Abstract
The expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)2 in the irradiated brain was examined to test how a single high dose radiation, similar to that used for intraoperative radiation therapy given to the normal cerebrum, can affect the vascular endothelium. After a burr hole trephination in the rat skull, the cerebral hemisphere was exposed to a single 10 Gy dose of gamma rays, and the radiation effect was assessed at 1, 2, 4, 6, and 8 weeks after irradiation. His-tological changes, such as reactive gliosis, inflammation, vascular proliferation and necrosis, were correlated with the duration after irradiation. Significant VEGF and FGF2 expression in the 2- and 8-week were detected by enzyme-linked immunosorbent assay quantification in the radiation group. Immunohistochemical study for VEGF was done and the number of positive cells gradually increased over time, compared with the sham operation group. In conclusion, the radiation injuries consisted of radiation necrosis associated with the expression of VEGF and FGF2. These findings indicate that VEGF and FGF2 may play a role in the radiation injuries after intraoperative single high-dose irradiation.
Keywords: Radiotherapy; Vascular Endothelial Growth Factor A; Fibroblast Growth Factor 2; Radiation Injuries, Experimental
INTRODUCTION
Central nervous system (CNS) damage resulting from irradiation is most often characterized by vascular abnormalities, demyelination and, ultimately, necrosis. In the intraoperative radiotherapy (IORT), Matsutani et al. reported that localized necrosis developed in 32% of patients (1), and we previously observed radiation necrosis in the normal rat brain 8 weeks after experimental IORT using 10 Gy (2). Although the pathogenesis of radiation necrosis is not fully understood, it is possible that vascular endothelial cell injury is one of several major culprits. Acute vascular changes within 24 hr after irradiation include increased endothelial cell swelling, vascular permeability and edema, lymphocyte adhesion and infiltration, and apoptosis (3, 4). Late vascular effects occur weeks to months after irradiation and include capillary collapse, thickening of basement membranes, scarring and fibrosis, telangiectasias, and a loss of clonogenic capacity. Despite the importance of radiation effects on the angiogenesis in the CNS, there are limited knowledge and in vivo data on the role of growth factors.
Growth factors currently appear to be the central mediators of angiogenesis and vascular permeability. The fibroblast growth factor (FGF) family is composed of at least nine related mitogens that affect a variety of cells of neuroectodermal or mesenchymal origin. FGF1 or acidic FGF, and FGF2 or basic FGF, have 53% sequence homology (5). They have a strong affinity for heparin and are often associated with the heparin sulfate proteoglycans present in the basement membrane and extracellular matrix. FGF2, also described as a fibroblast mitogen and isolated from bovine pituitary glands and brains, is known to be synthesized by a variety of tumor and endothelial cells (6). It is found in reactive astrocytes and neurons (7, 8). Since it has been known as a potent mitogen for astroglial cells, this factor is also known to be capable of stimulating the replication of multiple cell types including astrocytes (9). Furthermore, FGF2 is a direct angiogenic factor that promotes every phase of the angiogenic process and induces in vitro synthesis of plasminogen activator, a serine protease that plays a critical role in the angiogenetic process and other processes (10).
Vascular endothelial growth factor (VEGF) is a 36,000 to 46,000 Da dimeric glycoprotein with a potent and specific activity for endothelial cell proliferation in vivo (11). VEGF, also known as vascular permeability factor, was originally found to be secreted by tumor cells causing venules and small veins to become hyperpermeable to circulating macromolecules (12). In CNS tumors, expression of VEGF is associated with tumor vascularity and peritumoral edema (13). Its expression is upregulated in the presence of hypoxia, and VEGF mRNA is preferentially expressed in tumor cells on the periphery of necrotic areas, i.e. areas of hypoxia (14). Because radiation necrosis also exists in hypoxic regions on the peripheral necrotic areas, it is conceivable that VEGF may play a critical role in single high-dose irradiation.
In this study, we investigated whether VEGF and FGF2 are upregulated by single high-dose irradiation, similar to that used for IORT given to the normal brain. Our results were consistent with the hypothesis that the majority of delayed vascular changes observed in the irradiated normal brain are due to irradiation-induced upregulation of VEGF and FGF2.
