Abstract
AIM: To investigate if and how programmed death type-1 (PD-1) expression affects the natural course of hepatitis B virus (HBV) infection.
METHODS: Sixty-four patients in different natural stages of chronic HBV infection were enrolled in this study. PD-1 expression in total T cells was detected by flow cytometry. Levels of total CD8+ T cell responses and proliferation in relation to PD-1 expression levels were analyzed with intracellular staining and PD-1/PD-L1 blockage.
RESULTS: The PD-1 expression in T cells was dynamically changed during the natural course of chronic HBV infection, did not significantly increase in the immune tolerance phase, and returned to normal in the inactive virus carrier stage. Blockage of the PD-1/PD-L1 pathway could not affect the T-cell response in the immune tolerance and inactive virus carrier stages of chronic HBV infection. However, it could significantly restore the T-cell response in the immune clearance stage of chronic HBV infection. Furthermore, the PD-1 expression level in T cells was associated with the alanine aminotransferase level during the immune clearance stage of chronic HBV infection.
CONCLUSION: The PD-l/PD-L1 pathway plays a different role in T-cell response during the natural course of chronic HBV infection.
Keywords: Programmed death type-1, Hepatitis B virus, Chronic hepatitis B, Natural stage, CD8+ T cell, Serum viral load, Programmed death ligand, T cell response
INTRODUCTION
During chronic hepatitis B virus (HBV) infection, a dynamic balance between viral replication and host immune response is pivotal to the pathogenesis of liver disease. In accordance with immune characteristics, chronic HBV infection can be clinically categorized into three periods, namely immune tolerance phase, immune clearance phase, and immune stable phase or inactive virus carrier phase[1,2]. It has been widely accepted that adaptive immune responses, particularly cellular immune responses, mediate the clearance of HBV[3-6]. Unfortunately, HBV-specific T-cell function is impaired in patients with chronic HBV infection characterized by low levels of antiviral cytokines, impaired cytotoxic T lymphocyte activity, and persistent viremia[7,8]. However, the mechanism underlying this T-cell malfunction in chronic HBV infection has not been completely understood[9].
The immunologic receptor programmed death type-1 (PD-1), a 55 kDa transmembrane protein containing an immunologic receptor tyrosine-based inhibitory motif, was originally isolated from a T-cell line exhibiting a high sensitivity to apoptosis[10]. The PD-1/PD-L1 pathway has been well documented to play a negative role in the regulation of activation and proliferation of T-cells and production of cytokines[11-13]. There is evidence that the PD-1 pathway plays an important role in inhibiting the function of virus-specific CD8+ T-cells in chronic viral infection involving human immunodeficiency virus (HIV)[14-16], hepatitis C virus (HCV)[17,18], and HBV[19].
Although reports are available on the changes in expression levels of PD-1 and T-cell responses in patients with HBV infection[20], the change pattern of PD-1 expression in the natural course of chronic HBV infection has not yet been presented. Understanding such changes in PD-1 expression and T-cell responses in the course of chronic HBV infection is crucial in the management of HBV carriers. For this reason, we analyzed the PD-1 expression in T cells and tested the role of the PD-1/PD-L1 pathway in the regulation of T-cell response during different stages of chronic HBV infection. Our results suggest that activated PD-1 signaling is closely related with T-cell malfunction in the immune clearance phase but not in the other two phases of chronic HBV infection.
MATERIALS AND METHODS
Subjects
Sixty-four patients with chronic HBV infection (53 males and 11 females), enrolled in this study, were positive for HBsAg and anti-HBc but negative for antibodies (Abs) to HCV, delta virus (HDV), HIV-1 and -2, and other symptoms of chronic liver damage. The patients were observed for more than 48 wk during which liver function and serum DNA level were tested once every 3 mo. Of the 64 patients, 9 were in the immune tolerance stage with the presence of HBeAg, high serum DNA level, normal serum alanine aminotransferase (ALT) and minimal or no evident inflammation on liver biopsy, 10 were in the inactive virus carrier stage and negative for HBeAg and positive for anti-HBe antibody with undetectable or low HBV DNA level, and 45 were in the immune clearance phase with persistent elevated serum ALT level and positive serum HBV DNA. The morphology of liver was examined by ultrasonography or computerized tomography, which showed no radiologic or histological evidence of cirrhosis. None of the patients received antiviral therapy and/or immune regulate therapy before they were admitted (clinical information is listed in Table 1). Twelve healthy blood donors served as normal controls.
