Abstract
Diagnostic utility of E-cadherin (E-CD) and cytokeratin (CK) subtype profiling in effusion cytology was investigated, employing immunocytochemistry on cellblock sections available from 211 metastatic carcinomas (MC), 6 mesotheliomas and 73 reactive mesothelial hyperplasias (MH). E-CD and monoclonal carcinoembryonic anti-gen (mCEA) stained 85% (120/141) and 65% (138/211) of MC, respectively. E-CD staining of MC was frequently heterogeneous (76/120) and absent in all anaplastic carcinomas (0/2). E-CD stained none (0/57) of MH while mCEA and epithelial membrane antigen (EMA) stained 12% (9/73) and 32% (16/32) of MH, respectively. Of 6 mesotheliomas, E-CD focally stained in 2 while mCEA stained none and EMA stained all. CK20 and CK17 stained none of MH or mesotheliomas. CK20 stained 15% of MC and CK 17 stained 22% of MC. CK5/6 and high molecular weight CK stained all mesotheliomas, 56% and 88% of MH, 26% and 39% of MC, respectively. MC showed predominant CK7+/20- expression, with the exceptions of MC from mucinous type of colon/rectum and ovary showing predominant CK20 positive. E-CD may be a useful positive marker for MC in effusion cytology, although it may focally stain in some mesotheliomas. Any positive staining for CK20 of MC suggests MC from the gastrointestinal tract or ovary among others.
Keywords: Pleural Effusion; Ascites; Carcinoma; Mesothelioma; Neoplasms, Mesothelial; Cadherins; Keratin; Cytokeratin 20; Keratin 7
INTRODUCTION
Distinction between mesothelial proliferations (hyperplasia or mesothelioma) and metastatic carcinomas, especially adenocarcinomas has been one of the most difficult problems in cytology as well as in surgical pathology. Various histochemical and immunohistochemical stains have been applied to solve this problem, as adjuncts to morphologic differential features including ultrastructural findings. Recently, several new antibodies including cadherins and cytokeratin subtypes have been reported to be useful in differential diagnosis between mesothelial proliferation and metastatic adenocarcinoma (1-6).
Cadherins are a multi-gene family of transmembrane glycoproteins and play an important role in sorting cells into specialized tissues during morphogenesis (7). E-cadherin (E-CD) exists in the adherence junctions of epithelium, mediating tight cell-to-cell adhesion, and is expressed by almost all kinds of epithelial cells (8). Although cadherin expression may be altered in tumorigenesis, there is an indication that their expression, predominantly localized at the lateral cell borders, may be useful in tracing the histogenetic origin of tumors. Particularly, E-CD has been suggested as a useful marker for distinguishing adenocarcinoma from mesothelioma (1, 9). E-CD was strongly and diffusely expressed in most cases of carcinomas (20-40%) while it was weakly and focally expressed in neoplastic or reactive mesothelial proliferation (0-6%) (1, 2, 5, 10).
Cytokeratin (CK) filaments are the intermediate filaments forming the skeleton of epithelial cells, providing support to maintain cell integrity and the structure of epithelial tissues. Human epithelia can present 20 different types of CKs (11). Not a single CK subtype, but a panel of CK subtypes, which includes both positive and negative markers for mesothelial cells, should be employed for distinguishing mesothelioma from adenocarcinomas. The expression of pan CK, in contrast to that of E-CD, does not clearly indicates the presence of carcinoma cells in effusion because mesothelial cells express a broad range of CKs (12). CK5/6 is one of the positive markers for mesothelial proliferation (mesothelioma) with high sensitivity (80-100%) and sufficient specificity for practical use in distinguishing it from metastatic adenocarcinomas (4, 13). High molecular weight CK (34bE12), composed of CKs 1, 5, 10 and 14, labels reactive and neoplastic mesothelial cells, basal cells, and squamous, ductal and complex epithelia (14-16). The tissue distribution of CK17 appears to be relatively limited to certain myoepithelial and basal cells of complex epithelia, and subsets of hair shaft epithelia (17). Therefore, the investigation of the utility of CK17 as a potential differential marker seems to be worthwhile.