MATERIALS AND METHODS
One hundred Sprague-Dawley rats weighing 350-400 g were used irrespective of their sex. The rats were given water and food ad libitum until the experiment was conducted.
Single high-dose irradiation and forebrain extirpation
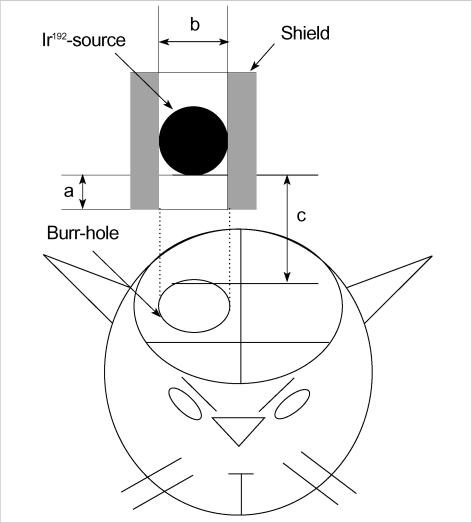
One percent ketamine (50 mg/kg), known to have no radioprotective effect, was intraperitoneally injected and then the heads were fixed to a head holder (SN-8N Semichronic Head holder, Narishige Co., Tokyo, Japan) in a ventral decubitus position. A longitudinal incision was made on the scalp along the median line. Under operative microscopy, an 8-mm diameter hole was drilled on the right parietal area to expose the dura mater. Because it was technically impossible to conduct intraoperative irradiation using electron beams on a tiny rat brain, iridium-192 (GammaMed 12i, MDS Nordion, Canada), which has the same characteristics as the electron beam and is commonly used for high-dose rate brachytherapy, was used instead as a source of radiation. A shield was made of Lipowitz metal (Cerroben®, MED-TEC, Orange, IA, U.S.A.), and then a 0.6-cm hole was made in the middle to ensure that the iridium source was located 0.5 cm above the hole. The source was located sufficiently distant so that the impact of diffused beams on the irradiation site could be minimized, a 1-cm diameter portion on the surface of the rat brain could be exposed to radiation, and intraperitoneally 10 Gy could be irradiated by a single irradiation on the brain surface 2 cm away from the source (Fig. 1). The 10 Gy dose was selected because it is known as the lowest dose to have a radiation effect during intraoperative irradiation. After irradiation, the scalp was sutured in an aseptic condition with absorbable thread. Fifty rats were divided into 5 groups of 10 each, and their forebrains were extirpated at 1, 2, 4, 6, and 8 weeks after irradiation. As the sham operation (control) group, 50 non-irradiated rats also underwent forebrain extirpation at the same time intervals as the irradiation group.
Fig. 1.
Schematic drawing of a burr hole trephination and irradiation. a: diameter of radiation tube, 6 mm; b: distance from Ir-192 source to shield end, 5 mm; c: distance from source to brain surface, 20 mm (Not drawn to scale).
Histopathological examination
The extirpated brains were fixed in 10% buffered formalin for 6 hr to maintain their antigenicity. A coronal section slice of 5-mm anteroposterior diameter was made centered around the irradiated portion. According to the usual tissue processing procedure, tissues were passed through ethanol for dehydration and embedded in paraffin, after which a thin, 5-µm thickness section was made and H-E stained.