Table 1.
Demographic and clinical parameters of 4 groups
| Subjects |
Sex |
Age (yr) | ALT (U/L) | eAg (+) |
HBV DNA |
||||
| Male | Female | ND | < 103 | 103-107 | > 107 | ||||
| NC | 8 | 4 | 23.5 ± 4.5 | 24.5 ± 9.7 | - | 12 | - | - | - |
| Immune tolerance | 7 | 2 | 26.0 ± 6.4 | 42.4 ± 28.9 | 9 | 0 | 0 | 2 | 7 |
| Immune clearance | 37 | 8 | 33.3 ± 8.8 | 298.0 ± 289.4 | 33 | 0 | 0 | 25 | 20 |
| Inactive carrier | 9 | 1 | 39.1 ± 12.1 | 23.8 ± 10.7 | 0 | 0 | 10 | 0 | 0 |
NC: Normal control; ND: Not done; ALT: Alanine aminotransferase; HBV: Hepatitis B virus.
All the patients and normal controls were Chinese. Our study was approved by the local ethics committee, and all patients provided their written informed consent.
Virology assessment
HBsAg, HBeAg, anti-HBs, anti-HBc, anti-HBe, and antibodies to HCV, HDV, HIV-1, and HIV-2 were detected by enzyme linked immunosorbent assay with commercially available kits (Sino-American Biotechnology Company, SABC). Serum HBV-DNA level was measured by fluorescent quantitative PCR with commercially available kits (PE/B/MJ/L, Shenzhen, China).
Isolation of peripheral blood mononuclear cells
EDTA- and heparin-anticoagulated blood (5-7 mL) was collected from each patient and used either directly for fluorescence-activated cell sorting (FACS) or for peripheral blood mononuclear cell (PBMC) isolation. PBMC (2 × 106-6 × 106) were isolated by Ficoll-Hypaque density gradient centrifugation, washed twice in phosphate-buffered saline, and analyzed immediately.
Flow cytometry
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, and allophycocyanin (APC)-labeled monoclonal antibodies, respectively, according to their manufacturers’ instructions. Flow cytometry was performed using FACSCalibur (Becton Dickinson, San Jose, CA). FACS data were analyzed using CellQuest software (Becton Dickinson Rutherford, NJ). The PE-labeled anti-PD-1 monoclonal antibodies were obtained from BD PharMingen (BD Biosciences, San Jose, CA). FITC-anti-CD4 and APC-anti-CD8 antibodies were purchased from eBioscience (San Diego, CA).
PD-1/PD-L1 blockage
Fresh PBMC (1 × 105-5 × 105) isolated from patients with chronic HBV infection were incubated for 45 min at 37°C with anti-PD-L1 (10 g/mL) or isotype control antibody (IgG2b clone MPC 11; 10 μg/mL e-Bioscience, Boston, MA) or without anything, washed and co-incubated with the Dybeads CD3/CD28 T-cell expander (4 × 107/mL, Invitrogen) for 3 d. On the third day of incubation, the cells were stained with surface antibodies, perforin, and granzyme B, respectively, for flow cytometry analysis.
Intracellular perforin and granzyme B staining
In vitro expanded cells (0.2 × 106-0.3 × 106) were stained with anti-CD8 APC mAb (BD Biosciences) before they were fixed and permeabilized (Cytofix-Cytoperm by BD Biosciences) for intracellular staining (ICS) with anti-perforin-FITC (BD Biosciences) or anti-GRZ-A-FITC (BD Biosciences) mAbs.
