Abstract
Leaf trichomes protect plants from attack by insect herbivores and are often induced following damage. Hormonal regulation of this plant induction response has not been previously studied. In a series of experiments, we addressed the effects of artificial damage, jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Artificial damage and jasmonic acid caused significant increases in trichome production of leaves. The jar1-1 mutant exhibited normal trichome induction following treatment with jasmonic acid, suggesting that adenylation of jasmonic acid is not necessary. Salicylic acid had a negative effect on trichome production and consistently reduced the effect of jasmonic acid, suggesting negative cross-talk between the jasmonate and salicylate-dependent defense pathways. Interestingly, the effect of salicylic acid persisted in the nim1-1 mutant, suggesting that the Npr1/Nim1 gene is not downstream of salicylic acid in the negative regulation of trichome production. Last, we found that gibberellin and jasmonic acid had a synergistic effect on the induction of trichomes, suggesting important interactions between these two compounds.
Insect herbivores can substantially reduce the fitness of plants (Crawley, 1983; Marquis, 1992). Because encounters with herbivores can be unpredictable, plants often express low constitutive levels of resistance and then rapidly divert resources to resistance upon the onset of damage (Karban and Baldwin, 1997). Given the generality of this pattern, an understanding of plant induction responses is critical for interpreting ecological interactions between plants and their enemies and for engineering greater protection of crops from agricultural pests (Baker et al., 1997; Thaler et al., 1999).
Many plant species respond to insect damage by increasing the density and/or number of trichomes on new leaves (Myers and Bazely, 1991; Agrawal, 1998, 1999, 2000; Traw, 2002; Traw and Dawson, 2002a). This structural barrier is an important component of resistance to herbivores for plants in general (Levin, 1973; Agren and Schemske, 1994; Fernandes, 1994; Traw and Dawson, 2002b) and for the model plant, Arabidopsis, in particular (Mauricio and Rausher, 1997). The leaf trichome of Arabidopsis is non-glandular but has three sharp points that appear to reduce access of herbivores to the leaf surface (Mauricio and Rausher, 1997). Each trichome is a single epidermal cell supported at the leaf surface by a ring consisting of eight to 12 rectangular cells (Marks, 1997). Due to its large cell size (200–300 μm in length), the trichome of Arabidopsis is widely considered a model system for study of spatial regulation of cell type specification in plants (Haughn and Somerville, 1988; Marks, 1997; Hülskamp and Schnittger, 1998). Factors that regulate trichome production are, therefore, of broad interest. Previous research has identified an important role for the hormone, gibberellin, in constitutive trichome production in Arabidopsis (Chien and Sussex, 1996; Telfer et al., 1997; Perazza et al., 1998). In the current study, we show that two other major plant chemicals, jasmonic acid and salicylic acid, also influence trichome production in Arabidopsis.
Induction of resistance to herbivores and pathogens is generally regulated by a network of signal transduction pathways in which salicylic acid and jasmonic acid function as key signaling molecules (Glazebrook, 2001; Thomma et al., 2001; Kunkel and Brooks, 2002). Herbivore damage and artificial wounding both cause rapid increases in jasmonic acid (Bostock, 1999; Reymond et al., 2000), triggering systemic defenses against herbivores and necrotrophic pathogens. In contrast, infection by biotrophic pathogens causes rapid increases in salicylic acid (Gaffney et al., 1993; Ryals et al., 1994) and systemic expression of defenses against these pathogens. There is substantial evidence that salicylic acid negatively regulates the jasmonate-dependent pathway in many plants (Bostock et al., 2001; Thaler et al., 2002), including Arabidopsis (Spoel et al., 2003; Traw et al., 2003). Despite clear potential for negative cross-talk with respect to structural plant defenses, to our knowledge, no such pattern has been previously shown.
In this study, we first investigated whether artificial wounding increases the density or number of trichomes on newly produced leaves of Arabidopsis. We then asked whether jasmonic acid causes induction of trichomes and whether adenylation of jasmonic acid is necessary. Next, we asked whether salicylic acid has a negative effect on trichome production and whether salicylic acid negatively regulates the response of plants to jasmonic acid. In addition, we assessed the role of the Npr1/Nim1 gene in the induction of trichomes. Last, we applied gibberellin, jasmonic acid, and salicylic acid alone and in combination to assess how gibberellin interacts with jasmonic acid and salicylic acid.