Quantitative analysis of VEGF and FGF2 using enzyme-linked immunosorbent assay (ELISA)
Using the quantitative sandwich ELISA method with an ELISA kit (Quantikine®, R&D Systems, Minneapolis, MN, U.S.A.), we quantified the expression of VEGF and FGF2 in the irradiated right hemispheres. To extract the protein, the paraffin-embedded tissues were sectioned at 10-µm thickness, and processed in 100% xylene for 5-10 min at room temperature for deparaffinization. Then they were centrifuged at 13,000 rpm for 3 min twice, and left in 95% acetone at room temperature for 5 min. Again, they were centrifuged at 13,000 rpm for 3 min twice and washed in distilled water for 10 min. After 100 µL of protein lysis buffer was added to each tube, they were left at 4℃ for 1 hr, and then centrifuged at 1,000 rpm for 30 min. Then, the upper layer was separated for the quantitation of protein according to the Bradford method. The specimen was diluted with a coating buffer to make 2 µg/mL, after which 100 µL/well was put into a 96-well plate. The plate was covered, incubated at 37℃ for 2 hr, and then washed with 200 µL/well phosphate-buffered saline Tween-20 (PBST) 3 times at intervals of 3-4 min. With an addition of 3% bovine serum albumin at 200 µL/well, the plate was further incubated for 2 hr at 37℃, and again washed 3 times with PBST. The plate was incubated with primary antibody diluted at 1:200 with PBST (100 µL/well) at 37℃ for 2 hr, and washed 3 times with PBST. Then, it was incubated with secondary antibody diluted at 1:1,500 with PBST (100 µL/well) at 37℃ for 2 hr, and washed 3 times with PBST. Finally, with an addition of substrate peroxidase buffer (100 µL/well), it was left at room temperature for 20 min for a reaction, and 50 µL/well of 5N H2SO4 was added to stop the reaction. By measuring the concentration with a precision microplate reader with an ultraviolet length of 450 nm (Molecular Devices, Sunnyvale, CA, U.S.A.), VEGF and FGF2 were quantified. The expression of VEGF and FGF2 over time after irradiation was measured in each experimental group (irradiated right cerebral hemisphere), and then the values were compared with bilateral hemispheres of the sham operation group.
Immunohistochemical staining of VEGF and FGF2
A paraffin section was heated in an incubator at 56-58℃ for 30 min, and then deparaffinized and dehydrated at room temperature. To restrict the endogenous peroxidase activity, the section was incubated with methanol and 0.3% hydrogen peroxide for 10 min, and then washed 2-3 times with phosphate-buffered saline (PBS, pH 7.4) for 5 min. Next, it was processed with a blocking solution (non-immune sheep serum, DAKO kit 1:5, Carpinteria, CA, U.S.A.) at room temperature for 20 min. The specimen was incubated with monoclonal anti-VEGF (Mouse monoclonal Ig G2a, Santa Cruz Biotechnology, CA, U.S.A.) and FGF2 (Polyclonal rabbit IgG, Oncogene, Cambrige, MA, U.S.A.) diluted at 1:100 in PBS at room temperature for 1 hr. Goat anti-mouse antibody, biotin-labeled secondary antibody, and avidin-biotin complex were administered for 1 hr each. After a 10-min incubation with 3.3-diaminobenzidine tetrahydrochloride as a substrate for a color reaction, the specimen was stained with Mayer's hematoxylin, sealed up with a glycerol mounting medium (DAKO-PATTS), and observed under an optical microscope. As a positive control for the expression of VEGF and FGF2, the spleen and tonsillar tissues were used. With the help of a pathologist, the cells, with their nuclei stained dark brown over the whole scope of the coronal section on the irradiated cerebral cortex, were checked using an optical microscope at a 200 magnification. The expression of VEGF and FGF2 over time after irradiation was compared between experimental groups.
Statistical analyses
The expression of VEGF and FGF2 over time after irradiation was statistically analyzed using Sigmastat for Windows version 1.0 (Jandel Corp., San Rafael, CA, U.S.A.). One-way ANOVA and unpaired t-test were used to verify the measurements within each experimental group, and unpaired t-test was used to verify the differences in measurements between the radiation and control groups. Results are expressed as mean±SD for ELISA analysis and mean±SE for immunohistochemical staining analysis.
RESULTS
Histopathology over time after irradiation
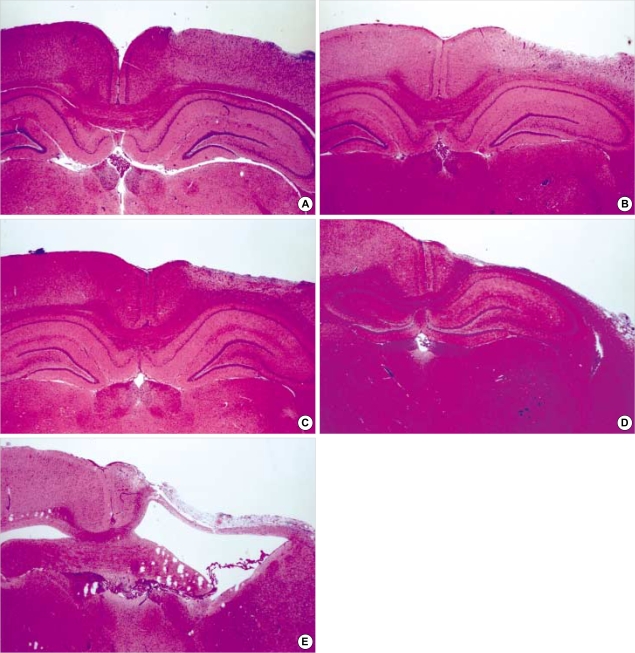
A gradual loss of the outermost molecular layer was shown over time after irradiation, and early cerebral change 1 and 2 weeks after intraoperative irradiation included reactive gliosis, edema, and appearance of inflammary cells. Subacute change 6 and 8 weeks after intraoperatvie irradiation included severe loss of the outermost molecular layer, fibrosis, appearance of inflammatory cells, proliferation of vascular endothelial cells, collapse of the white matter and necrosis (Fig. 2).
