Abstract
AIM: To evaluate whether crypt abscesses from inflammatory bowel disease (IBD) patients contain bacteria and to establish their nature.
METHODS: We studied 17 ulcerative colitis patients, 11 Crohn’s disease patients, 7 patients with acute self-limited colitis (ASLC) and normal colonic biopsies from 5 subjects who underwent colonoscopy for colon cancer screening. A fluorescent in situ hybridization technique was applied to colonic biopsies to assess the microbiota composition of the crypts and crypt abscesses.
RESULTS: Crypts colonized by bacteria were observed in 42.9% and 3.6% of ASLC and IBD patients, respectively (P = 0.019). Crypt abscesses colonized by bacteria were observed in 28.6% and 0.0% of ASLC and IBD patients, respectively (P = 0.035).
CONCLUSION: These results do not support the hypothesis that crypt abscesses in IBD are the result of localized dysbiosis arising from persistence of living bacteria colonizing the crypts.
Keywords: Inflammatory bowel diseases, Crohn’s disease, Ulcerative colitis, Crypt abscess, Microbiota
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are the 2 most common types of inflammatory bowel disease (IBD). Although their pathophysiology is still unknown, the gut microbiota is considered to play a crucial role[1]. The microbiota close to the mucosa differs from the luminal microbiota[2]. We and others described a luminal and mucosal dysbiosis in IBD[3-8] including a high proportion of unusual bacteria[6,8] and a restricted microbial biodiversity[7,9]. Nevertheless, no difference in the dominant microbiota was observed between inflamed and non-inflamed mucosa in CD and UC[5,10].
It would be of great importance to establish the early events leading to IBD onset or to perpetuation of inflammation. Crypt abscesses are early lesions observed in IBD, particularly in UC[11], consisting of dilated crypts containing polymorphonuclear cells. They can also occur in acute self limited colitis (ASLC)[12], collagenous and lymphocytic colitis[13], diverticula-associated colitis[14] and diversion colitis[15]. Defensins are antimicrobial peptides secreted in intestinal and colonic crypts and recent studies pointed out a defensin secretion defect in IBD patients[16-20]. The aim of this study was to evaluate if crypt abscesses in UC, CD and ASLC patients contained bacteria and, if so, to establish their nature i.e. investigate localized dysbiosis in this specific ecosystem.
MATERIALS AND METHODS
Patients
We studied 35 patients with acute colitis and crypt abscesses at histological examination of colon biopsy: 17 UC patients, 11 CD patients and 7 patients with ASLC (bacteria involved: Shigella sonnei, Campylobacter sp. and no identified pathogenic bacteria in the 5 other cases). We also analyzed normal colonic biopsies from 5 subjects who underwent colonoscopy for colon cancer screening. The characteristics of the patients are described in Table 1. Rectocolonic biopsies containing crypt abscesses were analyzed.
Table 1.
Characteristics of the patients
| UC | CD | ASLC | |
| n | 17 | 11 | 8 |
| Male (%) | 47.1 | 54.5 | 50.0 |
| Mean age (± SE, yr) | 42.0 ± 3.3 | 44.0 ± 3.9 | 45.6 ± 10.5 |
| Mean disease duration (± SE, mo) | 75 ± 23 | 50 ± 27 | NA |
| Montreal classification (%) | |||
| E1 | 0 | NA | NA |
| E2 | 70.6 | NA | NA |
| E3 | 29.4 | NA | NA |
| L1 | NA | 0 | NA |
| L2 | NA | 63.6 | NA |
| L3 | NA | 36.4 | NA |
| Analyzed segment (%) | |||
| Rectum | 64.7 | 36.4 | 71.4 |
| Left colon | 35.3 | 45.4 | 14.3 |
| Right colon | 0.0 | 18.2 | 14.3 |
| Treatment (%) | |||
| 5-aminosalicylic acid | 35.3 | 18.2 | 0.0 |
| Corticosteroids | 35.3 | 27.3 | 0.0 |
| Azathioprine | 17.6 | 18.2 | 0.0 |
| Infliximab | 0.0 | 18.2 | 0.0 |
UC: Ulcerative colitis; CD: Crohn’s disease; ASLC: Acute self-limited colitis; NA: Not available.