In this study, expression of E-CD and several cytokeratin subtypes was investigated in a variety of carcinomas and mesotheliomas using cellblock sections from effusion specimens, and was compared to the expression of monoclonal carcinoembryonic antigen (mCEA) and epithelial membrane antigen (EMA).
MATERIALS AND METHODS
The cytological materials of malignant effusion and mesothelial hyperplasia available formalin-fixed and paraffin-embedded effusion cellblocks were included in this study during 3 yr from 1997 to 1999 in daily routine sign-out in Samsung Seoul Hospital. Two hundred and eleven cases of metastatic carcinoma, 6 cases of mesothelioma and 73 cases of reactive mesothelial hyperplasia were selected for this study. Hematoxylin-eosin (H&E) stained smears, Papanicolau stained smears, H&E stained cellblock sections, and available tissue sections of all cases were reviewed.
The metastatic carcinomas consisted of anaplastic carcinoma (4 cases) from the ovary (1 case), pancreas (1 case), thyroid (1 case) and unknown primary site (1 case); adenocarcinoma (186 cases) from the Ampulla of Vater (1 case), bile duct (12 cases), breast (16 cases), colon/rectum (13 cases), endometrium (7 cases), lung (30 cases), ovary (38 cases), pancreas (8 cases), stomach (55 cases), and thyroid (1 case); adenosquamous carcinomas (3 cases) from the ovary (1 case), pancreas (1 case), and thyroid (1 case); hepatocellular carcinomas (2 cases); small cell carcinomas from the lung (5 cases); and squamous cell carcinomas (10 cases) from the esophagus (4 cases), lung (3 cases), and uterine cervix (3 cases). Adenocarcinomas from the breast consisted of ductal type (7 cases), lobular type (3 cases) and not otherwise specified (6 cases). Adenocarcinomas from the ovary consisted of mucinous (2 cases) and non-mucinous carcinoma (26 cases).
Immunohistochemical staining was performed in consecutive unstained sections taken from each cellblock, employing the avidin-biotin peroxidase complex method (DAKO LSAB kit-peroxidase, DAKO corp. Denmark) using several monoclonal antibodies summarized in Table 1. Not all cases had been stained with all antibodies because cellblocks were too thin to yield enough number of sections and a few sections were rejected on the unavailability of carcinoma cells and/or technical grounds. Cells were considered positive if at least one intact cell showed an unequivocal intercellular membranous staining for E-CD, cytoplasmic and membranous staining for all CK subtypes and EMA, and cytoplasmic staining for mCEA. The denominators of the fractions given show the numbers of cases assessed for each antibody
Table 1.
Antibodies used in this study
CEA, carcinoembryonic antigen; M.W., molecular weight; EMA, epithelial membrane antigen; MW, microwave pretreatment; E, enzyme pretreatment with protease K.
RESULTS
Comparison of E-CD with mCEA and EMA as a marker for carcinoma
Metastatic carcinoma, with the exception of anaplastic carcinoma, were positive for E-CD, mCEA and EMA (Table 2). E-CD stained 120 out of 141 cases of metastatic carcinoma (85%), which included the majority cases of adenocarcinoma (87%) and squamous cell carcinoma (83%). Adenosquamous carcinoma (2/2), hepatocellular carcinoma (1/1) and small cell carcinoma (2/3) show also positive for E-CD. E-CD stained adenocarcinomas from various primary sites, such as bile duct, breast (all ductal type), colon/rectum, endometrium, lung, pancreas, stomach, and thyroid. In addition, ovarian adenocarcinomas, both mucinous (1/1) and 90% non-mucinous types (19/21); serous adenocarcinoma (16/17), endometrioid adenocarcinoma (2/2), undifferentiated carcinoma (1/2), which are often difficult to be differentiated from mesothelioma, showed diffuse E-CD expression in the majority cases (Fig. 1A).