Fig. 2.
Morphologic changes after irradiation (H&E, ×12). According to the time intervals, loss of cortical thickness is increased and reactive gliosis, proliferation of vascular endothelial cells, and cystic necrosis become prominent. (A) 1 week, (B) 2 weeks, (C) 4 weeks, (D) 6 weeks, (E) 8 weeks.
Quantitative analyses of VEGF and FGF2 over time after irradiation
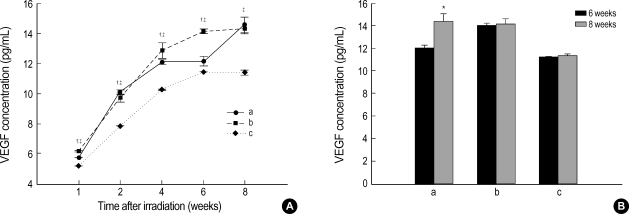
The expressions of VEGF and FGF2 in the radiation and sham operation groups were quantified. At 1, 2, 4, 6, and 8 weeks, the concentration of VEGF in the radiation group was 5.74±0.01 pg/mL, 10.09±0.14 pg/mL, 12.08±0.14 pg/mL, 12.12±0.25 pg/mL, and 14.56±0.52 pg/mL, respectively, as compared with 6.18±0.05 pg/mL, 9.70±0.30 pg/mL, 12.86±0.51 pg/mL, 14.14±0.13 pg/mL, and 14.29±0.36 pg/mL in the right hemisphere of the sham operation group (craniectomy site) and 5.18±0.01 pg/mL, 7.85±0.01 pg/mL, 10.27±0.02 pg/mL, 11.42±0.02 pg/mL, and 11.43±0.09 pg/mL in the left hemisphere (non-craniectomy site) (Fig. 3A). Whereas the concentration of VEGF in the radiation group was significantly higher than in the right hemisphere of sham operation group at 2 weeks, the expression showed a decrease at 1, 4, and 6 weeks (p<0.05). Although the concentration of VEGF at 8 weeks was higher than at 6 weeks in both groups, there was a significantly steep slope of expression in the radiation group between 6 and 8 weeks (p<0.05). As compared with the left hemisphere of the sham operation group, the concentration of VEGF in the radiation group was significantly increased at 1, 2, 4, 6 and 8 weeks (p<0.05).
Fig. 3.
(A) Expression of VEGF according to the time after irradiation. Whereas the level of expression of VEGF was significantly higher than in the right hemisphere(craniectomy site) of the sham operation group at 2 weeks, the expression showed a significant decrease at 1, 4, and 6 weeks (unpaired t-test, p<0.05). As compared with the left hemisphere (non-craniectomy site) of the sham operation group, the concentration of VEGF in the radiation group was significantly increased at 1, 2, 4, 6 and 8 weeks (p<0.05). (B) Although the concentration of VEGF at 8 weeks was higher than at 6 weeks in both groups, there was a significantly steep slope of expression in the radiation group between 6 and 8 weeks (unpaired t-test, p<0.05). a: right hemisphere (craniectomy site) in the radiation group; b: right hemisphere (craniectomy site) in the sham operation group; c: left hemisphere (non-craniectomy site) in the sham operation group.
