Abstract
The adaptation of the respiratory metabolism in roots of soybean (Glycine max L. Merr. cv Ransom) treated with herbicides that inhibit the enzyme acetolactate synthase (ALS) was analyzed. A new gas phase dual-inlet mass spectrometry system for simultaneous measurement of 34O2 to 32O2 and O2 to N2 ratios has been developed. This system is more accurate than previously described systems, allows measurements of much smaller oxygen gradients, and, as a consequence, works with tissues that have lower respiration rates. ALS inhibition caused an increase of the alternative oxidase (AOX) protein and an accumulation of pyruvate. The combination of these two effects is likely to induce the activation of the alternative pathway and its participation in the total respiration. Moreover, the start of the alternative pathway activation and the increase of AOX protein were before the decline in the activity of cytochrome pathway. The possible role of AOX under ALS inhibition is discussed.
There are four main classes of herbicides whose first mechanism of action is the inhibition of the enzyme acetolactate synthase (ALS; EC 4.1.3.18, also known as acetohydroxyacid synthase): imidazolinones, sulfonylureas, triazolopyrimidines, and pyrimidinylsalicilyc acids, with imidazolinones and sulfonylureas the first to be commercialized. ALS is the first common enzyme in the biosynthesis of branched-chain amino acids (BCAAs): Val, Leu, and Ile. This enzyme catalyzes the condensation of either two molecules of pyruvate to form acetolactate in the Leu and Val pathway or one molecule of pyruvate with one molecule of 2-ketobutyrate to form 2-aceto-2-hydroxybutyrate as the first step in the Ile biosynthesis (Singh, 1999). These herbicides cause a significant growth inhibition that is due more to a slower cell division than to an inhibition of cell expansion, although plants stay green for several weeks before death (Wittenbach and Abell, 1999). However, the precise mechanisms that link ALS inhibition with plant death have not been clarified yet. Plants respond quickly to ALS inhibitors by increasing protein turnover to renew BCAAs, and even the critical BCAA pool does not decline to a level that would affect protein synthesis (Wittenbach and Abell, 1999; Royuela et al., 2000). Carbohydrate accumulation in leaves and roots is one of the main symptoms of ALS-inhibiting herbicides in plants treated with imazethapyr (IM), an imidazolinone (Shaner, 1991; Royuela et al., 2000). Gaston et al. (2002) also demonstrated that the increase of soluble carbohydrates in roots can even precede that of starch in leaves, supporting the hypothesis that sugar accumulation in leaves can be due to a decrease in sink strength. In this context, it is surprising to notice that despite the cessation of plant growth and the accumulation of carbohydrates in roots, total root respiration rate (Vt) is unaffected or slightly affected (Ray, 1982; Gaston et al., 2002), indicating the possible occurrence of an impaired and/or regulatory mechanism of respiration in plants treated with ALS inhibitors. Thus, an induction of aerobic fermentation in pea (Pisum sativum) plants treated with IM has been described recently (Gaston et al., 2002). Aubert et al. (1997) showed that these herbicides induced the alternative oxidase (AOX) capacity in sycamore (Acer pseudoplatanus) suspension cells. They concluded that the induction of AOX synthesis was an indirect effect likely to be due to the effects of herbicides on cell metabolism. However, the relationship between AOX synthesis and ALS inhibition and its physiological significance in a whole plant system remain unclear.
AOX is an ubiquinol oxidase, alternative to the cytochrome oxidase, found in plant mitochondria and in some fungi and protists. Electron transfer from ubiquinol to the cytochrome oxidase is coupled to two sites of proton translocation source of ATP production, whereas the alternative pathway is effectively non-phosphorylating and releases energy as heat. Among the several factors known to regulate the activity of AOX (redox state of the enzyme, reduction state of the ubiquinol pool, and level of AOX protein), a remarkable feature is that the reduced form of the enzyme is further activated by α-ketoacids, pyruvate in particular (Millar et al., 1993; Umbach and Siedow, 1996). The physiological role of this pathway remains to be clarified except in the specialized case of promoting thermogenesis during flowering in aroid spadices (Meeuse, 1975; Moore and Siedow, 1991). However, several hints on the function of AOX have been achieved during the last decade and tend to indicate that AOX might have a function related to stress situations; for example, cold (Purvis and Shewfelt, 1993; Gonzàlez-Meler et al., 1999), oxygen radicals (Purvis, 1997; Yip and Vanlerberghe, 2001), phosphate starvation (Parsons et al., 1999; Gonzàlez-Meler et al., 2001), pathogen infection (Simons et al., 1999), and also in fruit ripening (Sluse and Jarmuszkiewicz, 2000; Considine et al., 2001) and hypersensitive response after pathogen infection (Xie and Chen, 2000; Ordog et al., 2002). Recently, Vanlerberghe et al. (2002) and Robson and Vanlerberghe (2002), using different death-inducing compounds, suggested a possible role of AOX in programmed cell death, either preventing or attenuating whole plant death. These studies may indicate that AOX alleviates the effect of stresses on plant performance by avoiding the over-reduction of the electron transfer chain preventing the generation of reactive oxygen species or allowing a limited synthesis of ATP when the cytochrome pathway is restricted.