Table 2.
E-cadherin, monoclonal CEA and epithelial membrane antigen expression in effusion cellblock
f, focal positive; ND, not done; *7 cases out of 7 cases of ductal carcinoma and one case of mammary adenocarcinoma, not otherwise specified; **one case of mucinous, 19 cases of non-mucinous and 3 cases of adenocarcinoma, not otherwise specified, out of one case of mucinous, 21 cases of non-mucinous and 3 cases of adenocarcinoma, not otherwise specified.
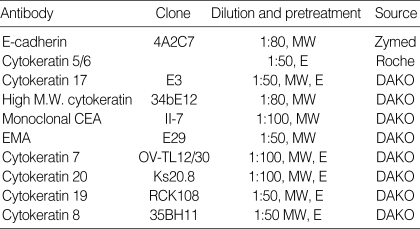
Fig. 1.
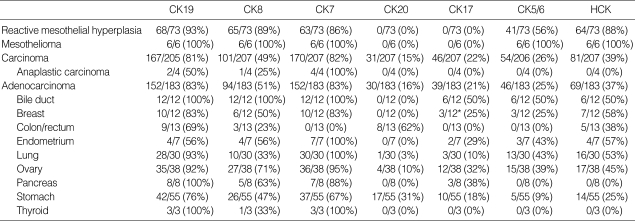
E-CD staining in metastatic adenocarcinoma (×400): metastatic papillary serous carcinoma of the ovary exhibits typical intercellular staining (A). Metastatic pulmonary adenocarcinoma exhibits a somewhat heterogeneous staining (B) or a partial loss of staining (C). Metastatic adenocarcinoma from common bile duct exhibits rather exaggerated, cytoplasmic staining (D). Metastatic signet ring cell carcinoma (arrows) of the colon Inset: cytoplasmic mucin in tumor cells, H&E (E) and metastatic undifferentiated carcinoma (arrows) of the ovary (F) exhibit none. Note many inflammatory cells are in background.
In 76 out of 120 cases (63%) of carcinomas showing E-CD expression, E-CD stain was focal and/or weak with heterogeneous pattern among carcinoma cells from the same case, particularly when they were singly dissociated (Fig. 1B, C). Infrequently, E-CD stain appeared exaggerated or altered in cytoplasmic staining (Fig. 1D). About 15% of metastatic carcinomas, including all anaplastic carcinomas, undifferentiated and signet ring cell carcinoma, showed no expression of E-CD (Fig. 1E, F).
Compared to E-CD, mCEA infrequently stained adenocarcinomas from the endometrium, thyroid, and ovary. mCEA also stained small cell carcinomas less frequently than E-CD. Compared to E-CD, EMA was more sensitive, but a much less specific positive marker for metastatic carcinoma. EMA stained all cases of metastatic carcinoma.
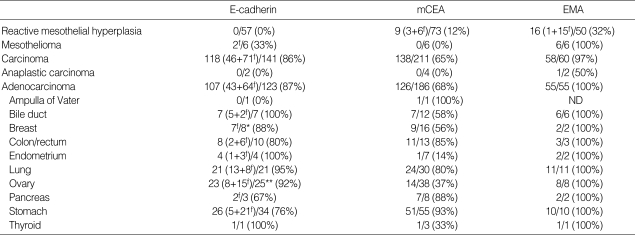
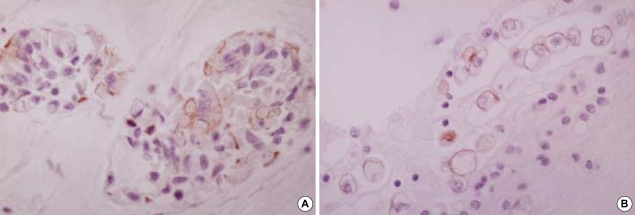
E-CD stained none of reactive mesothelial hyperplasias (0/57) (Fig. 2A) while mCEA and EMA stained 12% and 32% of reactive mesothelial hyperplasias, respectively. E-CD also stained 2 out of 6 cases of mesothelioma (30%), with focal membranous pattern (Fig. 2B, C) while mCEA stained none (0%), and EMA stained all cases (100%).