*p<0.05 by unpaired t-test within the radiation group; †p<0.05 by unpaired t-test compared between radiation and sham operation groups; ‡p<0.05 by unpaired t-test compared between right hemisphere of the radiation group and left hemisphere of the sham operation group.
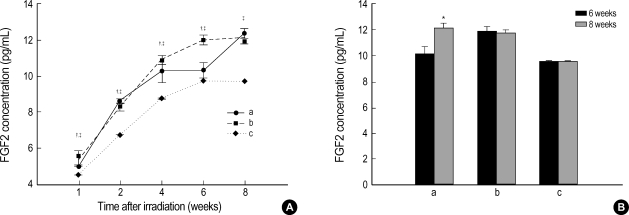
At 1, 2, 4, 6, and 8 weeks, the concentration of FGF2 in the radiation group was 4.99±0.04 pg/mL, 8.47±0.08 pg/mL, 10.18±0.59 pg/mL, 10.22±0.42 pg/mL, and 12.14±0.32 pg/mL, respectively, as compared with 5.48±0.38 pg/mL, 8.20±0.19 pg/mL, 10.79±0.22 pg/mL, 11.91±0.30 pg/mL, and 11.85±0.14 pg/mL in the right hemisphere of the sham operation group and 4.46±0.01 pg/mL, 6.65±0.01 pg/mL, 8.65±0.01 pg/mL, 9.60±0.01 pg/mL, and 9.60±0.01 pg/mL in the left hemisphere (Fig. 4A). Whereas the concentration of FGF2 in the radiation group was significantly higher than that in the right hemisphere of the sham operation group at 2 and 8 weeks, the expression was reduced at 1, 4, and 6 weeks (p<0.05). While the concentration of FGF2 at 8 weeks was less than that at 6 weeks in the right hemisphere of the sham operaion group, there was a significantly steep increase of expression in the radiation group between 6 and 8 weeks, similar to that in the VEGF study (p<0.05). As compared with the left hemisphere of the sham operation group, the concentration of FGF2 in the radiation group was significantly increased at 1, 2, 4, 6 and 8 weeks (p<0.05).
Fig. 4.
(A) Expression of FGF2 according to the time after irradiation. Whereas the level of expression of FGF2 was significantly higher at 2 and 8 weeks than in the right hemisphere (craniectomy site) of the sham operation group, the expression showed a significant decrease at 1, 4, and 6 weeks (unpaired t-test, p<0.05). As compared with the left hemisphere (non-craniectomy site) of the sham operation group, the concentration of FGF2 in the radiation group was significantly increased at 1, 2, 4, 6 and 8 weeks (p<0.05). (B) While the concentration of FGF2 at 8 weeks was lower than that at 6 weeks in the sham operation group, there was a significantly steep incline of expression in the radiation group between 6 and 8 weeks, similar to that in the VEGF study (unpaired t-test, p<0.05). a: right hemisphere (craniectomy site) in the radiation group; b: right hemisphere (craniectomy site) in the sham operation group; c: left hemisphere (non-craniectomy site) in the sham operation group.
*p<0.05 by unpaired t-test within the radiation group, †p<0.05 by unpaired t-test compared between radiation and sham operation groups, ‡p<0.05 by unpaired t-test compared between right hemisphere of the radiation group and left hemisphere of the sham operation group.
Immunohistochemical analyses of the expression of VEGF and FGF2
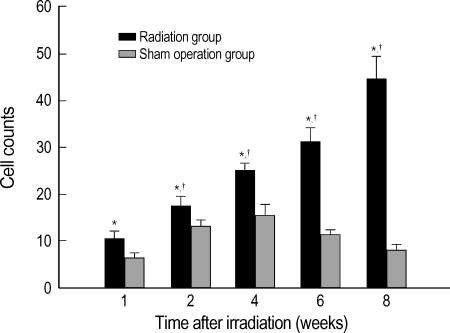
The cell count of immunohistochemical staining for VEGF in the radiation group, at 1, 2, 4, 6, and 8 weeks, was 10.38±1.45 cell/×200, 17.50±1.65, 25.00±1.46, 31.14±2.90, and 44.40±4.83, respectively, which showed a significant increase as compared with 6.25±1.18, 13.29±1.38, 15.50±2.57, 11.43±1.00, and 8.25±1.21 in the right hemisphere of the sham operation group (unpaired t-test, p<0.05), respectively. The expression of VEGF was significant increased within the radiation group over time after irradiation (p<0.05).