Given that respiration measured as O2 uptake is scarcely altered by ALS inhibitor herbicides and that pyruvate is the main substrate of ALS, there is a possibility that the accumulation and diversion of this metabolite from biosynthetic pathways to respiratory pathway is causing the continuation of the respiration rate. Moreover, pyruvate could act as an allosteric activator of AOX. Aubert et al. (1997) tested several metabolites known to be accumulated after ALS inhibition (α-oxobutyrate and α-aminobutyrate), but none of them activated AOX. However, pyruvate accumulation after ALS inhibition has not been studied yet in depth.
Because AOX can operate in parallel with the cytochrome pathway and compete for electrons with an unsaturated cytochrome pathway (Guy et al., 1989; Hoefnagel et al., 1995; Ribas-Carbo et al., 1995), the only reliable method to quantitatively measure the activities of the respiratory pathways is by the use of oxygen isotope fractionation (Δn) in which inhibitors are only required to determine the end points of the Δn by each pathway (Robinson et al., 1995; Day et al., 1996). An accurate measurement of O2 concentration and its isotopic composition is essential for the correct determination of the Δn. In this paper, we report the development of a gas phase dual-inlet system for simultaneous measurements of 34O2 to 32O2 and O2 to N2 ratios with intact plant tissues, similar to the one used for studies on marine organisms (Kiddon et al., 1993). This method increases the precision of measurement that, in turn, allows measurements to be made with much smaller oxygen gradients and, as a consequence, with tissues that have lower respiration rates.
We have applied this technique to further investigate the mode of action of ALS inhibitor herbicides in soybean (Glycine max L. Merr. cv Ransom): the possible accumulation of pyruvate and its effect on the electron transfer chain, in particular on AOX activity.
RESULTS
ALS Inhibition and Herbicide Symptoms. Plant Growth and Carbohydrate Content
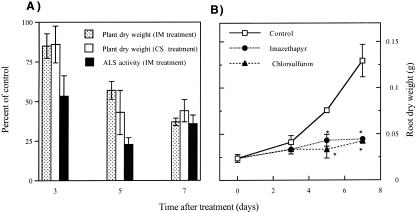
IM and chlorsulfuron (CS) supply at the selected concentrations (51.75 μm and 11.18 nm, respectively) caused similar effects on soybean. Plant growth was significantly inhibited by both herbicides (Fig. 1A). Whole plant dry weight represented 80% and 40% of control plants by d 3 and 7, respectively. Root growth was immediately halted in both treatments (Fig. 1B). IM treatment caused the inhibition of ALS activity (over 50%) by d 3 and stayed over 75% and 65% for days 5 and 7, respectively (Fig. 1A). Although this degree of ALS inhibition produces a lethal phenotype, the measurements presented in this study were carried out at the initial phase of toxicity, in which the plant viability was not compromised.
Figure 1.
A, Effect of ALS-inhibiting herbicides on soybean ALS activity (IM treatment) and total dry weight relative to control plants. Mean ± se (n = 5). Data, means, and ses are given as percentages of control plants. The control values for plant dry weight were: 187.5 ± 30.7, 375.8 ± 10.8, and 610.7 ± 55.9 mg at d 3, 5, and 7, respectively, and for ALS activity 379 ± 23, 564 ± 114, and 288 ± 35 nmol acetoin g–1 dry weight h–1 at d 3, 5, and 7, respectively. B, Effect of ALS-inhibiting herbicides on soybean root dry weight. Mean ± se (n = 5). *, Significant differences from the corresponding control (P ≤ 0.05).
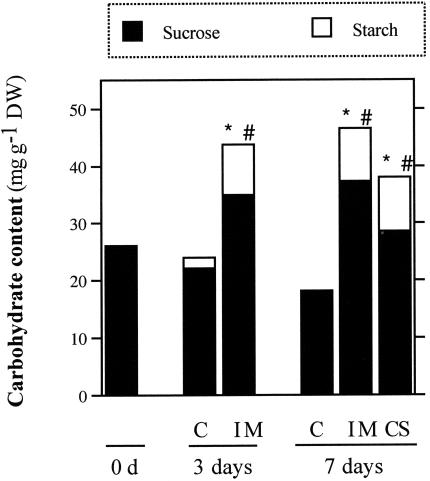
Figure 2 shows a significant increase in starch and Suc contents in roots of treated plants after d 3. It is remarkable that although starch content was very low in control roots, it significantly increased in roots of herbicide-treated plants. The occurrence of this increased concentration of carbohydrates is an indication of the sustained photosynthesis and photoassimilate transport, which ensure the viability of plants during the time course of the experiment.
Figure 2.
Effect of ALS-inhibiting herbicides on soybean root carbohydrate content: Suc, expressed as milligrams of Suc per gram dry weight; and starch, expressed as milligrams of Glc per gram dry weight. * and #, Significant differences in Suc and starch content, respectively, from the corresponding control (P ≤ 0.05).
