables. All included studies were weighted and pooled. The data were analyzed using Statsdirect (2.7.7; 9/13/2009). Relative risk (RR) and 95% confidence intervals (95% CI) were calculated using the Mantel-Haenszel and DerSimonian-Laird methods. The Cochran Q test was used to test heterogeneity. The event rate in the experimental (intervention) group against the event rate in the control group was calculated using L’Abbe plots as an aid to explore the heterogeneity of effect estimates. Funnel plot analysis was used as a publication bias indicator.
RESULTS
The electronic searches yielded 2691 items: eight from PubMed, 80 from Google, 246 from Embase, 21 from Scopus, and 2336 from Cochrane. Of these, 20 were scrutinized in full text, eight were considered eligible and had a well-defined global response outcome and were included in this analysis (Figure 1). The quality of the eligible studies was assessed by Jadad score. From eight studies, seven had Jadad scores ≥ 4[11,13,19,21-24] and the other study had a Jadad score of 3[25] (Table 1). These eight trials included 555 patients randomized to receive either mebeverine or placebo. 352 (63%) were women and 203 (37%) were men. All subtypes of IBS were represented. Abdominal pain was prevalent in only one study[24]. Patient’s characteristics, type, and dosage of mebeverine and placebo, duration of treatment, and outcomes (clinical improvement and the relief of abdominal pain) for each study are shown in Tables 2, 3, 4 and 5.
Table 2.
Characteristics of studies comparing mebeverine and placebo included in meta-analysis
| Study | Treatment duration (wk) |
Dosage |
IBS Subtype | Sex (F/M) |
Mean age (yr) |
||
| Placebo | Mebeverine | Placebo | Mebeverine | ||||
| Kruis et al[21] 1986 | 4-8-12-16 | Placebo open branch n = 40 | 100 mg qid n = 40 | All subtype | 23/17 | F = 43 | F = 43 |
| Wheat bran (12) | Wheat bran 15 g/d n = 40 | M = 41 | M = 41 | ||||
| Connell[13] 1965 | 12 | n = 20 | 400 mg/d n = 20 | All subtype | 25/15 | 40 | 40 |
| Tasman-Jones[22] 1973 | 4 | n = 12 | 400 mg/d n = 12 | All subtype | 14/10 | 43 | 43 |
| Berthelot et al[11] 1981 | 8 | n = 33 | 400 mg/d n = 36 | All subtype | 74/37 | 56 | 56 |
| Secco et al[19] 1983 | 4 | n = 15 | 400 mg/d n= 15 | All subtype | 15/15 | 45 | 45 |
| Enck et al[23] 2005 | 16 | Placebo n = 40 | n = 40 | All subtype | 43 | 36 | |
| Dietary treatment n = 40 | |||||||
Table 3.
Outcome results of studies comparing mebeverine with placebo included in meta-analysis
| Study |
Adverse effect |
Relief of abdominal pain |
Global or clinical improvement |
|||
| Placebo | Mebeverine | Placebo | Mebeverine | Placebo | Mebeverine | |
| Kruis et al[21] 1986 | - | - | 11/40 | 9/40 | 12/40 | 6/40 |
| Connell[13] 1965 | 3/22 | 2/22 | - | - | 1/22 | 11/22 |
| Tasman-Jones[22] 1973 | - | 7/24 | 15/24 | 7/24 | 15/24 | |
| Berthelot et al[11] 1981 | - | - | - | - | 24/33 | 31/36 |
| Secco et al[19] 1983 | - | - | 9/15 | 12/15 | - | - |
| Enck et al[23] 2005 | - | - | - | - | 16/40 | 8/40 |
Table 4.
Characteristics of studies comparing two dosage forms of mebeverine included in meta-analysis
| Study | Treatment duration (wk) |
Dosage |
IBS subtype | Sex (F/M) |
Mean age (yr) |
||
| Meb 200 mg bid | Meb 135 mg bid | Meb 200 mg | Meb 135 mg | ||||
| Gilbody et al[24] 2000 | 4-8 | n = 92 | n = 92 | Abdominal pain predominant | 142/42 | 34 | 32 |
| Inauen et al[25] 1994 | 3 | n = 24 | n = 24 | All subtype | 36/12 | 43 | 37 |
Meb: Mebeverine.
