Abstract
Spontaneous extrahepatic rupture of hepatocellular carcinoma (HCC) is a rare but serious complication that occurs with an incidence of between 5 and 15% of patients with HCC. It is thought to be preceded by rapid expansion due to intratumoral bleed-ing. Extrahepatic rupture of HCC has been reported as a rare complication of tran-scatheter arterial embolization (TAE). Although there have been reports of extrahepatic rupture of HCC after TAE, but there is no report regarding intratumoral hemor-rhage into HCC during TAE. We report a unique case of intratumoral hemorrhage into HCC during TAE presumably triggered by TAE. Although a rare complication, intratumoral hemorrhage into HCC after TAE should be considered in any patient with TAE due to HCC.
Keywords: Chemoembolization, Therapeutic; Carcinoma, Hepatocellular; Liver Neoplasms
INTRODUCTION
Because hepatocellular carcinoma (HCC) receives an abundant blood supply from the hepatic artery, transcatheter arterial embolization (TAE) is a useful treatment for unresectable HCC. Nonetheless, various vascular complications have been reported after successful TAE. Spontaneous hemorrhage of HCC is a critical and life-threatening complication, especially in the patients with chronic liver disease and coagulopathy. Knowledge of the imaging features of spontaneous hemorrhage of HCC is important because saving the patient's life is possible with proper management (1-5). Although there have been reports of spontaneous extrahepatic rupture of HCC after TAE (3, 4), to our knowledge, there is no report regarding intratumoral hemorrhage into HCC during TAE. We report a case of intratumoral hemorrhage into HCC presumably triggered by the first TAE.
CASE REPORT
A 64-yr-old man with chronic hepatitis B presented with a palpable abdominal mass. The laboratory findings were unremarkable except for a slightly elevated serum transaminase level (aspartate aminotransferase (AST): 53 U/L, alanine aminotransferase (ALT): 44 U/L). Ultrasound revealed two relatively well-demarcated large heterogeneous low echoic masses in both lobes of the liver. Contrast-enhanced abdominal CT also demonstrated two well-demarcated masses in both lobes of the liver. These masses showed enhancement in the arterial phase with wash-out in the portal and delayed phases (Fig. 1). Hepatocellular carcinoma was confirmed by ultrasound guided liver biopsy. The patient underwent TAE for treatment of the HCC. First, superior mesenteric arteriography was performed to visualize the portal circulation, but there was no evidence of portal vein thrombosis. A 5-F RH catheter (Cook, Bloomington, IN, U.S.A.) was placed in the proper hepatic artery. Hepatic angiography showed tumor staining in both lobes of the liver (Fig. 2). Embolization was carried out with a 3-F MicroFerret catheter (Cook, Bloomington, IN, U.S.A.).
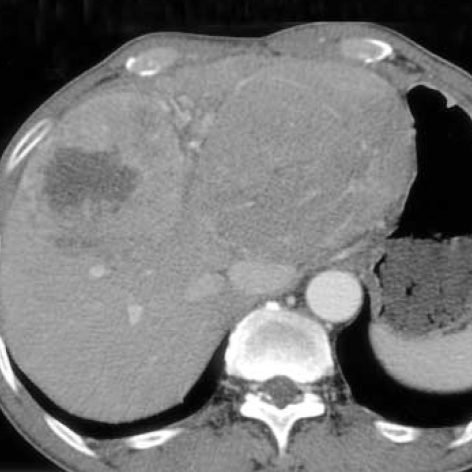
Fig. 1.
Contrast-enhanced CT scanning in the arterial phase shows two well-demarcated masses in both lobes of the liver. A mass in the right lobe shows the enhancement on the arterial phase with central low density. A mass in the left lobe shows homogeneous enhancement.