Fig. 2.
E-CD staining in mesothelial cells: reactive mesothelial cells exhibit none (A, ×400) and neoplastic mesothelial cells (arrow) (B, ×400).
Immunoreactivity for cytokeratin subtypes
Cytokeratins 19, 8, and 7 showed similar pattern of expression (Table 3). All three subtypes stained most cases of reactive mesothelial hyperplasia, ranging from 86 to 93%, and all cases of mesotheliomas (100%). CK19, CK8, and CK7 stained a variety of metastatic carcinomas, but with variable percentages (82%, 49%, and 81%, respectively).
Table 3.
Expression of cytokeratin subtypes in effusion cellblocks
CK, cytokeratin; HCK, high molecular weight cytokeratin; ND, not done. *3 cases of ductal carcinoma out of 7 cases of ductal carcinoma, 3 cases of lobular carcinoma and 2 cases of adenocarcinoma, not otherwise specified.
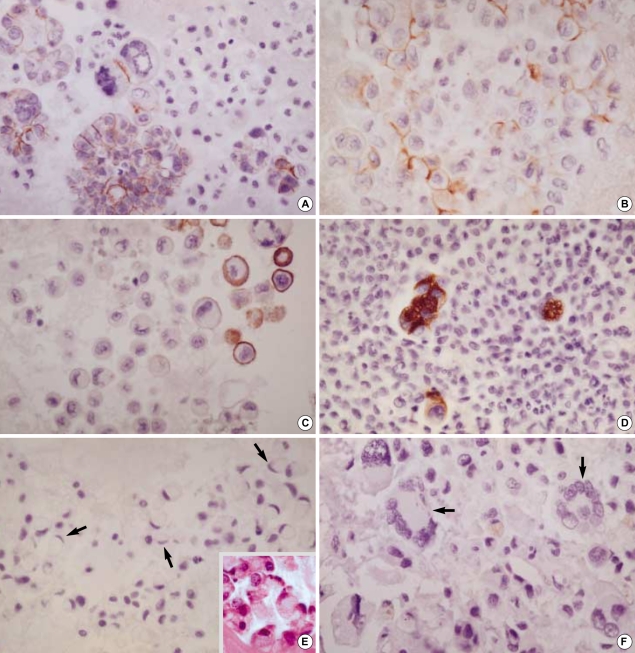
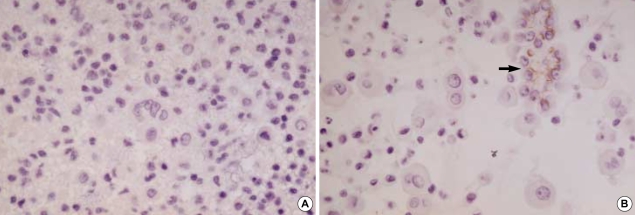
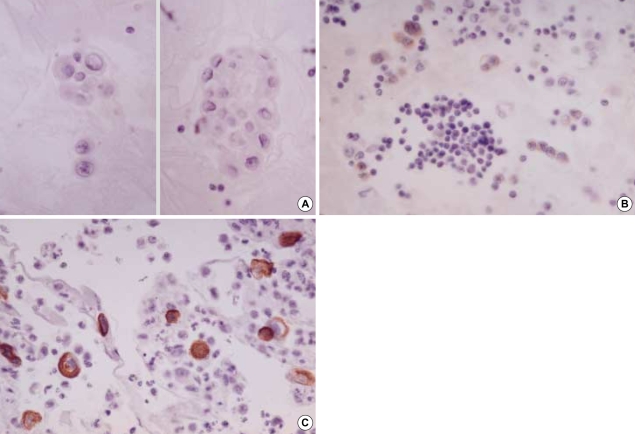
CK 20 stained none of the reactive mesothelial hyperplasias and mesotheliomas while it stained about 15% of metastatic carcinomas, which were limited to metastatic adenocarcinomas originated from the colon/rectum (8/13), lung (1/30), ovary (4/38) (Fig. 3A), and stomach (17/55) (Fig. 3B), and metastatic hepatocellular carcinomas (1/3). Similarly, CK 17 stained none of the reactive mesothelial hyperplasias or mesotheliomas (Fig. 4A) while it stained about 22% of metastatic carcinomas, including metastatic adenocarcinomas originated from the bile duct (6/12), breast (all ductal type, 3/12) (Fig. 4B), endometrium (2/7), lung (3/30), ovary (12/38), pancreas (3/8), and stomach (10/55), and metastatic squamous cell carcinomas (4/10) (Fig. 4C).