Pyruvate Content
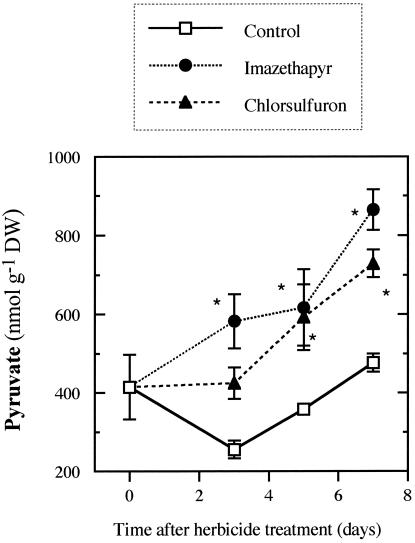
The supply of herbicides that inhibits ALS activity caused an increase of root pyruvate concentration, which is the main substrate of ALS activity (Fig. 3). The pattern of this increase was very similar in both herbicide treatments, although it was earlier in IM-treated plants. With IM, there was a significant increase in pyruvate level at d 3, whereas with CS, it was significant from d 5. Pyruvate content increased from 414 nmol g–1 dry weight at the beginning of the experiment to 865 and 729 nmol g–1 dry weight by d 7 for IM- and CS-treated plants, respectively, whereas it remained fairly constant in control plants (Fig. 3).
Figure 3.
Pyruvate content in roots of soybean treated with ALS-inhibiting herbicides. Mean ± se (n = 5). *, Significant differences from the corresponding control (P ≤ 0.05).
Respiratory Changes Caused by Herbicide Treatments
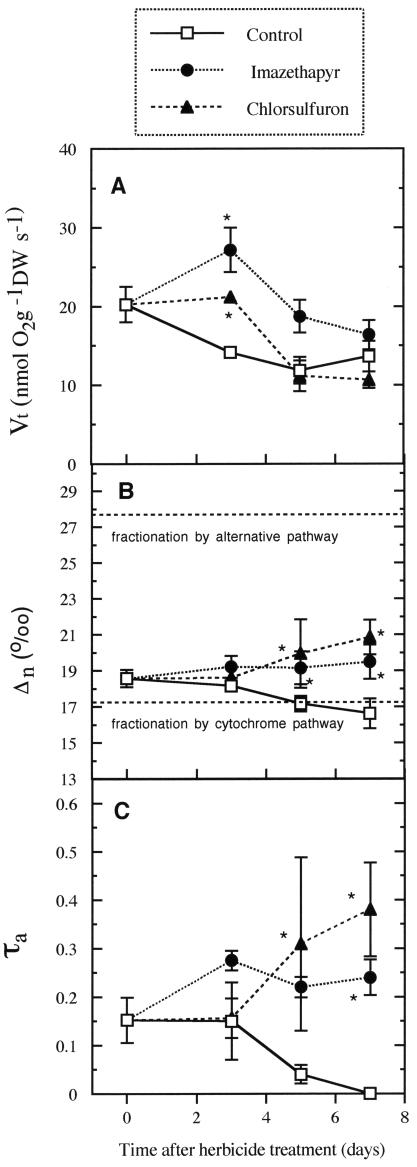
Vt of herbicide-treated plants was higher than that of control plants at d 3 and then decreased to values similar to those of control plants at d 7 (Fig. 4A).
Figure 4.
Vt (A), Δn (B), and τa through the alternative pathway (C), in roots of soybean treated with ALS-inhibiting herbicides. Mean ± se (n = 4–5). *, Significant differences from the corresponding control (P ≤ 0.05). Fractionation values for soybean roots were 17.35‰ ± 0.51‰ for the cytochrome pathway and 27.81‰ ± 0.02‰ for the alternative pathway.
Δn measured in the absence of inhibitors increased during the experimental time course in treated plants and was significantly higher than in control plants: CS-treated plants on d 5 and 7 were 20.36‰ and 21.30‰, respectively, and IM-treated plants on d 7 were 19.87‰ (Fig. 4B). However, Δn decreased in control plants throughout the experimental period from 18.56‰ on d 0 to 16.90‰ on d 7. The calculation of the electron partitioning between the cytochrome and alternative pathways (τa) requires each pathway's fractionation to be quantified. The Δn by the cytochrome (with salicylhydroxamic acid [SHAM]) and alternative (with potassium cyanide [KCN]) pathways in roots was determined to be 17.35‰ ± 0.51‰ (Δc) and 27.81‰ ± 0.02‰ (Δa), respectively. These values were used to calculate τa. Electron partitioning to the alternative pathway increased during the experiment in herbicide-treated plants: CS-treated roots maintained their electron partitioning through the alternative pathway at 0.15 during the first 3 d and then increased up to 0.38 at d 7 (Fig. 4C) and in IM-treated roots peaked at d 3 at 0.27, remaining constant thereafter (Fig. 4C). In control plants, τa decreased from 0.15 at d 3 to virtually zero by d 7 (Fig. 4C).