Tissues, histological examination and fluorescent in situ hybridization (FISH)
Histological examination and assessment of the bacterial composition of the crypts and crypt abscess microbiota were performed as previously described using FISH with one general probe (Eubacteria) and 6 group-specific probes, [Bacteroides-Prevotella, γ Proteobacteria, Bifidobacterium, Clostridium coccoides, Faecalibacterium prausnitzii (F. prausnitzii) and Lactobacillus-Enterococcus][21].
Histological examination: Colonic biopsy sections were deparaffined in xylene and successively rehydrated for 3 min in 100%, 96%, and 70% ethanol. They were then stained with hematoxylin and eosin for morphological assessment.
FISH: Prior to FISH, sections were deparaffinized, rehydrated, and postfixed in 4% paraformaldehyde for 5 min. Fixation was stopped in phosphate-buffered saline (PBS) 3 × and slides were washed twice for 1 min in PBS 1 ×. Tissue sections were incubated 10 min at room temperature with Tris-EDTA buffer containing 10 mg/mL of lysozyme and then washed using the hybridization solution (0.9 mol/L NaCl, 20 mmol/L Tris HCl, pH 8, 0.01% SDS, 30% formamide). Fixed tissue sections were then hybridized with the previous hybridization solution containing 4.5 ng/μL of one of the 5’-end-Cy3-labeled 16S rRNA targeted oligonucleotide probes. Hybridizations were performed at 35°C overnight in a microscope slide incubator and stringent washings were carried out at 37°C (2 × 15 min) in a buffer containing 65 mmol/L NaCl, 20 mmol/L Tris HCl, pH 8.0, 5 mmol/L EDTA, and 0.01% SDS to remove nonspecific binding. The sections were mounted with Vectashield [mounting medium with 4’,6’-diamidino-2-phenylindole (DAPI), Vector Laboratories, Burlingame, CA, USA]. DNA was stained with DAPI to visualize all cells.
Detection of crypt abscess-associated-bacteria
Bacteria were visualized with an epifluorescence microscope Leica DMRB using Cy3- and DAPI-specific filters at 100 ×, 400 ×, and 1000 × magnification and images were captured with Leica DFC 300 FX camera and FW 4000 software (Leica microsystemes SAS, Rueil-Malmaison, France). The entire mucosal surface (crypts and crypts abscess) of each colonic biopsy section was examined for the presence of bacteria. Pure cultured bacteria belonging to each group were hybridized as positive and negative controls for the FISH procedure.
Statistical analysis
We first performed a “patient analysis” considering that a patient had colonized crypts or crypt abscesses if at least one of his crypts or crypt abscesses was colonized by bacteria. As colonic biopsies harbored a different number of crypts or crypt abscesses, we also normalized the data by calculating in each patient group the ratio: total number of colonized crypts or crypt abscesses/total number of crypts or crypt abscesses. The χ2 test was performed for comparison of qualitative variables.