Fig. 3.
Metastatic ovarian mucinous carcinoma (A, ×400) and metastatic gastric signet ring cell carcinoma (B, ×400) show diffuse membranous staining for CK20.
Fig. 4.
Mesothelial cells exhibit none of stain for CK17 (A, ×400). In contrast, metastatic mammary duct carcinoma (B, ×200) and metastatic esophageal squamous cell carcinoma (C, ×400) exhibit diffuse membranous/cytoplasmic staining for CK17.
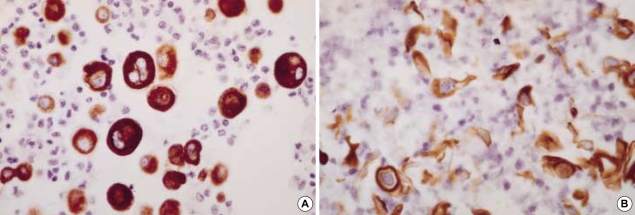
Both CK5/6 and high molecular weight keratin (HCK) stained most cases of reactive mesothelial hyperplasia (56% and 88%, respectively) and all 6 cases of mesothelioma (Fig. 5A), while they stained smaller portion of metastatic carcinomas (26% and 39%, respectively), which included adenocarcinoma from bile duct, breast, endometrium, lung, ovary and stomach. Notably, both CK5/6 and HCK stained metastatic carcinomas with squamous differentiation, such as adenosquamous cell carcinoma and squamous cell carcinoma (Fig. 5B).
Fig. 5.
Mesothelial cells (A, ×400) and metastatic squamous cell carcinoma of the lung (B, ×400) exhibit diffuse strong membranous/cytoplasmic staining for CK5/6.
Combined CK7/20 profile
Mesotheliomas and metastatic adenocarcinomas from the bile duct, breast, endometrium, lung, ovary (all non-mucinous type), pancreas and thyroid showed predominant CK7+/20-expression. Metastatic adenocarcinomas from the colon/rectum showed predominant CK7-/CK20+. Metastatic adenocarcinomas from the stomach showed all four profiles with slight predominance of CK7+/20-. Four cases of metastatic adenocarcinoma from the ovary with CK7+/CK20+ were all of the mucinous type.
DISCUSSION
The present study showed that E-cadherin was useful for distinguishing carcinoma cells from both reactive and neoplastic mesothelial cells in effusion cytology. In previous studies employing tissue sections, E-CD stained most of the pulmonary, breast duct (not lobular), ovary, colon, thyroid and prostate adenocarcinomas while it stained only about 6% of the mesotheliomas, with relatively weak and focal reactivity, suggesting that E-cadherin may be a useful marker in the differential diagnosis between epithelial mesotheliomas and adenocarcinomas of various origin (1, 2, 5, 9). In contrast, the study by Simsir et al. (10), which revealed E-CD expression in 12 out of 26 cases of mesothelioma on cellblock sections, suggested that E-CD might not useful in the differentiation of adenocarcinoma cells from neoplastic mesothelial cells as much as from reactive mesothelial cells.
