Abstract
Head and neck metastasis from glioblastoma is rare event usually seen in patients with previous and repeated surgery. We present the case of a 35 yr-old-female suffering from metastatic glioblastoma in cervical lymph node that was diagnosed by fine needle aspiration. During the last 4 yr, she had four separate craniotomies for the recurrent brain tumors. Cytological diagnosis was made by light microscopy with immunostaining with glial fibrillay acid protein. Chemotherapy with vincristine and procarbazine was performed. The cervical masses were decreased in size and some disappeared while the intracranial glioblastoma continued to grow during chemotherapy. We discuss possible explanations for these different courses after chemotherapy in extraneural metastatic glioblastoma and primary intracranial glioblastoma.
Keywords: Drug Therapy, Glioblastoma, Lymph Nodes, Neoplasm Metastasis
INTRODUCTION
Glioblastomas usually spread by local growth and infiltraion rather than by dissemination and metastasis, despite histologic appearances that may be just as malignant as systemic tumours that metastasize widely. Although the development of an extraneural metastasis from gliomas is rare event, the reports were accumulated (1, 3, 4, 6, 7, 9, 11, 12, 14, 15). We report a rare case of glioblastoma that metastasized to the cervical lymph node. It was diagnosed by fine needle aspiration and showed good response to chemotherapy consisted of procarbazine and vincristine while primary intracranial glioblastoma continued to grow during chemotherapy.
CASE REPORT
A 35-yr-old female presented with multiple neck masses. The patient complained recent aggravation of headache and nausea. During the last 4 yr, she had four separate craniotomies for recurrent brain tumors. She received radiation therapy (5,580 cGy) after the first operation. The last operation was performed before two months prior to this admission, and mass was confirmed as glioblastoma with malignant transformation from original anaplastic mixed glioma (Fig. 1A), which was consisting of ependymal and oligodendrogliomatous area with a small portion of astrocytic tumor in pathological finding. On physical examination, multiple subcutaneous masses of various sizes were palpable on the scalp and anterior neck that had been checked normally in previous follow-up period. MRI demonstrated extensive leptomeningeal spread to the left temporo-occipital region and also noted subcutaneous scalp masses in continuity with the intracranial mass through the burr hole site (Fig. 1B, C). MR study also showed multiple lymph adenopathies in the deep cervical region without continuity with scalp mass (Fig. 2). Pathological diagnosis was glioblastoma in the specimen from the last surgery (Fig. 3). Individual tumor cells showed variable cytologic features, including minigemistocytic epithelioid, clear, spindle and giant cells. Mitoses were seen frequently. And also extensive necrosis and endothelial proliferations were noted. Fine needle aspiration biopsy was done on the left antero-lateral neck mass, using a 19G needle. Although a tissue diagnosis was not performed exactly on the lymph node in patient, small amount of perilymph nodal tissue were obtained. Microscopically, small atypical cells infiltrated into the fibroadipose tissue of the cervical soft tissue and immunohistochemical stain showed positive immunoreactivity for glial fibrillay acid protein (GFAP) (Fig. 4). The cytological diagnosis was metastatic glioblastoma. After administration of vincristine and procarbazine, the cervical masses were decreased in size and some disappeared but at the same time the intracranial tumor continued to grow. The patient died 4 months later.
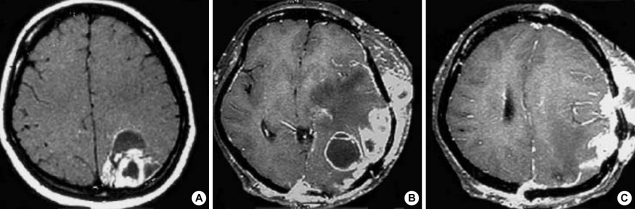
Fig. 1.
Radiologic findings. (A) Gd-enhanced axial T1-weighted MR images before first operation demonstrate a ring-enhanced mass in the left parietal region. (B, C) Gd-enhanced axial T1-weighted MR images show leptomeningeal spread of tumor, and subcutaneous scalp masses that are in continuity with the intracranial mass through the burr hole.
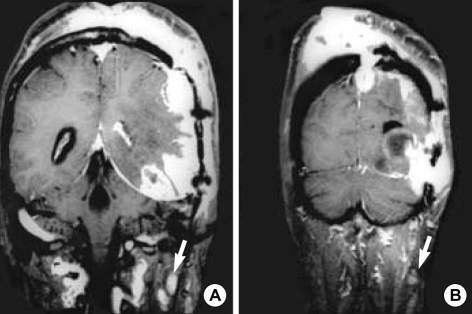
Fig. 2.
Radiologic findings. (A, B) Gd-enhanced coronal T1-weighted MR images of the brain show multiple lymph adenopathies in deep cervical area (arrows). These lymph adenopathies are separated from extended scalp masses.