Immunohistochemical staining for FGF2 showed severe cross reactivities, so that the results could not be interpreted.
DISCUSSION
IORT enables the intraoperative direct examination of tumors and delivery of a great amount of radiation on the tumor site, which can maximize the radiation effect and effectively prevent the recurrence of tumors (15). Although the risk for normal-tissue injury in the radiotherapeutic management of brain tumors is of major clinical concern, the specific cellular and molecular processes comprising the radioresponse of the CNS remain to be delineated. Furthermore, it has been speculated that normal brain damage by high dose irradiation during operation may be different from that by relatively low radiation, so-called conventional radiotherapy or single high dose irradiation before or after operation. Only a few studies have been done on radiation injuries after single high-dose irradiation, such as radiation necrosis and apoptosis. According to Calvo et al. (16), the incidence of necrosis in the cerebral white matter of the rat after high-dose irradiation is closely related to the amount of radiation and the lapse of time after irradiation, and these effects were associated with a more serious injury of cerebral tissues because of necrosis and proliferation of vascular endothelial cells. Our study also found that over the subacute stage after irradiation of 10 Gy on normal rat cerebral tissues, fibrous gliosis, appearance of inflammatory cells, proliferation of vascular endothelial cells, and necrosis gradually increased over time.
VEGF plays an important role in angiogenesis in the developing CNS (17), but its expression is significantly downregulated in the adult CNS after angiogenesis ceases. It is well established that under hypoxic conditions, upregulation of VEGF mRNA and protein occurs (18, 19). In our series, immunohistochemical studies disclosed strong VEGF-positivity in the radiated normal brain in the subacute stage. Hypoxia was found to be a potent inducer of VEGF expression in several organs including the CNS (20). In a tumor, VEGF is localized in the most ischemic and necrotic areas of the tissues, consistent with the notion that local hypoxia is a potent inducer of VEGF production (21). In non-neoplastic conditions, VEGF is secreted in significant quantities by activated macrophages and microglial cells in response to hypoxic conditions associated with ischemic stroke (22). Thus, VEGF up-regulation in the present study may provide further evidence for the presence of hypoxic condition associated with radiation-induced necrosis.
VEGF mRNA induction by radiation has been demonstrated recently in the neonatal rat spinal cord irradiated by a very high dose of 55 Gy, with an increase in the vascular density being observed at 4 to 5 weeks after irradiation (23). This is in contrast to other reports that showed a degenerative response of the vasculature in the adult CNS following irradiation (24). Although they suggested that the proliferative response observed in their study might be due to the very young age of the animals that were irradiated at postnatal day 0 and 3, we considered that tissue damage associated with angiogenesis and necrosis is one of causes of VEGF expression in subacute period. In the study of delayed cytokines response to brain irradiation without operation, the level of TNF-α mRNA is re-elevated during the subacute time period, 2-3 months after 25 Gy irradiation, and is still elevated at 6 months (25). This data suggest that cytokines such as TNF-α may be involved in late brain responses to irradiation and could contribute to clinical symptoms. In our study, ELISA and immunohistochemial studies showed that VEGF was strongly expressed since the subacute time period.