RESULTS
Artificial Wounding Increases Trichome Density and Number
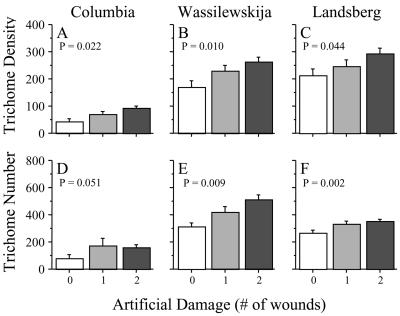
An effect of artificial wounding on trichome induction in Arabidopsis has not been previously reported. We performed such a test on three widely used accessions (Columbia, Wassilewskija, and Landsberg erecta) that serve as backgrounds for important mutants of Arabidopsis. We present trichome density because this metric is most relevant for defense against herbivores (Traw and Dawson, 2002b). We also report trichome number per leaf, because this variable reflects the actual commitment to trichomes by the plant (Roy et al., 1999; Traw, 2002). We found that trichome density and trichome number were higher on new leaves of artificially damaged plants than on the equivalent leaves of control plants (Fig. 1). All three accessions responded to artificial damage with significant induction of trichomes, with responses ranging from 25% to 117% of constitutive values. Because each accession was tested in a separate experiment, no statistical comparison of responses of the accessions was attempted.
Figure 1.
Effect of artificial damage on leaf trichome density (number per square centimeter) and trichome number of adaxial surface of a subsequent leaf. Sample sizes were n = 4, 4, and 24 per treatment for Columbia, Wassilewskija, and Landsberg, respectively. Error bars represent +1se. P values represent a planned pair wise contrast between control and the combined damaged treatments. Differences between the two levels of damage were not significant according to planned pair wise contrasts. We repeated each experiment with similar results.
Jasmonic Acid Increases Trichome Density and Number
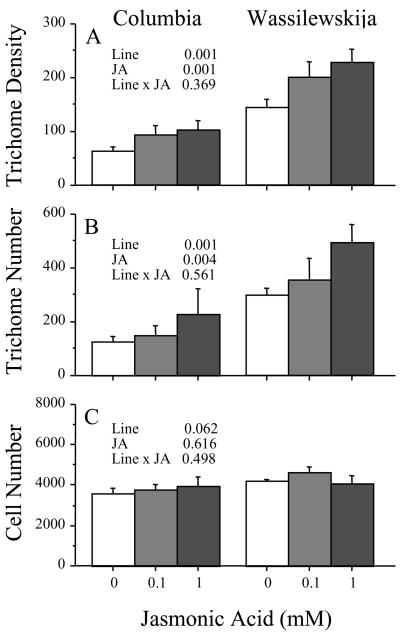
Artificial wounding and herbivory typically trigger jasmonate-dependent responses in plants (Bostock, 1999; Reymond et al., 2000). Having found that artificial wounding increased trichome production, we predicted that jasmonic acid would also cause up-regulation of trichomes. To test this prediction, we applied 0.6 mL of two concentrations (0.1 and 1 mm) of jasmonic acid or a water control to plants of two lines (Columbia and Wassilewskija) in a completely randomized experiment. For details of treatment application, trichome measurements, and statistical analysis see “Materials and Methods.” We found that jasmonic acid caused significant increases in both trichome density (F1,30 = 16.6, P < 0.001; Fig. 2A) and number (F1,27 = 8.6, P = 0.006; Fig. 2B). There was no difference in the effect of 0.1 and 1 mm jasmonic acid on trichome density (F1,30 = 0.98, P = 0.329; Fig. 2A), but the higher concentration did have a significantly greater effect on trichome number (F1,27 = 5.2, P = 0.029; Fig. 2B). Columbia and Wassilewskija responded similarly to jasmonic acid, as shown by the lack of a significant line by treatment interaction term in the analysis of variance for trichome density (Fig. 2A) or trichome number (Fig. 2B).
Figure 2.
Effect of 0.1 and 1 mm jasmonic acid on leaf trichome density, trichome number, and adaxial epidermal cell number for Columbia and Wassilewskija. Error bars represent +1se. P values shown for two-way ANOVA. We repeated the experiment with similar results.