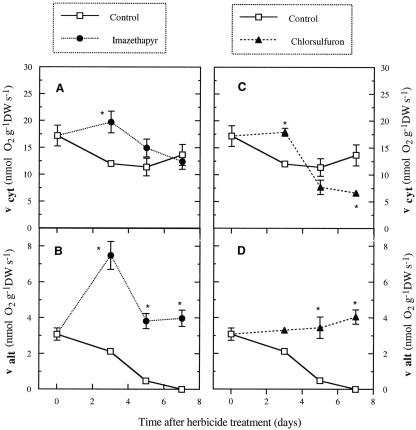
The activities of each respiratory pathway were also affected by herbicide treatments. The activity of the alternative pathway (valt) increased in treated plants throughout the course of the experiment. The pattern of activation was similar for both treatments, except for the marked increase in valt by d 3 in IM-treated plants (Fig. 5, B and D). In addition, in herbicide-treated plants, the activity of the cytochrome pathway (vcyt) was higher over the first 3 d than in control plants and then declined (Fig. 5, A and C). On the other hand, the initial decrease of Vt in control plants was associated with a decrease in both pathways: vcyt decreased during the first 3 d and slightly increased thereafter, and valt steadily declined throughout the experiment (Fig. 5).
Figure 5.
vcyt and valt of soybean roots treated with ALS-inhibiting herbicides: A and B for IM treatments and C and D for CS treatments. Mean ± se (n = 4–5). *, Significant differences from the corresponding control (P ≤ 0.05).
AOX Protein
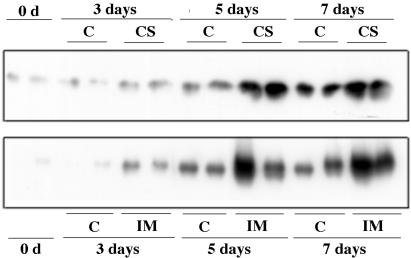
To study the profile of reduced and oxidized forms of AOX, Umbach and Siedow (1997) proposed the use of whole tissues instead of mitochondrial extracts because AOX protein undergoes oxidation of the sulfhydryl-disulfide system in the absence of sulfhydryl reagents in the latter. In this study, dithiothreitol was used as a reducer to obtain a single reduced form of the protein and, as a consequence, to enable the measurement of the total amount of protein by immunoblotting. A single AOX band of approximately 34 kD was obtained, which is in agreement with previous determinations of the apparent molecular size of soybean AOX species in the range of 33 to 39 kD (Finnegan et al., 1997; Fig. 6). The total amount of AOX protein in roots of herbicide-treated plants is significantly higher than in control plants throughout the experiment (Fig. 6). ALS inhibitors caused a subtle increase of the total amount of AOX protein by d 3 (approximately 1.6-fold for CS-treated plants and 2.5-fold for IM-treated plants), and this increase was substantially larger by d 5 (over 4-fold) and 7 (3-fold; Fig. 6).
Figure 6.
Immunoblots of AOX protein from whole-root tissue extracts from soybean at 0, 3, 5, and 7 d from the onset of herbicide treatment (C, control). Two lanes corresponding to two independent samples for each treatment are shown.
DISCUSSION
This report shows, for the first time to our knowledge, the development of a gas phase dual-inlet mass spectrometry system to measure the activities of respiratory pathways in plants with more precision and much smaller oxygen gradients and, as a consequence, with tissues that have lower respiration rates.
This approach permitted the observation that both herbicides caused an increase in the participation of valt in total respiration (Fig. 5). These results are consistent with previous observations in sycamore suspension cells treated with ALS-inhibiting herbicides, in which an increase of AOX capacity without modification of the cytochrome pathway was observed by polarographically monitoring O2 uptake (Aubert et al., 1997). The change in the partitioning of the electron transfer chain in soybean roots may have important implications in the adaptive response of plants to herbicides. Under ALS inhibition, there is an increase in the protein turnover that, in turn, causes an increase in the free amino acid pool (Shaner and Reider, 1986; Rhodes et al., 1987). In non-stressed conditions, protein turnover is generally considered as one of the most important maintenance processes that requires energy for protein synthesis. Under conditions in which energy requirement is strongly reduced for growth and ion uptake (which would be the case for ALS inhibitor-treated plants), ATP requirements for protein turnover increases and the maintenance of biomass becomes the most important energy requiring process in roots (Van der Werf et al., 1992). As such, the effect of ALS inhibitors should certainly alter the amount of energy allocated to growth and maintenance. The accumulation of carbohydrates is likely to be the ground for a sustained respiration rate in treated plants, through both cytochrome and alternative pathways during the first 3 d (Fig. 5). Afterward, as growth inhibition increases and maintenance respiration becomes more important, the alternative pathway increases its participation in the total respiration (Fig. 5, B and D).
The first indication that the inhibition of ALS activity could affect the alternative pathway was that herbicides caused an increase in AOX synthesis (Fig. 6; Aubert et al., 1997). It is well known that the total amount of AOX protein increases under several types of stress and, inherently, the total capacity of the pathway. However, an increase in the total amount of AOX protein does not always imply an increase of its activity (Lennon et al., 1997). In this study, the total amount of AOX content in the roots of control plants was also seen to increase throughout the experiment (Fig. 6), whereas the activity of the pathway decreased (Fig. 5). Several stress conditions induce AOX capacity in response to a cytochrome pathway restriction (chemical inhibitors, phosphate deficiency, anoxia, and senescence; Vanlerberghe et al., 1997; Parsons et al., 1999; Amor et al., 2000; Vanlerberghe et al., 2002). However in the present case, the induction of valt and the increase of AOX protein content were before the decline in vcyt in treated plants, indicating that alternative pathway does not always act as an electron bypass in response to the downstream restriction of the cytochrome pathway.