Table 5.
Outcome results of studies comparing two dosage forms of mebeverine included in meta-analysis
| Study |
Adverse effect |
Outcomes of therapeutic efficacy |
Relief of abdominal pain |
Global or clinical improvement |
||||
| Meb 200 mg | Meb 135 mg | Meb 200 mg | Meb 135 mg | Meb 200 mg | Meb 135 mg | Meb 200 mg | Meb 135 mg | |
| Gilbody et al[24] 2000 | 66/107 | 63/106 | 74/92 | 69/92 | 65/92 | 64/92 | 64/92 | 59/92 |
| Inauen et al[25] 1994 | No serious adverse effect | No serious adverse effects | 19/24 | 23/24 | 22/24 | 19/24 | ||
Efficacy of mebeverine compared to placebo
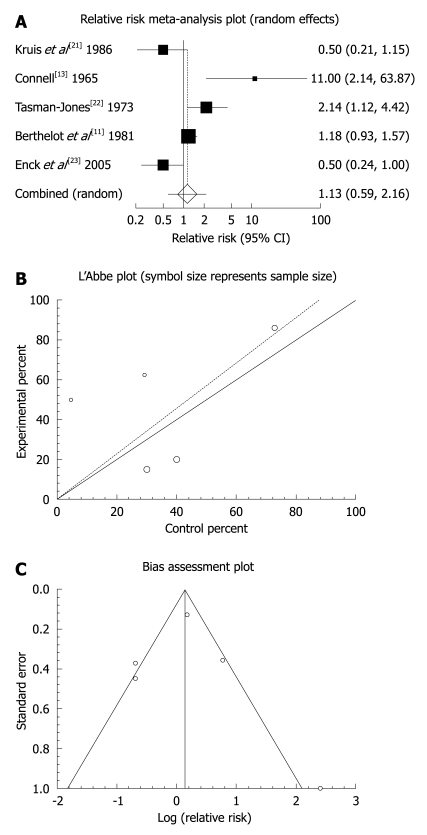
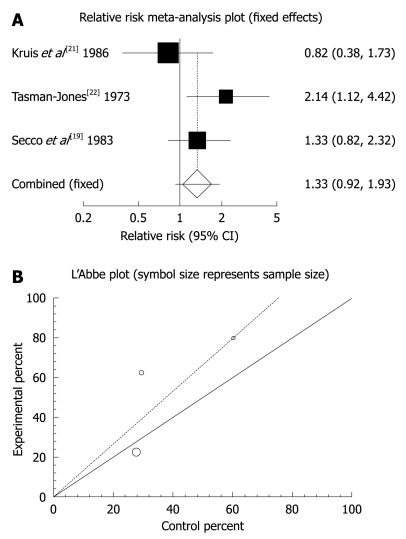
The summary RR for global or clinical improvement in five trials including[11,13,21-23] was 1.13 with a 95% CI of 0.59-2.16 and a non-significant RR (P = 0.7056, Figure 2A). The Cochrane Q test for heterogeneity indicated that the studies were heterogeneous (P = 0.0022, Figure 2B) and could not be combined, thus the random effects for individuals and summary of RR was applied. Regression of normalized effect vs precision for all included studies for clinical response among mebeverine vs placebo therapy was 0.217719 (95% CI: -5.538784 to 5.974221, P = 0.9118), and Kendall’s test on standardized effect vs variance indicated tau = 0.2, P = 0.8167 (Figure 2C). Summary RR for relief of abdominal pain in three trials[19,21,22] was 1.33 with a 95% CI of 0.92-1.93, a non-significant RR (P = 0.129, Figure 3A). The Cochrane Q test for heterogeneity indicated that the studies were homogenous (P = 0.1871, Figure 3B) and could be combined, thus fixed effects for individuals and summary of RR was applied. Regression of normalized effect vs precision for all included studies for clinical response among mebeverine vs placebo therapy could not be calculated because of too few strata.
Figure 2.
Individual and pooled relative risk (A), heterogeneity indicators (B), and publication bias indicators (C) for the outcome of “global or clinical improvement” in the studies comparing mebeverine vs placebo therapy.
Figure 3.
Individual and pooled relative risk (A) and heterogeneity indicators (B) for the outcome of “relief of abdominal pain” in the studies comparing mebeverine vs placebo therapy.