Adenylation of Jasmonic Acid Is Not Required
The jasmonic acid response mutant (jar1-1) produces jasmonic acid but does not adenylate it (Staswick et al., 2002) and therefore lacks induction of some jasmonate-mediated anti-fungal resistance traits (Staswick et al., 1992, 1998). If the Jar1-1 gene is required for jasmonate-dependent induction of trichomes, then we expected that the jar1-1 mutant would not induce trichomes following the application of jasmonic acid, whereas its wild-type background, Columbia, would. To test this prediction, we treated plants of the jar1-1 mutant and Columbia with 0.6 mL of 0.45 mm jasmonic acid or a water control in a completely randomized experiment. We chose 0.45 mm because it was intermediate between the two concentrations we had previously tested on Columbia. Jasmonic acid caused an increase in trichome density of 55.9% for the jar1-1 mutant and an increase of 52.7% for Columbia. Trichome number increased by 27.1% for the jar1-1 mutant and 34.6% for Columbia. In a two-way analysis of variance, these effects of jasmonic acid were significant for both trichome density (F1,11 = 16.9, P = 0.001) and trichome number (F1,11 = 7.4, P = 0.021). The lack of difference in the response of the jar1-1 mutant and its background was shown by the absence of a significant line by treatment effect for either trichome density (F1,11 = 0.01, P = 0.939) or trichome number (F1,11 = 0.39, P = 0.545). This result suggests that the Jar1-1 gene is not required for the induction of trichomes.
Salicylic Acid Decreases Trichome Density and Number
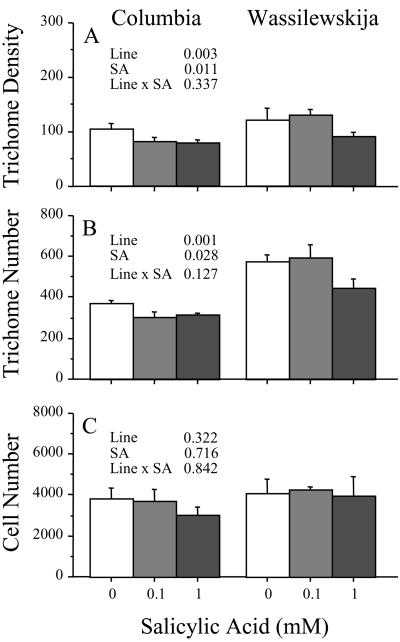
Given that the jasmonate and salicylate pathways generally exhibit negative cross-talk in Arabidopsis (Spoel et al., 2003; Traw et al., 2003; but see van Poecke and Dicke, 2002), we predicted that exogenous salicylic acid would reduce trichome density and number. To test this prediction, we applied two concentrations (0.1 and 1 mm) of salicylic acid or a water control to plants of two lines (Columbia and Wassilewskija) in a completely randomized experiment. We found that salicylic acid caused significant decreases in both trichome density (F1,33 = 7.9, P = 0.008; Fig. 3A) and number (F1,33 = 4.6, P = 0.039; Fig. 3B). There was no difference in the effect of 0.1 and 1 mm salicylic acid on trichome density (F1,33 = 2.6, P = 0.115; Fig. 3A) or trichome number (F1,33 = 3.3, P = 0.076; Fig. 3B). Columbia and Wassilewskija responded similarly to salicylic acid, as shown by the lack of a significant line by treatment interaction term in the analysis of variance for trichome density (Fig. 3A) or trichome number (Fig. 3B). We repeated the experiment with similar results.
Figure 3.
Effect of 0.1 and 1 mm salicylic acid on leaf trichome density, trichome number, and adaxial epidermal cell number for Columbia and Wassilewskija. Error bars represent +1se. P values shown for two-way ANOVA. We performed the experiment twice with similar results.
No Effect of Jasmonic Acid or Salicylic Acid on Epidermal Cell Number
Our finding that jasmonic acid and salicylic acid alter leaf trichome number prompted us to ask whether the response was due to a change in the proportion of epidermal cells receiving the trichome fate or whether there were simply more epidermal cells per leaf. To address this question, we estimated cell numbers on a subset of leaves treated with 0.1 or 1.0 mm jasmonic acid or salicylic acid. We found no evidence of differences in epidermal cell numbers between control leaves and leaves treated with jasmonic acid (Fig. 2C) or salicylic acid (Fig. 3C), thus trichome induction apparently resulted from an increased probability that epidermal cells become trichomes.