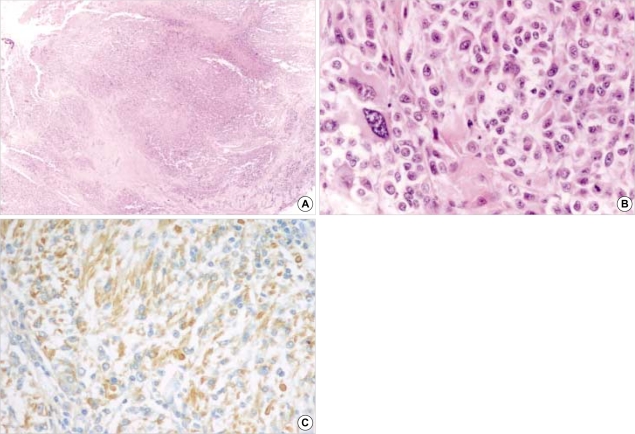
Fig. 3.
Pathologic findings of intracranial glioblastoma. (A, B) Biopsy specimen taken from the last surgery confirms as glioblastoma that had variable histologic features, mitosis, endothelial proliferation and necrosis (A; H&E, ×40: B; H&E, ×400). (C) Immunohistochemical stain demonstrates positive immunoreactivity for GFAP (GFAP immunostain, ×200).
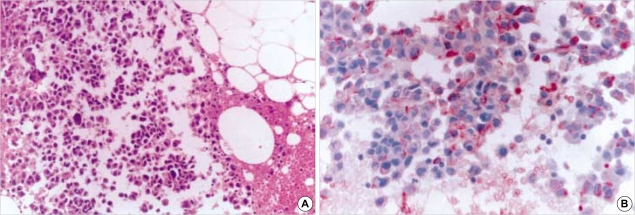
Fig. 4.
Fine needle aspiration smear from metastatic glioblastoma in cervical lymph node. (A) Photomicrograph reveals small atypical cells infiltrated into the fibroadipose tissue in cervical soft tissue (H&E, ×200). (B) Immunohistochemical stain demonstrates positive immunoreactivity for GFAP (GFAP immunostain, ×400).
DISCUSSION
The incidence of extraneural metastases from primary central nervous system tumors is around 0.5% (11). In gliomas it is estimated to approach 20-25 cases per five-year period (3). The most frequent metastatic gliomas are medulloblastoma and malignant astrocytoma/glioblastoma, followed by ependymoma (7).
Metastatic spread of malignant astrocytomas is not unusual and mainly localized to the neuroaxial spread by way of the cerebrospinal fluid (CSF). The incidence of CSF spread in glioblastomas was reported about 20% in autopsy series (5, 16). However, the incidence of symptomatic neuroaxial spread is almost certainly lower than the incidence seen at postmortem (8, 13). Vertosick et al. (13) reported that in 600 cases of supratentorial glioblastoma seen over a 10 yr period, only 11 patients with symptomatic brain stem or spinal cord metastases were identified. Although extraneural metastases of glioblastomas occur in less than 1% of cases, glioblastomas account for about two thirds of the neuroepithelial tumors that metastasize extraneurally (11). The lungs, pleura, lymph nodes, bone marrow, bone and liver are the most common recipients of distant metastases. Among the lymph node metastases, 62% were situated in the cervical areas, often ipsilateral to the site of craniotomy but sometimes bilateral (9).
A variety of hypotheses have been advanced to explain the rarity of extraneural spread of glioblastomas. It has been suggested that glioblastomas are prevented from metastasizing by the impassable dura, by the extracellular matrix, by the tough basement membrane that surrounds intracerebral blood vessels, and by the lack of true lymphatics in the brain (9, 15). Bernstein and Woodard (2) showed that the vital basement membrane was a deterrent to intravasation of glioblastoma cells into the blood vessels.
Extraneural metastases of glioblastoma are most commonly with surgical procedures that may have a chance to access to extracerebral structure, such as ventricular shunting or repeated craniotomies (9). In most cases of lymph node involvement the patients has undergone repeated craniotomies (6), and presumably the tumor gains access to lymphatics by dural or scalp extension through the surgical defect (15).
In addition to clinical history, immunocytochemistry sustained the cytological diagnosis of metastatic glioblastoma on lymph node. The smear from cervical lymph node showed the characteristic features for high-grade gliomas, such as abundant cellularity, necrosis and glomeruloid capillaries (6). The individual tumor cells were small and displayed marked pleomorphism (6, 12). Immunocytochemistry revealed numerous cells with strong immumoreactivities to GFAP antibody (1, 6). Primary intracranial glioblastoma is relatively easy to recognize histologically. However, in the case of extraneural metastasis, a differential diagnosis with other small cell tumors, such as small cell carcinoma, poorly differentiated carcinoma, embryonal rhabdomyosarcoma and neuroblastoma, should be considered (6).