Because pyruvate is the main substrate of ALS activity, it would be expected that in plants treated with these ALS inhibitor herbicides, pyruvate concentrations might be increased, as observed in Figure 3. Furthermore, other ALS inhibitor herbicides have been shown to increase pyruvate levels in Salmonella typhimurium (Epelbaum et al., 1996) and in maize (Zea mays; Hwang et al., 1997). On the other hand, there is much in vitro evidence that suggests the importance of pyruvate as an activator of AOX in mitochondrial extracts or partially purified AOX enzyme (Day et al., 1994; Umbach et al., 1994). Pyruvate stimulation of AOX activity results from an increase in the amount of AOX protein in the reduced form (active form; (Hoefnagel et al., 1997; Millar et al., 1997). Nevertheless, there is no conclusive evidence for the role of pyruvate in vivo (Millar et al., 1998; Millenaar et al., 1998). Several stresses cause pyruvate accumulation, usually by limiting the electron transfer chain (anaerobiosis, phosphate starvation, and chemical inhibitors; Good and Muench, 1993; Vanlerberghe et al., 1997; Juszczuk and Rychter, 2002). However, conversely to these stresses, in the present study, pyruvate increase is caused by ALS inhibition. As a consequence, there is a combination of two effects: (a) an upsurge in the synthesis of AOX protein (Fig. 6), which increases the total capacity of the alternative pathway; and (b) a higher pyruvate concentration in the herbicide-treated roots (Fig. 3) that favors the participation of the alternative pathway.
This regulatory feature reinforces the possible role of AOX as a protective enzyme, preventing fermentation of accumulated pyruvate (Day et al., 1995). Previous work showed that in IM-treated pea roots, a species with low AOX activity, aerobic fermentation was elicited (Gaston et al., 2002). It is likely that the low activity of AOX in peas could not prevent the engagement of these fermentative activities by the accumulation of pyruvate. The protective role of AOX has been described previously as a response to environmental stress or in the mitochondria-dependent programmed cell death (Vanlerberghe et al., 2002). To further investigate how much AOX enables the plant to cope with ALS-inhibiting herbicides will require further experiments with plants that cannot express AOX.
To summarize, we add a new insight into the mode of action of ALS-inhibiting herbicides related to changes in mitochondrial electron partitioning, especially engaging the alternative pathway. Whether the increase in the AOX pathway enhances the adaptive response to ALS inhibition or is directly involved in the death process remains to be clarified.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Soybean (Glycine max L. Merr. cv Ransom) seeds were treated with 0.5% (v/v) NaOCl for 10 min and swelled in distilled water for 2 h with continuous bubbling of air. Seeds were germinated in a 1:1 (w/v) mixture of sand:perlite in plastic trays and placed in a greenhouse under controlled conditions. Water was applied daily. Nine-day-old seedlings were transferred to hydroponic tanks filled with nutrient solution (Rigaud and Puppo, 1975; supplemented with 10 mm KNO3) and placed in a growth chamber. Growth conditions were 30°C/24°C on a 16-/8-h (light/dark) regime at 300 μmol photons m–2 s–1. Plants were acclimatized to the new growth conditions 4 d, and then they were divided into three groups: one control and two herbicide treatments. ALS inhibitors were: IM, an imidazolinone herbicide; and CS, a sulfonylurea herbicide. They were applied to the nutrient solution at concentrations of 51.75 μm and 11.18 nm for IM and CS, respectively. These concentrations were chosen after observing the similar effects on soybean growth. The treatment lasted 7 d, with plants being harvested at days 0, 3, 5, and 7. Length and dry weight of roots and shoots were measured.
ALS Activity
ALS activity was measured in vivo following the method of Lee and Owen (2000). Leaves from the apical meristem were excised under water to avoid cavitation and petioles were immediately immersed in 6 mL of 800 μm 1,1-cyclopropanedicarboxylic acid (CPCA). Leaves were allowed to transpire and absorb the feeding solution for 4 h in light (300 μmol m–2 s–1). CPCA inhibits the activity of keto-acid reductoisomerase (EC 1.1.1.86) leading to acetolactate accumulation. A parallel set of leaves was fed with water to correct the basal level of acetolactate. Leaves were removed from the feeding solution and immediately frozen in liquid nitrogen and stored at –80°C. Acetolactate was extracted three times with 80% (v/v) methanol at 80°C for 20 min each and determined after its conversion to acetoin by incubation with 0.6 n H2SO4 at 60°C for 15 min. Acetoin was determined according to Westerfeld (1945). The acetolactate produced during the 4 h of incubation with CPCA was corrected with the acetolactate content of the tissue when no inhibitor was used (feeding with water). ALS activity is expressed as nanomoles acetolactate per gram dry weight.