Tolerability of mebeverine compared to placebo
Adverse effects were rare or unknown in four of the six studies where mebeverine was compared to placebo. In two studies, 24% (15/62) of the mebeverine group and 22.5% (14/62) of the placebo group reported adverse effects[13,23].
Efficacy of mebeverine 200 mg compared to mebeverine 135 mg
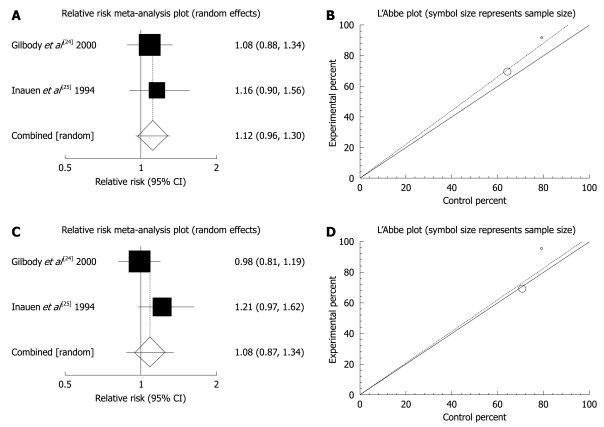
The summary RR for global or clinical improvement in two trials[24,25] was 1.12 with a 95% CI of 0.96-1.3 and a non-significant RR (P = 0.168, Figure 4A). The Cochrane Q test for heterogeneity indicated that the studies were homogenous (P = 0.6654, Figure 4B) and could be combined, but because of few included studies, the random effects for individuals and summary of RR was applied. Regression of normalized effect vs precision for all included studies for clinical response among mebeverine vs placebo therapy could not be calculated because of too few strata. Summary RR for relief of abdominal pain in two trials[24,25] was 1.08 with a 95% CI of 0.87-1.34, a non-significant RR (P = 0.463, Figure 4C). The Cochrane Q test for heterogeneity indicated that the studies were homogenous (P = 0.1398, Figure 4D) and could be combined, but because of few included studies, the random effects for individuals and summary of RR were applied. Regression of normalized effect vs precision for all included studies for clinical response among mebeverine vs placebo therapy could not be calculated because of too few strata.
Figure 4.
Individual and pooled relative risk and heterogeneity indicators for the outcome of “global or clinical improvement (A, B)” and “relief of abdominal pain (C, D)” in the studies considering mebeverine 200 mg compared to mebeverine 135 mg.
Tolerability of mebeverine 200 mg compared to mebeverine 135 mg
Of the two studies comparing mebeverine 200 mg to mebeverine 135 mg, only one of them reported adverse effects in about 61.5% (66/107) of the mebeverine 135 mg group and 59.5% (63/106) of the mebeverine 200 mg group[24]. The other study found no serious adverse effects in the trial[25].
DISCUSSION
The results of this meta-analysis demonstrate that the clinical improvement and relief of abdominal pain observed for mebeverine is not statistically significant compared to placebo.
It is well tolerated without any significant adverse effects. The meta-analysis also showed that mebeverine 200 mg is as effective as mebeverine 135 mg in the clinical improvement and relief of abdominal pain. The results also indicated no significant adverse effects for mebeverine 200 mg.
Although placebo effects in IBS clinical trials that measure a global outcome, are highly variable[26], the last meta-analysis on myorelaxants indicated that compounds like mebeverine are superior to placebo for global improvement of IBS and reducing pain. This drug showed significant efficacy on global assessment despite a high placebo effect (38% global improvement). The efficacy was also significant and in the same range for pain relief[9]. The present results are also consistent with a previous report that indicated low incidence of side effects[11].