RESULTS
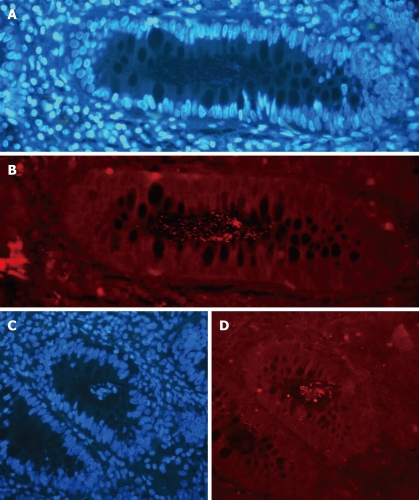
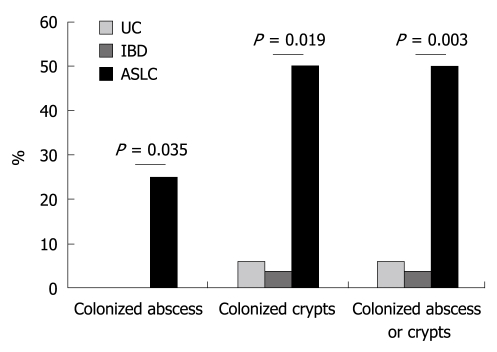
The FISH technique allowed detection of crypts and crypt abscesses colonized by bacteria as shown in Figure 1. Crypts colonized by bacteria (general probe targeting Eubacteria) were observed in 42.9% and 3.6% of ASLC and IBD patients (only UC patients), respectively (P = 0.019, Figure 2). No colonized crypt was observed in biopsies from CD patients. Crypt abscesses colonized by bacteria were observed in 2 patients with ASLC (28.6% of total ASLC patients) and in no biopsy from the 28 IBD patients (P = 0.035, Figure 2). Neither colonized crypts nor crypt abscesses were observed in colonic biopsies from control patients.
Figure 1.
Colonized crypt (A, B) and colonized crypt abscess (C, D). A, C: 4’,6’-diamidino-2-phenylindole (DAPI); B, D: Hybridization with the Eubacteria probe.
Figure 2.
Proportion of patients with at least one colonized crypt abscess, one colonized crypt or at least one crypt abscess or one crypt colonized by bacteria. UC: Ulcerative colitis; IBD: Inflammatory bowel disease; ASLC: Acute self-limited colitis.
Global and normalized analysis
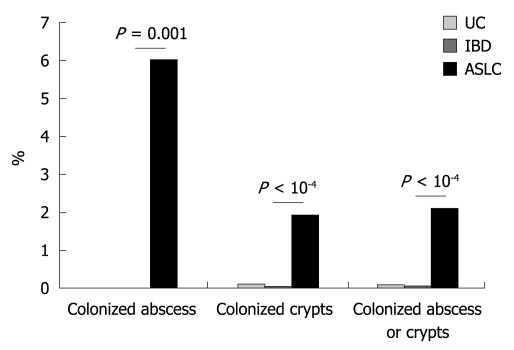
We observed 2317, 2499 and 2405 crypts in UC, CD and ASLC groups, respectively. None were colonized by bacteria (general probe for Eubacteria) in CD patients whereas 2 (0.09%) and 46 (1.91%) were colonized in UC and ASLC patients, respectively (Figure 3). We observed 121, 76 and 100 crypt abscesses in UC, CD and ASLC patients, respectively. Among these, 6 were colonized by bacteria in ASLC patients (6.0%) and none in UC and CD patients (Figure 3).
Figure 3.
Proportion of crypt abscesses and crypts harboring bacterial colonization.
Group/species-specific probe analysis
In order to determine what kind of bacteria was present in colonized crypts and crypt abscesses, we performed FISH analysis using 6 group- or species-specific probes on the sample previously identified to harbor colonized crypts or crypt abscesses. No fluorescent signal was detected in any of the crypt abscesses analyzed with the 6 specific probes. No bacteria-colonizing crypts were recognized by the F. prausnitzii or the Lactobacillus-Enterococcus probes. The 2 colonized crypts in UC patients contained Enterobacteria (detected by the gamma Proteobacteria probe) whereas 39.1%, 8.7%, 4.3% and 2.2% of the colonized crypts in ASLC patients contained bacteria from the Bacteroides (Bacteroides-Prevotella probe), the Clostridium coccoides, the Enterobacteria and the Bifidobacteria groups, respectively.
DISCUSSION
This study shows for the first time that crypt abscesses from IBD patients do not contain bacteria (aseptic abscess); colonic crypts of these patients are less often colonized than those from ASLC patients. These results do not support the hypothesis of the role of localized dysbiosis in the pathogenesis of crypt abscesses.