Starch and Suc Determinations
Frozen samples of roots (0.2 g fresh weight) were extracted in boiling 80% (v/v) ethanol. Ethanol soluble extracts were dried in a Turbovap LV evaporator (Zymark Corporation, Hopkinton, MA), and soluble compounds were redissolved with 4 mL of distilled water, mixed, and centrifuged at 2,300g for 10 min. The ethanol insoluble residue was extracted for starch as in MacRae (1971). Glc produced from starch and Suc were determined by high-performance capillary electrophoresis in a PACE system 5500 (Beckman Instruments, Fullerton, CA). The background buffer was 10 mm benzoate (pH 12.0) containing 0.5 mm tetradecyl-trimethyl-ammonium bromide. The applied potential was –15 kV, and the capillary tubing had an internal diameter of 50 μm and was 31.4/38.4 cm long. The indirect UV detection wavelength was set at 225 nm.
Pyruvate Extraction and Determination
Samples of roots (0.4 g fresh weight) were harvested and rapidly frozen by addition of 0.6 n trichloroacetic acid (TCA) in diethyl-ether at –100°C and stored at –80°C until extraction. Samples were homogenized with 1.5 mL of cold 5% (w/v) TCA and centrifuged at 2,000g for 10 min. To remove TCA, the supernatant was washed three times with diethyl-ether saturated with water. The extracts were filtered through a microfilter (0.22 μm, Millex-GV, Millipore, Bedford, MA). Pyruvate was determined by ion chromatography in a DX-500 system (Dionex Corporation, Sunnyvale, CA) in a Dionex IonPac AG11+AS11 column by gradient separation (0.2–15 mm NaOH in 15 min).
Respiratory Measurements on Intact Tissues with a Gas Phase Dual-Inlet System
Vt, vcyt, and valt in roots were determined using a closed gas phase system connected to a dual-inlet mass spectrometer as described below. The measuring system consisted of a 3-mL closed cuvette where the plant tissue was placed and from which 200 μL of air was sequentially withdrawn and fed into the mass spectrometer sample bellows. Both the 34O2 to 32O2 and O2 to N2 ratios from the air analyzed were directly obtained from an isotope ratio mass spectrometer (Finnigan Delta S, Thermo Finnigan, San Jose CA) operating in dual-inlet mode and by comparison with a standard air sample. The stainless steel cuvette was equipped with two inlets: one connected to a 1-mL air-tight syringe and the other to the mass spectrometer sample bellows through a capillary tube (127-μm i.d.) with a pneumatically controlled on-off microneedle valve. The sampled air went through a liquid N2 trap for water and CO2 removal. To avoid any drop in the cuvette's pressure during the experiment, the air was well mixed using the air-tight syringe, which was left with 1 mL of air. Throughout the experiment, the syringe was used to mix the air. At the beginning of every measurement, 200 μL of the syringe was placed in the cuvette to maintain its atmospheric pressure. The system was regularly tested for leaks by filling the cuvette with He and measuring samples over three times the experimental time span. No oxygen signals were observed. The time between successive samples was 20 min, and the length of a full experiment would vary between 90 and 120 min. The system was previously tested using alfalfa (Medicago sativa) seedlings (purchased alfalfa sprouts) in the presence of KCN or SHAM, which gave values of 25.4‰ (Δa) and 19.7‰ (Δc). These values are very similar to the values observed with previous systems (Robinson et al., 1995).
Root samples (0.2–0.3 g fresh weight) were placed in the 3-mL stainless steel closed cuvette. Roots were carefully surface dried before measurements so as to minimize diffusion resistance to tissue gas exchange. All experiments were carried out at controlled room temperature (23°C). During inhibitory treatments to measure fractionation values through each pathway, either 1.0 mm KCN (in 1 mm TES, pH 8) or 10 mm SHAM (in water from a 1.0 m stock solution in dimethylsulphoxide) were applied by sandwiching the plant tissues between medical wipes soaked with the corresponding inhibitor. No recovery from inhibitor treatment was observed because respiratory rates remained constant throughout the experiment. All stocks were freshly prepared before measurement. In addition, for KCN experiments, a piece of tissue wetted with KCN was present in the cuvette (Gonzàlez-Meler et al., 2001). Calculations of the isotopic fractionation were made as described by Guy et al. (1989) and Ribas-Carbo et al. (1995) without Ar correction. The electron partitioning between the two pathways in the absence of inhibitors was calculated as described by Guy et al. (1989) without forcing the slope to intercept the origin. Over the course of the experiment, the root samples consumed at least 10% of the initial oxygen (21%). r2 Values of all unconstrained linear regressions between –lnf and ln(R/Ro) (with at least five data points) were al least 0.995, corresponding to an error in the estimation of less than 0.5‰ (Ribas-Carbo et al., 2000).
Isolation, Blotting, and Immunodetection of the AOX Protein
One hundred to 150 mg fresh weight frozen soybean roots were ground in liquid nitrogen using a mortar and pestle in presence of 1 mL of extraction buffer (50 mm Trizma, 5 mm EDTA, 1% [w/v] SDS, and protease inhibitors [Sigma, St. Louis]). The mixture was centrifuged at 20,000g for 15 min. The protein concentration of the supernatant was estimated by the method of Lowry et al. (1951), and no difference among treatments or over the course of the experiment was found. As such, the gels were loaded on a protein content basis. The samples were mixed with 3× sample buffer (0.5 m phosphate buffer [pH 7], 30% [w/v] glycerol, 7.5% [w/v] SDS, 100 mm dithiothreitol, and 0.75 mm Bromphenol blue) before loading 75 μg of protein to a 10% (w/v) SDS-PAGE gel (Laemmli, 1970). After separation by electrophoresis, proteins were transferred to Immobilon PVDF transfer membranes (Millipore Corporation, Bedford, MA). AOX proteins were tagged with AOX-recognizing monoclonal antibodies (Elthon et al., 1989) at a dilution of 1:500 (w/v), followed by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody at a dilution of 1:1,000 (w/v; DAKO, Boehringer Mannheim/Roche, Basel), and detected by chemiluminescence. Total protein was quantified by scanning densitometry (Phoretix 1D International Ltd., Newcastle-upon-Tyne, UK) using Ponceau staining as loading control.