The previous result reported immunoreactivity of CEA, EMA, E-CD, N-CD, and CK5/6, shows similar results with our ones: E-CD stained all cases of peritoneal or ovarian serous carcinomas, which mCEA rarely stained (18). E-CD reactivity in mesothelioma was limited to a few cases while EMA strongly stained all.
The results showing E-CD negative staining in about 15% of carcinoma including anaplastic type, its focal positive staining in about 50% of mesothelioma, and its frequent heterogeneous reactivity in the same carcinoma, may represent not only some limitations of E-CD in terms of diagnostic utility, by being the potential sources of false positive/negative results, but also some pathological implication of E-CD expression. As the previous studies revealed, decreased E-cadherin expression in carcinomas, resulting to a heterogeneous reactivity or complete negativity immunohistochemically, seemed to correlate with cellular dedifferentiation/anaplasia, and disintegration of carcinoma cell nests, frequently leading to cytologically dissociated single cells (19, 20). The observation that E-CD stained carcinoma cells focally and/or weakly with a heterogeneous pattern in more than half of cases might be related to the fact that this study employed cases with relatively high tumor stage, presenting with the malignant effusion.
E-cadherin is thought to be indispensable for the formation and maintenance of carcinoma cell nests such as the glandular differentiation of colorectal tumor cells, just as it is indispensable for the formation and maintenance of normal epithelial tissues (21). E-cadherin loss or dysfunction has been described in a variety of human and experimental neoplasms, and correlated with increased tumor stage and decreased patient survival, with only a few exceptions (22-24). It has been thought that E-cadherin loss or dysfunction, probably resulting from down regulation or mutation of cadherin genes during tumorigenesis, causes loss of cell-to-cell adhesion in carcinoma cell clusters, which leads to increased invasiveness of carcinoma cells (26). The intact E-cadherin is considered as a suppressor of invasion (27).
Focal E-CD stain in mesotheliomas might be related with papillary architecture of mesothelioma. In a study regarding E-CD overexpression in papillary urothelial carcinoma, it has been suggested that papillary structure may be one of the architectures requiring the strongest intercellular adhesion mediated by E-CD (28). In our 6 cases of mesothelioma, E-CD staining was mainly localized to papillary clusters. E-CD expression may indicate particular subtypes of tumor. E-CD expression has been noted in ductal breast carcinoma, but not in lobular carcinoma (29). Among various renal epithelial neoplasia, diffuse and strong expression of E-CD has been known to be unique for chromophobe renal carcinoma and oncocytoma (30).
CK5/6 seems to have some limitation as a positive marker for epithelial mesothelioma. In this study, CK5/6 stained about 25% of adenocarcinomas including ovarian carcinoma, which is often difficult to differentiate from mesothelioma. It also stained squamous and adenosquamous carcinomas. It has been known that most urothelial carcinomas express CK5/6, too. CK5/6 does not distinguish mesotheliomas from reactive mesothelial hyperplasias. HCK expression was similarly distributed among tumor types, with higher sensitivity for detecting squamous differentiation than CK5/6.
In contrast to both CK5/6 and HCK, CK17 was a negative marker for mesothelial proliferation. According to the study by Miettinen et al. (17), CK17 may stain normal myoepithelial and basal cells of complex epithelia, some hair shaft epithelia, and a wide range of carcinomas, including squamous cell carcinoma, and adenocarcinomas with complex glandular differentiation. In this study, CK17 stained none of reactive or neoplastic mesothelial proliferation. Therefore, CK17 may be used as a positive marker for metastatic carcinoma in effusion cytology, especially for carcinoma with squamous or complex glandular differentiation, but a marker with relatively low sensitivity. As already indicated in the previous report (17), CK17 stain may also be useful for separating mammary ductal carcinoma from lobular carcinoma, just like E-CD, as well as for separating gastric adenocarcinoma from colorectal adenocarcinoma.