Responses to the irradiation after craniectomy include several tissue reactions such as inflammation, cerebral edema by traumatic brain injury and ischemia except radiation injury. VEGF expression in inflammatory cells, including plasma cells and macrophages, has been previously described in the tumors and in non-neoplastic conditions of the brain, such as in the retina of diabetics, cerebral infarct, cerebral abscess, or after nervous system trauma (26). VEGF is increased in CSF after pediatric traumatic brain injury and the peak VEGF occur at 22.4 hr after injury (27). By 72 hr after injury, the mean VEGF concentration maintains approximately 1.8 times of control and remains elevated 8 days after injury. In ischemic conditions, there was a significant increase in the expression of serum VEGF, which reach a peak after 7 days and remain elevated after 14 days (28). Therefore, it is conceivable that the increase of expression of VEGF of the control group is related to various tissue reactions, including inflammation, brain tissue injury due to craniectomy, and focal ischemia and can remain elevated after 8 weeks. On the other hand, the volume of irradiated-hemisphere was markedly reduced at 6 and 8 weeks and necrotic tissue that does not have VEGF-expressing cells, such as astrocytes, microglial cells, and macrophages, was prominent. Therefore, the concentration of VEGF to the unit volume using the ELISA method could showed a greater increase in the control group than in the radiation group in 6 and 8 weeks. To eliminate these reactions, we also used non-operated hemisphere (left hemisphere) in the sham operated rats as the other control. This result showed that the expression of VEGF was also increased in the left cerebral hemispheres, although the increase was smaller than that in the right hemispheres. Although the mechanism is unclear, these results suggest the possibilities of inflammation caused by craniectomy or migration of macrophages to the opposite hemispheres from the right hemisphere or inflow of cytokines through the tissue fluid. The concentration of VEGF between 6 and 8 weeks rapidly increased in the radiation group, whereas that in the control group stabilized. These results indiate that the VEGF expression is markedly increased during this period after experimental IORT, when we considered that the number VEGF-expressing cells of unit area in the irradiated group is smaller than control. Furthermore, there was a gradual increase in cells that expressed VEGF in the irradiated white matter except necrotic tissue over time.
FGF2 was first demonstrated in the bovine corneal basement membrane and developing retinal capillaries (29). Endothelial cells synthesize and store FGF2 within the cells and their extracellular matrix, which in vivo includes the basement membrane of the microvascular wall. Folkman et al. hypothesized that tissue injury could release FGF2, thereby triggering angiogenesis (30). The FGF2 may also serve as a reservoir for the vascular mitogen. The FGF2 exists as either a stable, inactive molecule or a rapidly degradable, bioactive one (31). The active form of FGF2 stimulates endothelial cell mitosis, migration, and remodeling of basement membranes, which are three major steps in the process of angiogenesis. FGF has been shown to exert radioprotective effects in several non-CNS tissue systems in vivo and serves as an antiapoptotic survival factor for endothelial cells irradiated in vitro and in a lung model in vivo (4, 32). The present study showed that the concentration of FGF2 in the normal brain was also increased over time after irradiation. We thought that the increase of FGF2 in the control group is related to operation-induced tissue injury. Expression of FGF2 in the radiation group was increased at the subacute stage and these results suggest that FGF2 might play a major role in the normal brain after single high-dose irradiation.
In the present study, we found that the expressions of VEGF and FGF2 in the irradiated cerebral hemispheres were significantly increased over time since the subacute period when compared with those in the sham operation group. These results are consistent with the report that the functions of VEGF and FGF2 are not fixed, but rather have a multidirectional nature depending on given cells, tissues, and their conditions. In this manner, they relate to the proliferation of astrocytes, vascular endothelial cells, and necrosis usually shown after irradiation and contribute to the radiation injury. In summary, we demonstrated that the radiation injuries after IORT, as used for various malignant tumors these days, consist of radiation necrosis, which was associated with the expression of VEGF, and FGF2. Further elucidation of the roles of FGF2 and VEGF in relation to radiation injury, can find appreaches for the prevention of complications after single high-dose radiation therapy.
Fig. 5.
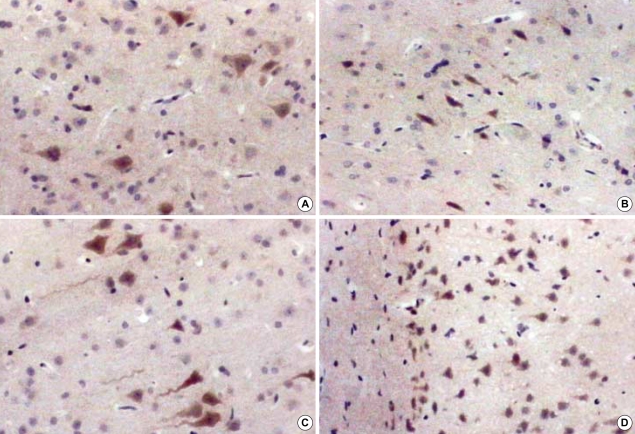
Immunohistochemical stain for VEGF of rat brain at 1 and 8 weeks after irradiation, ×400. In acute stage, there are no prominent changes in the radiation group compared to the sham operation group. According to the time interval, the cellularity becomes more prominent and stained cell counts are increased markedly in the white matter of the radiation group. (A) 1 week in the control group, (B) 1 week in the radiation group, (C) 8 weeks in the control group, (D) 8 weeks in the radiation group.