Proliferation of CD8 T cells
Freshly isolated peripheral lymphocytes were re-suspended at the concentration of 1 × 106 cells/mL in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (R10; Invitrogen) and stimulated with the Dybeads CD3/CD28 T cell expander (4 × 107 cells/mL, Invitrogen), with or without anti-PD-L1 Mab (10 g/mL) or isotype control antibody (IgG2b clone MPC 11; 10 μg/mL e-Bioscience, Boston, MA). On day 3, the cells re-suspended in 300 μL PBS were double-stained with anti-CD8-FITC and 7-amino-actinomycin D. Cellular data were acquired for analysis. The total number of cells in each well was calculated according to the following formula: Total number of cells = (number of live cells/ number of beads) × 105.
Statistical analysis
Wilcoxon matched paired test and Mann-Whitney test of SSPS 12.0 were used to assess the difference between different groups. Spearman correlation analysis of PD-1 expression and HBV viral titers or ALT level was performed. P < 0.05 was considered statistically significant. The test of significance was two sided.
RESULTS
PD-1 expression in CD8+ T cells during the natural course of chronic HBV infection
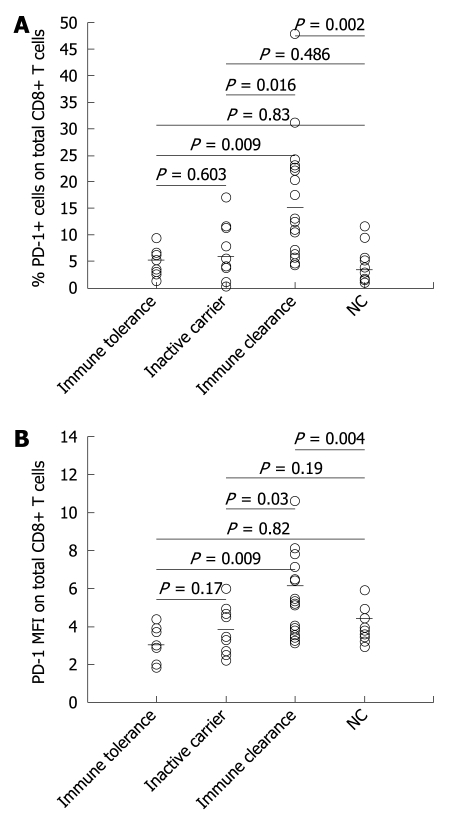
To determine the surface expression of PD-1 in total peripheral CD8+ T cells during the natural course of chronic HBV infection, the total number of peripheral T cells of 39 patients (9 in the immune tolerance phase, 10 in the inactive virus carrier phase, and 20 in the immune clearance phase) were analyzed by flow cytometry. The PD-1 expression levels in CD8+ T cells in the immune tolerance and inactive virus carrier stages of chronic HBV infection patients did not significantly differ from those of the normal controls. However, the PD-1 expression levels were significantly higher in the immune clearance stage of chronic HBV infection patients than in normal controls and in the other two natural stages of chronic HBV infection patients (Figure 1).
Figure 1.
Programmed death type-1 (PD-1) expression in total peripheral CD8+ T cells in the natural stages of chronic hepatitis B virus (HBV) infection. A: The dot plots showing the percentage of PD-1+ CD8+ T cells in different natural stages of chronic HBV infection patients and normal controls and the horizontal bars indicating the median percentage of positive PD-1 in CD8+ T cells; B: The dot plots showing the PD-1 mean fluorescence intensity (MFI) expression in total CD8+ T cells, and the horizontal bars indicating the median PD-1 MFI expression level in CD8+ T cells. NC: Normal control.
PD-1 expression level was positively correlated with serum ALT level in the immune clearance stage of chronic HBV infection
The PD-1 expression level was significantly higher in the immune clearance stage of chronic HBV infection patients than in normal controls and in the other two natural stages of chronic HBV infection patients.