For those few glioblastomas with extraneural metastases, only palliative therapy is available when they cause symptoms although the detection of extraneural metastases is important for prediction of short survival. More discrete areas of tumor in extraneural sites such as bone can be treated with focal radiation. Soft tissue metastases sometimes respond in dramatic fashion to systemic chemotherapy (10). We decided to introduce chemotherapeutic agents to this patient because surgery was not considered any more. Steinbok et al. (12) reported that cervical tumor decreased in size, indicating sensitivity to lomustine, but intracranial tumor was not responded, as in our case. Possible explanations of this phenomenon are inadequate drug delivery to the intracranial tumor, different sensitivities of intracranial and extraneural tumors, or a combination of these factors. Whereas the primary intracranial glioblastoma was made up of large and small cells, the metastatic tumor contained only small cell population. Therefore, small cell population of the glioblastoma might be more sensitive to the chemotherapeutic agents than was the brain tumor as a whole (12).
Presumably, extraneural spread to cervical lymph node developed through the lymphatic system from the scalp mass after repeated craniotomies in this patient. We think that fine needle aspiration and cytology is a simple and reliable diagnostic method and chemotherapy may be helpful in metastatic glioblastoma. To reveal the reason of different response to the chemotherapy between intracranial and metastatic glioblastoma, more clinical studies are needed.
References
- 1.Ates LE, Bayindir C, Bilgic B, Karasu A. Glioblastoma with lymph node metastases. Neuropathology. 2003;23:146–149. doi: 10.1046/j.1440-1789.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein JJ, Woodard CA. Glioblastoma cell do not intravasate into blood vessels. Neurosurgery. 1995;36:124–132. doi: 10.1227/00006123-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: a case report and review of the literature. Neurosurgery. 1985;17:413–418. doi: 10.1227/00006123-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Datta CK, Weinstein JD, Bland JE, Brager PM, Stewart MA. A case of cervical lymph node metastasis resulting from glioblastoma multiforme. W V Med J. 1998;94:276–278. [PubMed] [Google Scholar]
- 5.Erlich SS, Davis RL. Spinal subarachnoid metastasis from primary intracranial glioblastoma multiforme. Cancer. 1978;42:2854–2864. doi: 10.1002/1097-0142(197812)42:6<2854::aid-cncr2820420647>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez Campora R, Otal Salaverri O, Vazquez Ramirez F, Salguero Villadiego M, Galera Davidson H. Metastatic glioblastoma multiforme in cervical lymph nodes: report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1993;37:938–942. [PubMed] [Google Scholar]
- 7.Hoffman HJ, Duffner PK. Extraneural metastases of central nervous system tumors. Cancer. 1985;56:1778–1782. doi: 10.1002/1097-0142(19851001)56:7+<1778::aid-cncr2820561309>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Kim ES, Kang SS, Lee JK, Kim TS, Jung S, Kim JH, Kim SH, Lee JH. Spinal metastases of cerebral glioblastoma : case report. J Korean Neurosurg Soc. 1998;27:660–666. [Google Scholar]
- 9.Pasquier B, Pasquier D, N'Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45:112–125. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Salcman M. Glioblastoma multiforme and anaplastic astrocytoma. In: Kaye AH, Laws ER Jr, editors. Brain tumors. 2nd ed. New York: Churchil Livingstone; 2001. pp. 493–524. [Google Scholar]
- 11.Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969;31:50–58. doi: 10.3171/jns.1969.31.1.0050. [DOI] [PubMed] [Google Scholar]
- 12.Steinbok P, Dolman CL, Goldie JH. Variation in response to CCNU of glioblastoma multiforme in brain and cervical lymph node: case report. J Neurosurg. 1985;62:918–921. doi: 10.3171/jns.1985.62.6.0918. [DOI] [PubMed] [Google Scholar]
- 13.Vertosick FT, Jr, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: a clinical series. Neurosurgery. 1990;27:516–521. doi: 10.1097/00006123-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Vural G, Hagmar B, Walaas L. Extracranial metastasis of glioblastoma multiforme diagnosed by fine-needle aspiration: a report of two cases and a review of the literature. Diagn Cytopathol. 1996;15:60–65. doi: 10.1002/(SICI)1097-0339(199607)15:1<60::AID-DC12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Wallace CJ, Forsyth PA, Edwards DR. Lymph node metastases from glioblastoma multiforme. AJNR Am J Neuroradiol. 1996;17:1929–1931. [PMC free article] [PubMed] [Google Scholar]
- 16.Yung WA, Horten BC, Shapiro WR. Meningeal gliomatosis: a review of 12 cases. Ann Neurol. 1980;8:605–608. doi: 10.1002/ana.410080610. [DOI] [PubMed] [Google Scholar]