Dysbiosis is involved in the perpetuation of inflammation in IBD but it is presently not known whether it occurs early in the disease process and whether a localized dysbiosis in a specific ecological niche could trigger early inflammatory events. No previous study searched for bacteria inside crypt abscess in IBD or in other conditions. Swidsinski et al[22] also looked at colonic crypts and found bacteria inside the crypts more frequently in IBD patients than in control subjects without inflammation or infection. In the current study, we did not find any colonized crypts in healthy subjects, and colonized crypts in IBD patients were infrequent. The differences between these 2 studies could arise from the population studied but also from the method used for the fixation of the colon biopsy. Indeed Swidsinski et al[22] used a nonaqueous Carnoy solution which focuses at preserving the mucus layer on the surface epithelium while we used 4% buffered formalin. Nevertheless, in our hands, nonaqueous Carnoy solution and formalin gave similar results (unpublished data).
One can hypothesize that the results may be different in a cohort of newly diagnosed patients without any antiinflammatory or immunomodulatory therapy. It is not possible to address this question. Nevertheless, the fact that 53.6% of the IBD patients in our cohort received no treatment or only 5-aminosalicyclic acid, and that in 36% the IBD diagnosis was performed in the previous year suggested that the results would be similar in naive patients.
In ASLC, pathogenic bacteria induce an acute and transient breakdown of the intestinal barrier. On the other hand, many pathogenic bacteria involved in ASLC developed a strategy to escape the host immune system[23,24]. Alteration of the intestinal barrier could lead to deep penetration of gut microbiota bacteria (and also pathogenic bacteria) into the crypts where they could proliferate. In the current study, bacteria found in crypts from ASLC patients belonged to various phylogenetic bacterial groups of the normal gut microbiota, suggesting a non-specific intestinal barrier breakdown in this pathology, allowing the penetration of any bacteria in the crypts.
Our results do not support the hypothesis that crypt abscesses in IBD are the result of persistent localized dysbiosis with a focused reaction against high numbers of living bacteria specifically colonizing the crypts. On the other hand, the absence of entire bacteria in crypt abscesses does not rule out the implication of bacteria in their onset as one may hypothesize that bacteria could stimulate the genesis of crypt abscesses, with recruitment of polymorphonuclear leukocytes which would then destroy them but also contribute to chronic epithelial lesions. The dysbiosis in IBD seems to affect the surface of the whole mucosa (diffuse mucosal dysbiosis) and more studies should be performed to understand its specificity and the ways to influence it.
COMMENTS
Background
Ulcerative colitis (UC) and Crohn’s disease (CD) are the 2 most common types of inflammatory bowel disease (IBD). Although their pathophysiology is still unknown, the gut microbiota is considered to play a crucial role. The microbiota close to the mucosa differs from the luminal microbiota. We and others described a luminal and mucosal dysbiosis in IBD. Nevertheless, no difference in the dominant microbiota was observed between inflamed and non-inflamed mucosa in CD and UC.
Research frontiers
It would be of great importance to establish the early events leading to IBD onset or to perpetuation of inflammation. Crypt abscesses are early lesions observed in IBD, particularly in UC, but they can also occur in acute self limited colitis. The aim of this study was to search for a localized dysbiosis in crypt abscesses from UC, CD and acute self-limited colitis patients.
Innovations and breakthroughs
The results do not support the hypothesis that crypt abscesses in IBD are the result of localized dysbiosis arising from persistence of living bacteria colonizing the crypts.
Terminology
Dysbiosis: Breakdown in the balance between putative species of ‘‘protective’’ vs ‘‘harmful’’ intestinal bacteria.
Peer review
This is an interesting and original study, which attempts to characterize dysbiosis associated with cryptitis and crypt abscesses in patients with early IBD. The results suggest that these lesions are in fact sterile.