Statistical Analysis
Data are reported as the mean ± se of at least four replications in two independent experiments for each parameter, as described in the figure legends. The results were compared statistically by using a Fisher's test, and differences were considered significant when P values were ≤0.05.
Acknowledgments
We gratefully thank Larry Giles for all his technical support and Dr. Cesar Arrese-Igor and Dr. Brent Helliker for their helpful thoughts and grammar corrections. IM was a gift from BASF Española S.A. (Barcelona, Spain), and CS was a gift from DuPont Ibérica (Barcelona, Spain). We alsothank Dr. Thomas E. Elthon for his generous gift of the AOX antibodies and Gustavo Garijo for his technical assistance.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.027805.
This research was supported by the Ministerio de Ciencia y Tecnología (project nos. AGL–2000–0974, AGL–2001–1994 and BFI2002-00772), by the Ruth Lee Kennedy-Fulbright Commission (grant to S.G.), by the Universidad Pública de Navarra (grant to S.G.), and by the Ministerio de Educación y Ciencia (grant to A.Z.). This is Carnegie Institution of Washington Publication no. 1553.
References
- Amor Y, Chevion M, Levine A (2000) Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett 477: 175–180 [DOI] [PubMed] [Google Scholar]
- Aubert S, Bligny R, Day DA, Whelan J, Douce R (1997) Induction of alternative oxidase synthesis by herbicides inhibiting branched-chain amino acid synthesis. Plant J 11: 649–657 [Google Scholar]
- Considine MJ, Daley DO, Whelan J (2001) The expression of alternative oxidase and uncoupling protein during fruit ripening in mango. Plant Physiol 126: 1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT (1996) The cyanide-resistant oxidase: to inhibit or not to inhibit, that is the question. Plant Physiol 110: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Millar AH, Wiskich JT, Whelan J (1994) Regulation of alternative oxidase activity by pyruvate in soybean mitochondria. Plant Physiol 106: 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Whelan J, Millar AH, Siedow JN, Wiskich JT (1995) Regulation of the alternative oxidase in plants and fungi. Aust J Plant Physiol 22: 497–509 [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol 89: 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Chipman DM, Barak Z (1996) Metabolic effects of inhibitors of two enzymes of branched-chain amino acid pathway in Salmonella typhimurium. J Bacteriol 178: 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA (1997) Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol 114: 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston S, Zabalza A, González EM, Arrese-Igor C, Aparicio-Tejo PM, Royuela M (2002) Imazethapyr, an inhibitor of the branched-chain amino acid biosynthesis, induces aerobic fermentation in pea plants. Physiol Plant 114: 524–532 [DOI] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Ribas-Carbo M, Giles L, Siedow JN (1999) The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol 120: 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Thomas RB, Giles L, Siedow JN (2001) Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ 24: 205–215 [Google Scholar]
- Good AG, Muench DG (1993) Long term anaerobic metabolism in root tissue: metabolic products of pyruvate metabolism. Plant Physiol 101: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177: 483–491 [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Millar AH, Wiskich JT, Day DA (1995) Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch Biochem Biophys 318: 394–400 [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Rich PR, Zhang Q, Wiskich JT (1997) Substrate kinetics of the plant mitochondrial alternative oxidase and the effects of pyruvate. Plant Physiol 115: 1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IT, Lee HJ, Cho KY, Chun JC (1997) Safening effects of 1,8-naphthalic anhydride in corn plants treated with bensulfuron-methyl and imazaquin. J Pestic Sci 22: 6–11 [Google Scholar]
- Juszczuk IM, Rychter AM (2002) Pyruvate accumulation during phosphate deficiency stress of bean roots. Plant Physiol Biochem 40: 783–788 [Google Scholar]
- Kiddon J, Bender ML, Orchardo J (1993) Isotopic fractionation of oxygen by respiring marine organisms. Global Biogeochem Cycles 7: 679–694 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lee JM, Owen MDK (2000) Comparison of acetolactate synthase enzyme inhibition among resistant and susceptible Xanthium strumarium biotypes. Weed Sci 48: 286–290 [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN (1997) The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol 115: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Far AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- MacRae JC (1971) Quantitative measurement of starch in very small amounts of leaf tissue. Planta 96: 101–108 [DOI] [PubMed] [Google Scholar]
- Meeuse BJD (1975) Thermogenic respiration in aroids. Annu Rev Plant Physiol 26: 117–126 [Google Scholar]
- Millar AH, Finnegan PM, Whelan J, Drevon JJ, Day DA (1997) Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ 20: 1273–1282 [Google Scholar]