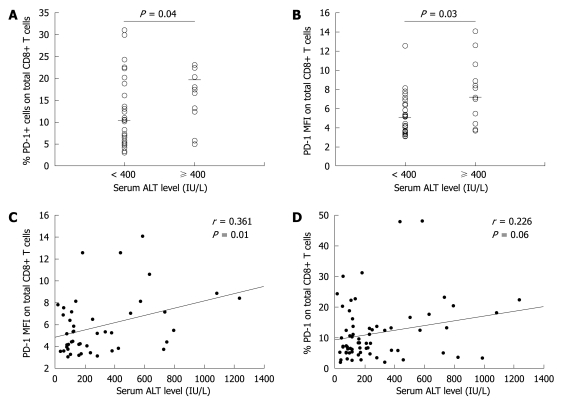
The patients were divided into two groups according to their ALT level. Based on the exacerbation or flare of hepatitis B[21] and general indications of antiviral therapy for CHB with interferon[22], we used 10 times the upper limit of normal ALT level (10 × ULT, 400 IU/L) as the cut-off value in dividing the patients. Patients with their ALT level higher than 400 IU/L were defined as the high immune response group, while those with their ALT levels lower than 400 IU/L were defined as the low immune response group. The PD-1 expression level in CD8+ T cells was much higher in the high immune response group than in the low immune response group (P < 0.05, Figure 2A and B).
Figure 2.
PD-1 expression levels in the total T cells in the immune clearance stage of chronic HBV infection and liver inflammatory groups. A: The dot plots showing the percentage of positive PD-1 in CD8+ T cells in high and low liver inflammatory groups; B: The dot plots showing the PD-1 MFI expression level in the total CD8+ T cells in high and low liver inflammatory groups; C: Positive correlation between PD-1 MFI expression and alanine aminotransferase (ALT) levels in CD8+ T cells; D: Positive PD-1 percentage and ALT level in CD8+ T cells.
Further analysis showed that the PD-1 expression level was positively correlated with the ALT level in total CD8+ T cells (P < 0.05, Figure 2C and D).
PD-1 expression level was not correlated with serum HBV DNA level in total T cells during the immune clearance phase of chronic HBV infection
In this study, no significant correlation was found between the percentage and mean fluorescence intensity (MFI) of PD-1 expression and the serum viral load in total T cells. Similarly, no difference was observed in PD-1 expression in CD4+ T cells and CD8+ T cells between the HBe+ and HBe- groups.
Relation between CD8+ T cell response and PD-1 expression level in the immune clearance stage of chronic HBV infection
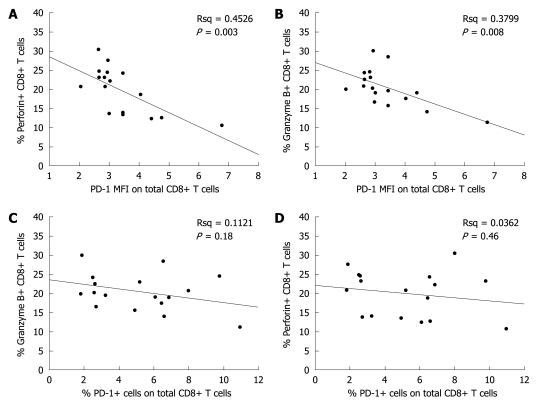
The relation between total CD8+ T cell response and PD-1 expression level was analyzed with ICS, which showed that the CD8+ T cell response level was inversely correlated with PD-1 MFI (Figure 3A-D).
Figure 3.
Relation between PD-1 expression level in CD8+ T cells and CD8+ T-cell responses in the immune clearance stage of chronic HBV infection. A: Inverse relation between the percentage of perforin and PD-1 MFI in positive CD8+ T cells; B: Inverse relation between the percentage of granzyme B and PD-1 MFI in positive CD8+ T cells; C: No correlation between the percentages of granzyme B and PD-1 in positive CD8+ T cells; D: No significant correlation between the percentages of perforin and PD-1 in positive CD8+ T cells.