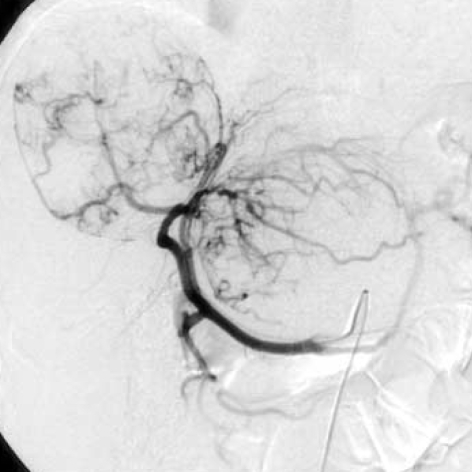
Fig. 2.
Heptic angiography shows tortuous vessels with staining of masses in both lobes of the liver.
The infusion suspension for chemotherapy consisted of 16 mg/m2 of mitomycine and 10 mL of iodized oil (Lipiodol; Guerbet, Aulnarysous-Bois, France). This suspension was infused from the proper hepatic artery. Then transient embolization of the right hepatic artery was performed using absorbable gelatin sponge material (J & J Medical, Gargrave, Skipton, U.K.). After embolization of the right hepatic artery, embolization of the left hepatic artery was performed using the same method. Hepatic angiography showed intratumoral gush of contrast material (Fig. 3). After emergent embolization of the distal left hepatic artery performed using a 20×3 mm sized HILAL coil (Cook, Bloomington, IN, U.S.A.), no more extravasation of contrast material was seen on hepatic angiography.
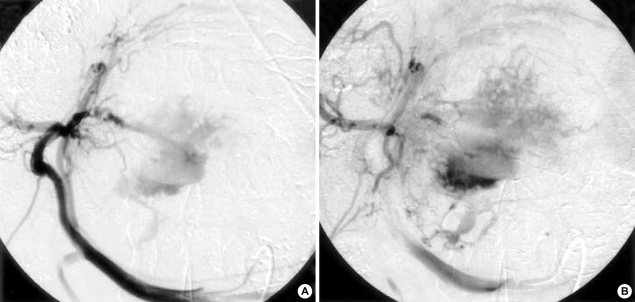
Fig. 3.
Serial hepatic angiography after embolization of the right hepatic artery shows intratumoral gush of contrast material in the left lobe.
After TAE, the patient showed transient elevation of his serum transaminase level (AST: 350 U/L, ALT: 290 U/L) for three days. No additional symptoms or signs were detected. On follow-up contrast-enhanced abdominal CT, the left lobe mass showed massive necrosis with decreased size.
DISCUSSION
Spontaneous extrahepatic rupture of HCC is a rare but serious complication that occurs with an incidence of between 5 and 15% of patients with HCC (1-4). Extrahepatic rupture is usually fatal, especially in patients with underlying liver cirrhosis who may have severe coagulation deficiencies, as surgical treatment or management of bleeding is difficult in most cases. Therefore, extrahepatic rupture of HCC usually has a high mortality rate and the prognosis remains poor (1, 2).
There are many complications after TAE, including fever, abdominal pain, nausea, vomiting, cholecystitis, gastric ulcer, hepatic failure, renal dysfunction, and agranulocytosis caused by anticancer agents. A major complication rate of 5.3% has been reported in these patients (5). Although the incidence of extrahepatic rupture of HCC after TAE ranges from 0.4-0.9%, the mechanism is unclear but may be related to acute ischemic necrosis of the capsule of superficial tumors, hepatic venous obstruction due to tumor invasion, increased pressure inside the tumor induced by TAE, or vascular injury secondary to TAE (2, 3). Development of acute necrotizing angiitis with resultant infiltration of inflammatory cells and thickening of the arterial wall has been reported in arterial embolization with gelatin sponge particles. The arterial wall becomes fragile because of inflammatory and reparative change (3).
In our case, it is intratumoral hemorrhage into the HCC during TAE. The mechanism of intratumoral hemorrhage into the HCC after TAE is unclear and there are no previous reports. Aso et al. (6) reported five patients who developed multiple intrahepatic aneurysms after TAE. In their report, the aneurysms located in the third to sixth order branches of the hepatic arteries and aneurysms were found 25 to 45 days after initial TAE. In their report, TAE was a direct or indirect cause of aneurysms formation. They speculated that the development of multiple intrahepatic aneurysms following TAE was due to mechanical stimulation on angiography from the catheter and guide wire or angiitis caused by the antineoplastic agent and infection due to contamination during TAE.