As demonstrated by previous reports on tissue sections (31, 32), a combined CK7 and CK20 profile seems to be useful to identify the origin of tumor cells in effusions as an adjunct to clinical information and morphological study, mostly due to the fact that CK20 is very specific for metastatic gastrointestinal and ovarian mucinous adenocarcinomas (33, 34).
In summary, E-CD expression which is frequently heterogeneous in metastatic carcinoma, may be a useful positive marker for metastatic carcinomas in effusion cytology, but one needs to be cautious about its possible expression in mesothelioma. CK17 and CK20 may be negative markers for reactive or neoplastic mesothelial cells while CK5/6 and HCK may be positive markers. Any positive staining among CK5/6, HCK, and CK17 may be noted in metastatic carcinomas with squamous and/or complex glandular epithelial differentiation. CK20-positive staining seems to be restricted to metastatic adenocarcinomas from the gastrointestinal tract and ovary, as well as among other sites of origin.
References
- 1.Peralta Soler A, Knudsen KA, Jaurand MC, Johnson KR, Wheelock MJ, Klein-Szanto AJ, Salazar H. The differential expression of N-cadherin and E-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas. Hum Pathol. 1995;26:1363–1369. doi: 10.1016/0046-8177(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 2.Leers MP, Aarts MM, Theunissen PH. E-cadherin and calretinin: a useful combination of immunochemical markers for differentiation between mesothelioma and metastatic adenocarcinoma. Histopathology. 1998;32:209–216. doi: 10.1046/j.1365-2559.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 3.Lim SJ, Kim GY, Kim YW, Park YK, Lee J, Yang MH, Won NH. Usefulness of E-cadherin expression in malignant effusion. Korean J Cytopathol. 1999;10:121–126. [Google Scholar]
- 4.Ordonez NG. Value of cytokeratin 5/6 immunostaining in distinguishing epithelial mesothelioma of the pleura from lung adenocarcinoma. Am J Surg Pathol. 1998;22:1215–1221. doi: 10.1097/00000478-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol. 2000;24:598–606. doi: 10.1097/00000478-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Kim BH, Kwon OJ. Immunocytochemical expression of E-cadherin in cell blocks of serous effusions. Korean J Cytopathol. 2001;12:81–88. [Google Scholar]
- 7.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 8.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. [PubMed] [Google Scholar]
- 9.Peralta Soler A, Knudsen KA, Tecson-Miguel A, McBrearty FX, Han AC, Salazar H. Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum Pathol. 1997;28:734–739. doi: 10.1016/s0046-8177(97)90184-2. [DOI] [PubMed] [Google Scholar]
- 10.Simsir A, Fetsch P, Mehta D, Zakowski M, Abati A. E-cadherin, N-cadherin, and calretinin in pleural effusions: the good, the bad, the worthless. Diagn Cytopathol. 1999;20:125–130. doi: 10.1002/(sici)1097-0339(199903)20:3<125::aid-dc3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985;52:243–256. [PubMed] [Google Scholar]
- 12.Battifora H. Pleura. In: Sternberg SS, editor. Diagnostic Surgical Pathology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 1117–1145. [Google Scholar]
- 13.Clover J, Oates J, Edwards C. Anti-cytokeratin 5/6: a positive marker for epithelioid mesothelioma. Histopathology. 1997;31:140–143. doi: 10.1046/j.1365-2559.1997.2150833.x. [DOI] [PubMed] [Google Scholar]
- 14.O'Malley FP FP, Grignon DJ, Shum DT. Usefulness of immunoperoxidase staining with high-molecular-weight cytokeratin in the differential diagnosis of small-acinar lesions of the prostate gland. Virchow Arch A Pathol Anat Histopathol. 1990;417:191–196. doi: 10.1007/BF01600133. [DOI] [PubMed] [Google Scholar]