- Millar AH, Owen K, Atkin R, Menz I, Henry B, Farquhar G, Day DA (1998) Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol 117: 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA (1993) Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett 329: 259–262 [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H (1998) The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol 118: 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AL, Siedow JN (1991) The regulation and nature of the cyanide resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059: 121–140 [DOI] [PubMed] [Google Scholar]
- Ordog SH, Higgins VJ, Vanlerberghe GC (2002) Mitochondrial alternative oxidase is not a critical component of plant viral resistance but may play a role in the hypersensitive response. Plant Physiol 129: 1858–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons HL, Yip JYH, Vanlerberghe GC (1999) Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiol 121: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis AC (1997) Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol Plant 100: 165–170 [Google Scholar]
- Purvis AC, Shewfelt RL (1993) Does the alternative pathway ameliorate chilling injury in sensitive plant tissues?. Physiol Plant 88: 712–718 [DOI] [PubMed] [Google Scholar]
- Ray TB (1982) The mode of action of chlorsulfuron: a new herbicide for cereals. Pest Biochem Physiol 17: 10–17 [Google Scholar]
- Rhodes D, Hogan AL, Deal L, Jamieson GC, Haworth P (1987) Amino acid metabolism of Lemna minor L.: II. Responses to chlorsulfuron. Plant Physiol 84: 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN (1995) Electon partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol 109: 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Robinson SA, Gonzàlez-Meler MA, Lennon AM, Giles L, Siedow JN, Berry JA (2000) Effects of light on respiration and oxygen isotope fractionation in soybean cotyledons. Plant Cell Environ 23: 983–989 [Google Scholar]
- Rigaud J, Puppo A (1975) Indole-3-acetic catabolism by soybean bacteroids. J Gen Microbiol 88: 223–228 [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA (1995) Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen isotope discrimination. Aust J Plant Physiol 22: 487–496 [Google Scholar]
- Robson CA, Vanlerberghe GC (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathway of programmed cell death. Plant Physiol 129: 1908–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royuela M, González A, González EM, Arrese-Igor C, Aparicio-Tejo PM, González-Murúa C (2000) Physiological consequences of continuous, sublethal imazethapyr supply to pea plants. J Plant Physiol 157: 345–354 [Google Scholar]
- Shaner DL (1991) Physiological effects of the imidazolinone herbicides. In DL Shaner, SL O'Connor, eds, The Imidazonlinoe Herbicides. CRC Press, Inc., Boca Raton, FL, pp 129–137
- Shaner DL, Reider ML (1986) Physiological responses of corn (Zea mays) to AC 243, 997 in combination with valine, leucine, and isoleucine. Pest Biochem Physiol 25: 248–257 [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H (1999) Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol 120: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK (1999) Biosynthesis of valine, leucine, and isoleucine. In BK Singh, ed, Plant Amino Acids: Biochemistry and Biotechnology. Marcel Dekker, Inc., New York, pp 227–247
- Sluse FE, Jarmuszkiewicz W (2000) Activity and functional interaction of alternative oxidase and uncoupling protein in mitochondria from tomato fruit. Braz J Med Biol Res 33: 259–268 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN (1996) The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxidase with sulfhydryl reagents suggests that α-keto acid activation involves the formation of a thiohemiacetal. J Biol Chem 271: 25019–25026 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN (1997) Changes in the redox state of the alternative oxidase regulatory sulfhydryl/disulfide system during mitochondrial isolation: implications for inferences of activity in vivo. Plant Sci 123: 19–28 [Google Scholar]
- Umbach AL, Wiskich JT, Siedow JN (1994) Regulation of alternative oxidase kinetics by pyruvate and intermolecular disulfide and redox status in soybean seedling mitochondria. FEBS Lett 348: 181–184 [DOI] [PubMed] [Google Scholar]
- Van der Werf A, Vand den Berg G, Ravenstein HJL, Lambers H, Eising R (1992) Protein turnover: A significant component of maintenance respiration in roots? In H Lambers, LHW Van der Plas, eds, Molecular, Biochemical and Physiological Aspects of Plant Respiration. SPB Academic Publishing, The Hague, The Netherlands, pp 483–492
- Vanlerberghe GC, Robson CA, Yip JYH (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 129: 1829–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L (1997) Molecular genetic evidence of the ability of alternative oxidase to support respiratory carbon metabolism. Plant Physiol 113: 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161: 495–502 [PubMed] [Google Scholar]
- Wittenbach VA, Abell LM (1999) Inhibition of valine, leucine and isoleucine biosynthesis. In BK Singh, ed, Plant Amino Acids: Biochemistry and Biotechnology. Marcel Dekker, Inc., New York, pp 385–416
- Xie ZX, Chen ZX (2000) Harpin-induced hypersensitive cell death is associated with altered mitochondrial functions in tobacco cells. Mol Plant Microbe-Interact 13: 183–190 [DOI] [PubMed] [Google Scholar]
- Yip JYH, Vanlerberghe GC (2001) Mitochondrial alternative oxidase acts to dampen the generation of active oxygen species during a period of rapid respiration induced to support a high rate of nutrient uptake. Physiol Plant 112: 327–333 [DOI] [PubMed] [Google Scholar]