Blocking PD-1/PD-L1 engagement in the total CD8+ T cells during the immune clearance stage of chronic HBV infection
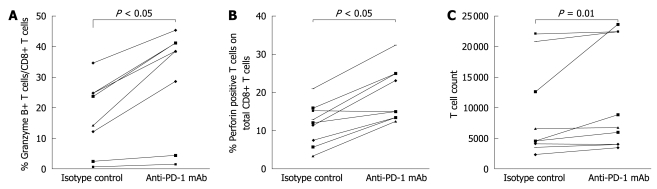
To investigate the role of PD-1 expression in CD8+ T cell responses in the natural stages of chronic HBV infection, expansion of CD8+ T cells and production of cytokines were detected 3 d after in vitro stimulation in the presence or absence of anti-PD-L1 antibody. The blockage of PD-1/PD-L1 significantly enhanced the expansion of T cells in the immune clearance stage of chronic HBV infection (Figure 4C), and increased the production of perforin and granzyme B by CD8+ T cells in the immune clearance, immune tolerance and inactive virus carrier stages of chronic HBV infection (Figure 4A and B).
Figure 4.
Effect of anti-PD-L1 antibody on the function of CD8+ T cells in the immune clearance stage of chronic HBV infection. A, B: Significant restoration of CD8+ T cell response after blockage of PD-1/PD-L1; C: Restoration of T-cell proliferation after blockage of PD-1/PD-L1.
DISCUSSION
As a negative co-stimulatory receptor, PD-1 interacts with its ligands, PD-L1 and PD-L2, to attenuate T-cell responses and appears particularly important in regulating T-cell tolerance. In chronic infection with viruses such as HIV and HCV[18,23], the PD-1 expression level remains high in virus-specific T cells. In our study, the PD-1 expression level in T cells was dynamically changed during the natural stage of chronic HBV infection, indicating that it is up-regulated when the host antiviral immune is activated and down-regulated when the virus is cleared and the antiviral immune is inactivated.
Among the natural stages of chronic HBV infection, the immune clearance stage is the most critical phase for the host in eliminating the virus. In this study, the PD-1 expression level in T cells was significantly higher in the immune clearance stage than in the other two stages of chronic HBV infection and in normal controls. The PD-1 expression level in CD8+ T cells was related with serum ALT level but not with serum viral load, which is not consistent with the reported findings[14,18,24]. The difference may be due to the different study groups and detection methods used. Furthermore, the PD-1 MFI in CD8+ T cells was inversely correlated with CD8+ T cell responses, and blockage of PD-1/PD-L1 interaction significantly restored the proliferation of T-cells and the secretion of antiviral cytokines in the immune clearance stage of chronic HBV infection, suggesting that the PD-1/PD-L1 pathway affects the progress of chronic HBV infection by regulating T-cell response in the immune clearance stage of chronic HBV infection.
CHB is characterized by loss of virus-specific CD8+ T cells and varying degrees of functional impairment of virus-specific T-cell responses. In patients with chronic HBV infection, the PD-1 is highly expressed in virus specific CD8+ T cells, and the spectrum of anti-HBV immunity can be improved by blocking the PD-1[19]. In this study, the PD-1 expression level in CD8+ T cells was up-regulated, and blocking the PD-1/PD-L1 pathway increased the proliferation of T-cells and restored the response of CD8+ T cells in the immune clearance stage but not in the immune tolerance and inactive virus carrier stages of chronic HBV infection, suggesting that the PD-1 inhibitory pathway particularly acts on the immune clearance stage of chronic HBV infection, and that the PD-1 expression level and the role of the PD-1/PD-L1 pathway are dramatically changed with the antiviral immune change in the natural stage of chronic HBV infection.