Footnotes
Supported by The Association Francois Aupetit
Peer reviewers: Dr. John K Marshall, Associate Professor of Medicine, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada; Dr. Daniel R Gaya, Gastrointestinal Unit, Molecular Medicine Centre, School of Molecular and Clinical Medicine, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh EH4 2XU, United Kingdom
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, Pochart P, Doré J, Marteau P. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- 2.Lepage P, Seksik P, Sutren M, de la Cochetière MF, Jian R, Marteau P, Doré J. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis. 2005;11:473–480. doi: 10.1097/01.mib.0000159662.62651.06. [DOI] [PubMed] [Google Scholar]
- 3.Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis. 2008;14:858–867. doi: 10.1002/ibd.20392. [DOI] [PubMed] [Google Scholar]
- 4.Lepage P, Seksik P, De La Cochetiere MF, Sutren M, Marteau P, Jian R, Dore J. Molecular study of the ileal and colonic mucosa associated flora in IBD. Gastroenterology. 2003;124:A477–A478. [Google Scholar]
- 5.Seksik P, Lepage P, de la Cochetière MF, Bourreille A, Sutren M, Galmiche JP, Doré J, Marteau P. Search for localized dysbiosis in Crohn's disease ulcerations by temporal temperature gradient gel electrophoresis of 16S rRNA. J Clin Microbiol. 2005;43:4654–4658. doi: 10.1128/JCM.43.9.4654-4658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol H, Lepage P, Seksik P, Doré J, Marteau P. Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative colitis. J Clin Microbiol. 2006;44:3172–3177. doi: 10.1128/JCM.02600-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 9.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol H, Lepage P, Seksik P, Doré J, Marteau P. Molecular comparison of dominant microbiota associated with injured versus healthy mucosa in ulcerative colitis. Gut. 2007;56:152–154. doi: 10.1136/gut.2006.109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Berre N, Heresbach D, Kerbaol M, Caulet S, Bretagne JF, Chaperon J, Gosselin M, Ramée MP. Histological discrimination of idiopathic inflammatory bowel disease from other types of colitis. J Clin Pathol. 1995;48:749–753. doi: 10.1136/jcp.48.8.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar NB, Nostrant TT, Appelman HD. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis) Am J Surg Pathol. 1982;6:523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Ayata G, Ithamukkala S, Sapp H, Shaz BH, Brien TP, Wang HH, Antonioli DA, Farraye FA, Odze RD. Prevalence and significance of inflammatory bowel disease-like morphologic features in collagenous and lymphocytic colitis. Am J Surg Pathol. 2002;26:1414–1423. doi: 10.1097/00000478-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 14.West AB, Losada M. The pathology of diverticulosis coli. J Clin Gastroenterol. 2004;38:S11–S16. doi: 10.1097/01.mcg.0000124005.07433.69. [DOI] [PubMed] [Google Scholar]
- 15.Komorowski RA. Histologic spectrum of diversion colitis. Am J Surg Pathol. 1990;14:548–554. doi: 10.1097/00000478-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehkamp J, Schmid M, Stange EF. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:370–378. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- 20.Fahlgren A, Hammarstrom S, Danielsson A, Hammarstrom ML. beta-Defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin Exp Immunol. 2004;137:379–385. doi: 10.1111/j.1365-2249.2004.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez N, Mangin I, Lepage P, Seksik P, Duong JP, Blum S, Schiffrin E, Suau A, Allez M, Vernier G, et al. Patchy distribution of mucosal lesions in ileal Crohn's disease is not linked to differences in the dominant mucosa-associated bacteria: a study using fluorescence in situ hybridization and temporal temperature gradient gel electrophoresis. Inflamm Bowel Dis. 2007;13:684–692. doi: 10.1002/ibd.20084. [DOI] [PubMed] [Google Scholar]
- 22.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braat H, McGuirk P, Ten Kate FJ, Huibregtse I, Dunne PJ, Hommes DW, Van Deventer SJ, Mills KH. Prevention of experimental colitis by parenteral administration of a pathogen-derived immunomodulatory molecule. Gut. 2007;56:351–357. doi: 10.1136/gut.2006.099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallon PG, Alcami A. Pathogen-derived immunomodulatory molecules: future immunotherapeutics? Trends Immunol. 2006;27:470–476. doi: 10.1016/j.it.2006.08.002. [DOI] [PubMed] [Google Scholar]