Salicylic Acid Inhibits Plant Response to Jasmonic Acid
Having observed differential effects of jasmonic acid and salicylic acid on trichome production, we asked whether these two elicitors interact in their effects on trichome production. If salicylic acid negatively regulates the jasmonate-dependent pathway (Spoel et al., 2003), then we would predict that plants treated with salicylic acid would show less induction of trichomes in response to jasmonic acid.
To test this prediction, we used an intermediate concentration (0.45 mm) for each elicitor. Plants received water, 0.45 mm jasmonic acid alone, 0.45 mm salicylic acid alone, or both 0.45 mm jasmonic acid and 0.45 mm salicylic acid. We applied these four treatments to plants of Columbia and Wassilewskija in a completely randomized experiment. For details of treatment application, trichome measurements, and statistical analysis, see “Materials and Methods.”
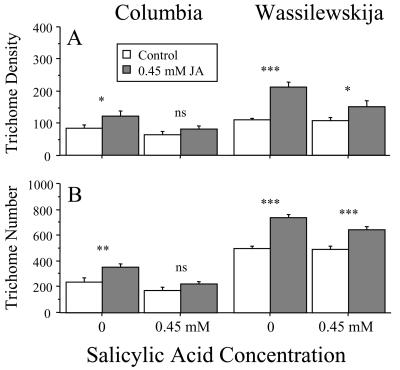
As in the previous experiments, jasmonic acid alone caused large increases in trichome density (Fig. 4A) and number (Fig. 4B). Averaged across the two lines, plants treated with jasmonic acid alone had 70.9% higher trichome density (F1,26 = 32.5, P < 0.001) and 67.8% higher trichome number (F1,26 = 53.8, P < 0.001) relative to control plants. However, when plants received both jasmonic acid and salicylic acid, they produced new leaves with only 18.3% higher trichome density (F1,26 = 2.4, P = 0.127) and 37.4% higher trichome number (F1,26 = 6.3, P = 0.018) relative to equivalent leaves of control plants. This reduced effect of jasmonic acid in the presence of salicylic acid was supported by a significant jasmonic acid by salicylic acid interaction term in the three-way analysis of variance for both trichome density (F1,26 = 5.6, P = 0.025; Table I) and number (F1,26 = 4.6, P = 0.040; Table I). The results were therefore consistent with the presence of negative cross-talk between the salicylate and jasmonate-dependent defense pathways in Arabidopsis.
Figure 4.
Effect of 0.45 mm jasmonic acid (JA) and 0.45 mm salicylic acid (SA) alone and in combination on mean leaf trichome density and number of Columbia and Wassilewskija. Each bar represents the mean of five replicates. Error bars represent +1se. We repeated the experiment with similar results. Significant planned contrasts between treatment and control means are indicated by asterisks. ***, P < 0.001; **, P < 0.01; *, P < 0.05. ns, Nonsignificant.
Table I.
Three-way analysis of variance testing the effects of jasmonic acid (Control, 0.45 mm) and salicylic acid treatment (Control, 0.45 mm) on leaf trichome density (no. / cm2) and no. of two lines (Columbia and Wassilewskija)
Significant P values are indicated in bold. df, degrees of freedom; MS, mean square; JA, jasmonic acid; SA, salicylic acid.
| Source
|
df
|
Trichome Density
|
Trichome No.