Fig. 6.
The changes of the mean VEGF staining cell counts under ×200 magnification after irradiation. Values are expressed as mean±standard errors.
*p<0.05 by one way ANOVA within the radiation group. †p<0.05 by unpaired t-test compared between radiation and sham operation groups.
References
- 1.Matsutani M, Nakamura O, Asai A. Intraoperative radiation therapy for glioblastoma multiforme. Saishin Igaku. 1986;41:1506–1513. [Google Scholar]
- 2.Kim JH, Chung YG, Kim HK, Kim CY, Lee HK, Lee KC. Pathological changes in the rat brain after experimental intraoperative radiation. J Korean Neurosurg Soc. 1997;26:1502–1512. [Google Scholar]
- 3.Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989;7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 4.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- 5.Zagzag D. Angiogenic growth factors in neural embryogenesis and neoplasia. Am J Pathol. 1995;146:293–309. [PMC free article] [PubMed] [Google Scholar]
- 6.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 7.Finklestein SP, Apostolides PJ, Caday CG, Prosser J, Philips MF, Klagsbrun M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res. 1988;460:253–259. doi: 10.1016/0006-8993(88)90370-8. [DOI] [PubMed] [Google Scholar]
- 8.Janet T, Grothe C, Pettmann B, Unsicker K, Sensenbrenner M. Immunocytochemical demonstration of fibroblast growth factor in cultured chick and rat neurons. J Neurosci Res. 1988;19:195–201. doi: 10.1002/jnr.490190204. [DOI] [PubMed] [Google Scholar]
- 9.Kniss DA, Burry RW. Serum and fibroblast growth factor stimulate quiescent astrocytes to re-enter the cell cycle. Brain Res. 1988;439:281–288. doi: 10.1016/0006-8993(88)91485-0. [DOI] [PubMed] [Google Scholar]
- 10.Moscatelli D, Rifkin DB. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988;948:67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 11.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 12.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 13.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 14.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 15.Chung YG, Kim CY, Lee HK, Lee KC, Chu JW, Choi MS. Preliminary experiences with intraoperative radiation therapy (IORT) for the treatment of brain tumors. J Korean Med Sci. 1995;10:449–452. doi: 10.3346/jkms.1995.10.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 17.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 18.Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischaemia in the rat. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its recepters. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 20.Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- 21.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 22.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Bartholdi D, Rubin BP, Schwab ME. VEGF mRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur J Neurosci. 1997;9:2549–2560. doi: 10.1111/j.1460-9568.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 24.Stewart PA, Vinters HV, Wong CS. Blood-spinal cord barrier function and morphometry after single doses of x-rays in rat spinal cord. Int J Radiat Oncol Biol Phys. 1995;32:703–711. doi: 10.1016/0360-3016(94)00594-B. [DOI] [PubMed] [Google Scholar]
- 25.Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- 26.Vaquero J, Zurita M, Cincu R. Vascular endothelial growth-permeability factor in granulation tissue of chronic subdural haematomas. Acta Neurochir (Wien) 2002;144:343–347. doi: 10.1007/s007010200047. [DOI] [PubMed] [Google Scholar]
- 27.Shore PM, Jackson EK, Wisniewski SR, Clark RS, Adelson PD, Kochanek PM. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery. 2004;54:605–612. doi: 10.1227/01.neu.0000108642.88724.db. [DOI] [PubMed] [Google Scholar]
- 28.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-β 1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 29.Hanneken A, Lutty GA, McLeod DS, Robey F, Harvey AK, Hjelmeland LM. Localization of basic fibroblast growth factor to the developing capillaries of the bovine retina. J Cell Physiol. 1989;138:115–120. doi: 10.1002/jcp.1041380116. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 31.Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28:1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 32.Ding I, Huang K, Wang X, Greig JR, Miller RW, Okunieff P. Radioprotection of hematopoietic tissue by fibroblast growth factors in fractionated radiation experiments. Acta Oncol. 1997;36:337–340. doi: 10.3109/02841869709001273. [DOI] [PubMed] [Google Scholar]