Another systematic review on the safety and tolerability of antispasmodics in the treatment of IBS also confirmed the low incidence of adverse effects associated with mebeverine incidence (0.1-0.6 events per patient-year of exposure) and the investigators provided a favorable judgment regarding tolerance of mebeverine in dosages of both 600 mg and 400 mg[27,28]. Among other active agents for the treatment of IBS, probiotics can be used only as supplements of standard therapy. In addition, low doses of antidepressants induce clinical response and reduce abdominal pain score in patients with IBS[1,2]. Selective serotonin reuptake inhibitors (SSRIs) are often better tolerated than tricyclic antidepressants and have anxiety reducing benefits with a potential value in IBS[7,29]. Despite this, results of a recent meta-analysis showed that SSRIs overall are not significantly better than placebo for the relief of individual IBS symptoms[2]. Recent trials have demonstrated that alosetron, a 5-HT3 receptor antagonist, is effective in the treatment of IBS in non-constipated female vs placebo and vs mebeverine[7,30]. However, mebeverine could still be useful, particularly in treating males and constipated female with IBS, and it could diminish stool frequency or improve global feeling in diarrhea predominant IBS patients[9,31]. Thus it can be concluded that mebeverine is more effective than placebo in the management of diarrhea- or constipation-predominant IBS, without significant adverse effects.
Moreover, mebeverine 200 mg bid was shown to be therapeutically equivalent to mebeverine 135 mg tid in treatment of abdominal pain in IBS without a higher incidence of adverse effects. Studies also confirmed that both formulations of mebeverine were regarded as effective in more than 80% of cases. Tolerability was also excellent, with only few adverse effects and compliance close to 100% for most of patients. Of course, reducing the number of daily doses from three to two is an advantage of the mebeverine SR capsule in terms of patients’ compliance[24,25,32].
Fortunately, all the included studies in the present meta-analysis were well randomized, had acceptable Jadad scores, and included all subtypes of IBS (diarrhea predominant, constipation predominant, pain predominant and alternating). Some general limitations are unavoidable in meta-analyses, such as dissimilarities among patient characteristics (age, sex, lifestyle, and compliance), different duration of treatment, and different IBS subtypes; however, in this meta-analysis the high homogeneity of the included trials helped us to reach convincing conclusions. Of course, it would have been better to individualize patients based on IBS subtype and sex and evaluate outcomes for each subtype and gender, but it was not always applicable in the present study. Indeed, there is a need for more controlled, randomized trials considering the above-mentioned limitations.
CONCLUSION
Although the effects of mebeverine on clinical improvement and relief of abdominal pain are not statistically significant, it could be considered clinically effective until more studies are added to this meta-analysis to increase the power of the conclusions. Comparing doses, the mebeverine capsule (200 mg bid) is effective and well tolerated without significant adverse effects and, in terms of compliance, it could be considered as an appropriate form of dosage.
Footnotes
Peer reviewer: Dr. Shahab Abid, Associate Professor, Department of Medicine, Aga Khan University, Stadium Road, PO Box 3500, Karachi 74800, Pakistan
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM
References
- 1.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi R, Nikfar S, Abdollahi M. Selective serotonin reuptake inhibitors for the management of irritable bowel syndrome: A meta-analysis of randomized controlled trials. Arch Med Sci. 2008;4:71–76. [Google Scholar]
- 3.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salari P, Abdollahi M. Current opinion in the pharmaceutical management of irritable and inflammatory bowel diseases: Role of ATP. Recent Pat Endocr, Metab Immune Drug Discovery. 2009;3:69–75. [Google Scholar]
- 5.Mathew P, Bhatia SJ. Pathogenesis and management of irritable bowel syndrome. Trop Gastroenterol. 2009;30:19–25. [PubMed] [Google Scholar]
- 6.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 7.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ. Drug therapy options for patients with irritable bowel syndrome. Am J Manag Care. 2001;7:S261–S267. [PubMed] [Google Scholar]