In our case, infection due to contamination seems unlikely because there was no time for an infection to form and no signs of infection were noted during or after the procedure. We speculate that the development of intratumoral hemorrhage into the HCC after TAE was due to mechanical stimulation during angiography from the catheter and guide wire or angiitis caused by the antineoplastic agent. Although, in our patients, TAE was performed by well-trained radiologists and the bleeding foci were observed in relatively peripheral hepatic arteries. It can be assumed that it was due to stimulation during angiography from the catheter and guide wire and that vascular damage occurred in the pathological vessels of the HCC after TAE, resulting in bleeding into the tumor, which subsequently caused hemorrhage. Another possibility is angiitis caused by the antineoplastic agent. In our patients, TAE was performed with a gelatin sponge, iodized oil, and antineoplastic agents. We speculate that these agents were the main cause of the intratumoral hemorrhage into the HCC. Howerever, further studies will be required to delineate the predisposing factors and mechanism of intratumoral hemorrhage into the HCC after TAE.
In the management of extrahepatic ruptured HCC, several options such as TAE, hepatic artery ligation, and hepatectomy, have been advocated (7). In our case, TAE was performed. The prognosis for extrahepatic ruptured HCC is poor because many of these patients have advanced disease at presentation. In comparison with the high mortality and poor prognosis of extrahepatic ruptured HCC with peritoneal hemorrhage, in our case, intratumoral hemorrhage into the HCC without peritoneal hemorrhage had a good prognosis as this patient exhibited transient elevation of his serum transaminase level and no additional symptoms and signs after TAE. Follow-up contrast-enhanced abdominal CT also demonstrated decreased size of two masses in left lobe of the liver.
Although a rare complication, intratumoral hemorrhage into HCC after TAE should be considered in any patient who has undergone TAE for HCC.
ACKNOWLEDGEMENT
We thank Bonnie Hami, M.A., University Hospitals of Cleveland, Cleveland, Ohio, U.S.A. for her editorial assistance in the preparation of this manuscript.
References
- 1.Chen CY, Lin XZ, Shin JS, Lin CY, Leow TC, Chen CY, Chang TT. Spontaneous rupture of hepatocellular carcinoma: a review of 141 Taiwanese cases and comparison with nonrupture cases. J Clin Gastroenterol. 1995;21:238–242. [PubMed] [Google Scholar]
- 2.Zhu LX, Wang GS, Fan ST. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996;83:602–607. doi: 10.1002/bjs.1800830507. [DOI] [PubMed] [Google Scholar]
- 3.Liu CL, Ngan H, Lo CM, Fan ST. Ruptured hepatocellular carcinoma as a complication of transarterial oily chemoembolization. Br J Surg. 1998;85:512–514. doi: 10.1046/j.1365-2168.1998.00664.x. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto Y, Kita Y, Takayama T, Kawauchi N, Minagawa M, Makuuchi M. Rupture of hepatocellular carcinoma after transcatheter arterial embolization: an unusual case. Hepato-gastroenterology. 1999;46:453–456. [PubMed] [Google Scholar]
- 5.Han MC, Park JH. Interventional radiology. Seoul: Ilchokak; 1999. pp. 161–166. [Google Scholar]
- 6.Aso N, Matsunaga N, Fukuda T, Sakamoto I, Ashizawa K, Aikawa H, Isomoto I, Hayashi K, Fukushima T, Morikawa M. Multiple intrahepatic aneurysms following transcatheter arterial embolization. Radiology. 1994;193:743–746. doi: 10.1148/radiology.193.3.7972817. [DOI] [PubMed] [Google Scholar]
- 7.Hong SW, Kim JH, Wang HJ, Kim MU. Risk factors and treatment strategy of ruptured hepatocellular carcinoma. Korean J Gastroenterol. 1998;32:749–756. [Google Scholar]