In conclusion, PD-1 expression participates in modulating the host antiviral immunity throughout the natural course of chronic HBV infection. However, in this study, we only examined the total number of CD8+ T cells but did not calculate the number of HBV-specific CD8+ T cells as targets. Further study is needed on the virus-specific anti-viral immunity.
COMMENTS
Background
Adaptive immune response, particularly cellular immune response, mediates the clearance of hepatitis B virus (HBV). Unfortunately, HBV-specific T-cell function is severely impaired in chronic HBV infection patients, characterized by low levels of antiviral cytokines, impaired cytotoxic T lymphocyte activity and persistent viraemia. The mechanism underlying T-cell malfunction in chronic HBV infection has not yet been completely understood.
Research frontiers
The programmed death type-1 (PD-1)/PD-L1 pathway plays a negative role in the regulation of activation and proliferation of T cells and production of cytokines. There is evidence that the PD-1 pathway plays an important role in inhibiting the function of virus-specific CD8+ T-cells in chronic viral infections involving human immunodeficiency virus, hepatitis C virus, and HBV. However, the change pattern of PD-1 expression in the natural course of chronic HBV infection has not been presented. The results of this study indicate that the PD-l/PD-L1 pathway plays a different role in T-cell response in the natural course of chronic HBV infection.
Innovations and breakthroughs
Recent reports have highlighted the importance of the PD-1 pathway in T-cell response in in different stages of chronic HBV infection. This study showed PD-1 expression participated in modulating host antiviral immunity throughout the natural course of chronic HBV infection.
Applications
By discussing the changes in expression of PD-1 and T cell responses in the natural course of chronic HBV infection, this study may represent a future strategy for therapeutic intervention in treatment of patients with HBV infection.
Terminology
PD-1: A 55 kDa transmembrane protein containing an immunologic receptor tyrosine-based inhibitory motif, which was originally isolated from a T-cell line exhibiting a high sensitivity to apoptosis. As a negative co-stimulatory receptor, PD-1 interacts with its ligands, PD-L1 and PD-L2, to attenuate T-cell responses and appears to be particularly important for regulating T-cell tolerance.
Peer review
By employing well-designed immunological techniques, the authors found that the expression of PD-1 was significantly up-regulated in T-cells of patients in the immune clearance phase of chronic HBV infection and was closely correlated with increasing serum ALT levels but not with serum HBV DNA in the same phase, which may contribute to the elucidation of the pathophysiology underlying the different natural courses of chronic HBV infection.
Footnotes
Supported by Grants from the “Yucai” Research Program of Changhai Hospital
Peer reviewer: Emanuel K Manesis, MD, Professor of Medicine, Athens University School of Medicine, Liver Unit, Euroclinic, 19 Mavromateon Street, 104 34 Athens, Greece
S- Editor Wang JL L- Editor Wang XL E- Editor Zheng XM
References
- 1.Pan CQ, Zhang JX. Natural History and Clinical Consequences of Hepatitis B Virus Infection. Int J Med Sci. 2005;2:36–40. doi: 10.7150/ijms.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi YH, Shi CH. Molecular characteristics and stages of chronic hepatitis B virus infection. World J Gastroenterol. 2009;15:3099–3105. doi: 10.3748/wjg.15.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61–66. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2:43–50. doi: 10.1016/s1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 6.Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386–1396. doi: 10.1016/s0016-5085(99)70289-1. [DOI] [PubMed] [Google Scholar]
- 7.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 12.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ, Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 17.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye P, Weng ZH, Zhang SL, Zhang JA, Zhao L, Dong JH, Jie SH, Pang R, Wei RH. Programmed death-1 expression is associated with the disease status in hepatitis B virus infection. World J Gastroenterol. 2008;14:4551–4557. doi: 10.3748/wjg.14.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005) Chin Med J (Engl) 2007;120:2159–2173. [PubMed] [Google Scholar]
- 23.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 24.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]