- 9.Poynard T, Naveau S, Mory B, Chaput JC. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:499–510. doi: 10.1111/j.1365-2036.1994.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie JA, Truelove SC. Comparison of various treatments for irritable bowel syndrome. Br Med J. 1980;281:1317–1319. doi: 10.1136/bmj.281.6251.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berthelot J, Centonze M. Controlled double blind trial of Duspatalin (mebeverine) against placebo, in the treatment of irritable colon. Gaz Med de France. 1981;85:2341–2343. [Google Scholar]
- 12.Prout BJ. The treatment of irritable bowel syndrome. Two doses of mebeverine compared. Practitioner. 1983;227:1607–1608. [PubMed] [Google Scholar]
- 13.Connell AM. Physiological and clinical assessment of the effect of the musculotropic agent mebeverine on the human colon. Br Med J. 1965;2:848–851. doi: 10.1136/bmj.2.5466.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baume P. Mebeverine, an effective agent in the irritable colon syndrome. Aust N Z J Med. 1972;2:34–36. doi: 10.1111/j.1445-5994.1972.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 15.Eisenburg J, Kruis W, Schüssler P, Weinzierl M. [The irritable colon syndrome. New therapeutic possibilities in the treatment of a frequent syndrome] Fortschr Med. 1978;96:2064–2070. [PubMed] [Google Scholar]
- 16.Hürzeller Castamer JC. Functional colitis: A multi centre study with mebeverine in 380 patients. Der Inf Arzl. 1986;2:54–56. [Google Scholar]
- 17.Liem KS, Timmermans HS. Final report on a double-blind placebo controlled tolerance study of mebeverine in healthy volunteer subjects. 1973, Duphar Report No. 56638/1482/7. [Google Scholar]
- 18.Lüttecke K. A three-part controlled study of trimebutine in the treatment of irritable colon syndrome. Curr Med Res Opin. 1980;6:437–443. doi: 10.1185/03007998009109464. [DOI] [PubMed] [Google Scholar]
- 19.Secco GB, Di Somma C, Arnulfo G, Ricci C. [Controlled clinical study of the action of mebeverine hydrochloride in the treatment of irritable colon] Minerva Med. 1983;74:699–702. [PubMed] [Google Scholar]
- 20.Jadad A. Randomized controlled trials. London: BMJ Books; 1998. [Google Scholar]
- 21.Kruis W, Weinzierl M, Schüssler P, Holl J. Comparison of the therapeutic effect of wheat bran, mebeverine and placebo in patients with the irritable bowel syndrome. Digestion. 1986;34:196–201. doi: 10.1159/000199329. [DOI] [PubMed] [Google Scholar]
- 22.Tasman-Jones C. Mebeverine in patients with the irritable colon syndrome: double blind study. N Z Med J. 1973;77:232–235. [PubMed] [Google Scholar]
- 23.Enck P, Klosterhalfen S, Kruis W. [Determination of placebo effect in irritable bowel syndrome] Dtsch Med Wochenschr. 2005;130:1934–1937. doi: 10.1055/s-2005-872605. [DOI] [PubMed] [Google Scholar]
- 24.Gilbody JS, Fletcher CP, Hughes IW, Kidman SP. Comparison of two different formulations of mebeverine hydrochloride in irritable bowel syndrome. Int J Clin Pract. 2000;54:461–464. [PubMed] [Google Scholar]
- 25.Inauen W, Halter F. Clinical efficacy, safety and tolerance of mebeverine slow release (200 mg) vs. mebeverine tablets in patients with irritable bowel syndrome. Drug Invest. 1994;8:234–240. [Google Scholar]
- 26.Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–340. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 27.Heading R, Bardhan K, Hollerbach S, Lanas A, Fisher G. Systematic review: the safety and tolerability of pharmacological agents for treatment of irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:207–236. doi: 10.1111/j.1365-2036.2006.02937.x. [DOI] [PubMed] [Google Scholar]
- 28.Boisson J, Coudert PH, Dupuis J, Laverdant CH, Toulet J. Long term tolerance of mebeverine. Actual Cardiol Angeiol Int (Paris) 1987;16:289–292. [Google Scholar]
- 29.Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig Dis Sci. 2008;53:108–115. doi: 10.1007/s10620-007-9830-4. [DOI] [PubMed] [Google Scholar]
- 30.Jones RH, Holtmann G, Rodrigo L, Ehsanullah RS, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:1419–1427. doi: 10.1046/j.1365-2036.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 31.Lu CL, Chen CY, Chang FY, Chang SS, Kang LJ, Lu RH, Lee SD. Effect of a calcium channel blocker and antispasmodic in diarrhoea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2000;15:925–930. doi: 10.1046/j.1440-1746.2000.02230.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Outryve M, Mayeur S, Meeus MA, Rosillon D, Hendrickx B, Ceuppens M. A double-blind crossover comparison study of the safety and efficacy of mebeverine with mebeverine sustained release in the treatment of irritable bowel syndrome. J Clin Pharm Ther. 1995;20:277–282. doi: 10.1111/j.1365-2710.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]