- 15.Wojno KJ, Epstein JI. The utility of basal cell-specific anti-cytokeratin antibody (34bE12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995;19:251–260. doi: 10.1097/00000478-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Moinfar F, Man YG, Lininger RA, Bodian C, Tavassoli FA. Use of keratin 34betaE12 as an adjunct in the diagnosis of mammary intraepithelial neoplasia-ductal type-benign and malignant intraductal proliferations. Am J Surg Pathol. 1999;23:1048–1058. doi: 10.1097/00000478-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Nobel MP, Tuma BT, Kovatich AJ. Keratin 17: Immunohistochemical mapping of its distribution in human epithelial tumors and its potential applications. Appl Immunohistochem. 1997;5:152–159. [Google Scholar]
- 18.Ordonez NG. Role of immunohistochemistry in distinguishing epithelial peritoneal mesotheliomas from peritoneal and ovarian serous carcinomas. Am J Surg Pathol. 1998;22:1203–1214. doi: 10.1097/00000478-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama Y, Hirohashi S. Cadherin intercellular adhesion molecule in hepatocellular carcinomas: loss of E-cadherin expression in an undifferentiated carcinoma. Cancer Lett. 1991;57:131–135. doi: 10.1016/0304-3835(91)90206-w. [DOI] [PubMed] [Google Scholar]
- 20.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- 21.Pignatelli M, Liu D, Nasim MM, Stamp GW, Hirano S, Takeichi M. Morphoregulatory activities of E-cadherin and beta-1 integrins in colorectal tumour cells. Br J Cancer. 1992;66:629–634. doi: 10.1038/bjc.1992.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto M, Niwa O, Nitta Y, Takeichi M, Yokoro K. Unstable expression of E-cadherin adhesion molecules in metastatic ovarian tumor cells. Jpn J Cancer Res. 1989;80:459–463. doi: 10.1111/j.1349-7006.1989.tb02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussemakers MJ, van Moorselaar RJ, Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM, Schalken JA. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992;52:2916–2922. [PubMed] [Google Scholar]
- 24.Kinsella AR, Green B, Lepts GC, Hill CL, Bowie G, Taylor BA. The role of the cell-cell adhesion molecule E-cadherin in large bowel tumour cell invasion and metastasis. Br J Cancer. 1993;67:904–909. doi: 10.1038/bjc.1993.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross JS, Figge HL, Bui HX, del Rosario AD, Fisher HA, Nazeer T, Jennings TA, Ingle R, Kim DN. E-cadherin expression in prostatic carcinoma biopsies: correlation with tumor grade, DNA content, pathologic stage, and Mod Pathol clinical outcome. Mod Pathol. 1994;7:835–841. [PubMed] [Google Scholar]
- 26.Tsuji K, Hirano T, Shibanuma H, Okada S, Kawate N, Konaka C, Ebihara Y, Kato H. Cytologic features based on the expression of E-cadherin and catenins in lung adenocarcinoma. Acta Cytol. 1999;43:381–389. doi: 10.1159/000331085. [DOI] [PubMed] [Google Scholar]
- 27.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross JS, Cheung C, Sheehan C, del Rosario AD, Bui HX, Fisher HA. E-cadherin cell-adhesion molecule expression as a diagnostic adjunct in urothelial cytology. Diagn Cytopathol. 1996;14:310–315. doi: 10.1002/(SICI)1097-0339(199605)14:4<310::AID-DC6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 30.Taki A, Nakatani Y, Misugi K, Yao M, Nagashima Y. Chromophobe renal cell carcinoma: An immunohistochemical study of 21 Japanese cases. Mod Pathol. 1999;12:310–317. [PubMed] [Google Scholar]
- 31.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 32.Wang NP, Zee S, Zarbo RJ, Bacchi CE, Gown AM. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99–107. [Google Scholar]
- 33.Ascoli V, Taccogna S, Scalzo CC, Nardi F. Utility of cytokeratin 20 in identifying the origin of metastatic carcinomas in effusions. Diagn Cytopathol. 1995;12:303–308. doi: 10.1002/dc.2840120404. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen M. Keratin 20: Immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995;8:384–388. [PubMed] [Google Scholar]