|
||||
|---|---|---|---|---|---|---|---|
| MS | F | P | MS | F | P | ||
| Line | 1 | 26,065 | 44.5 | 0.001 | 979,323 | 419.4 | 0.001 |
| JA | 1 | 20,893 | 35.6 | 0.001 | 157,903 | 67.6 | 0.001 |
| SA | 1 | 7,884 | 13.4 | 0.001 | 45,968 | 19.6 | 0.001 |
| Line × JA | 1 | 4,286 | 7.3 | 0.011 | 30,174 | 12.9 | 0.001 |
| Line × SA | 1 | 1 | 0.0 | 0.957 | 4,579 | 1.9 | 0.173 |
| JA × SA | 1 | 3,278 | 5.6 | 0.025 | 10,873 | 4.6 | 0.040 |
| Line × JA × SA | 1 | 566 | 0.9 | 0.334 | 211 | 0.1 | 0.765 |
| Residual | 26 | 585 | 2,334 | ||||
| Total | 33 | ||||||
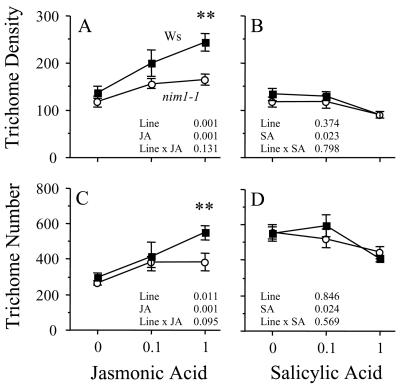
The nim1-1 Mutant Has Reduced Response to Jasmonic Acid
The non-inducible immunity (nim1-1) mutant is in the Wassilewskija background and does not induce defensive proteins following elicitation with biotrophic bacteria or application of salicylic acid (Delaney et al., 1995). The Npr1/Nim1 gene therefore plays a central role in the induction of resistance (Delaney, 2000; Glazebrook, 2001) and appears to act down-stream of salicylic acid in some instances of negative regulation of the jasmonate-dependent pathway (Spoel et al., 2003). For this reason, we predicted that trichome induction in the nim1-1 mutant would have an increased response to jasmonic acid relative to its background and no response to salicylic acid.
To test these predictions, we treated the nim1-1 mutant and Wassilewskija with 0, 0.1, or 1 mm jasmonic acid in one experiment and with 0, 0.1, or 1 mm salicylic acid in another experiment. For details of treatment application, trichome measurements, and statistical analysis, see “Materials and Methods.”
The nim1-1 mutant responded to jasmonic acid with a significant increase in trichome density (Fig. 5A) and trichome number (Fig. 5C). However, the induction response was significantly reduced relative to that of its background, Wassilewskija, at the higher concentration of jasmonic acid for both trichome density (F1,28 = 10.6, P = 0.002) and trichome number (F1,28 = 10.3, P = 0.003). The result was therefore the opposite of our prediction that the nim1-1 mutant would have an increased response to jasmonic acid and suggests that the Npr1/Nim1 gene may accentuate induction of trichomes following the application of jasmonic acid. Additionally, the nim1-1 mutant responded to salicylic acid with a significant decrease in trichome density (Fig. 5B) and trichome number (Fig. 5D), contrary to our second prediction that it would not respond to salicylic acid. Because the response of nim1-1 to salicylic acid did not differ from Wassilewskija, our results suggest that the Npr1/Nim1 gene does not contribute to the negative regulation of trichome induction by salicylic acid.
Figure 5.
Response of Wassilewskija (Ws) and nim1-1 mutant to 0, 0.1, or 1 mm jasmonic acid or salicylic acid. Each bar represents the mean of five replicates. Error bars represent ±1se. We repeated the experiments with similar results. Significant planned contrasts between treatment and control means are indicated by asterisks. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Gibberellin and Jasmonic Acid Have a Synergistic Effect on Trichome Induction
Gibberellin is a hormone that regulates plant growth and developmental events ranging from seed germination to the timing of flowering and senescence. Gibberellin appears to have a primary role in initiating Arabidopsis trichomes (Chien and Sussex, 1996; Telfer et al., 1997; Perazza et al., 1998). For example, the ga1-3 mutant is unable to produce gibberellin and does not produce trichomes. However, when gibberellin is added exogenously, trichome production is restored (Chien and Sussex, 1996). Additionally, wild-type plants are unable to produce trichomes following treatment with two gibberellin biosynthesis inhibitors, paclobutrazol (Chien and Sussex, 1996) and uniconazol (Perazza et al., 1998). Given our observation of positive effects of jasmonic acid on trichome production, we initiated study of how jasmonic acid and salicylic acid interact with gibberellin in the production of trichomes.
To address the interactions among jasmonic acid, salicylic acid, and gibberellin, we applied a 0.45 mm concentration of each compound alone and in all possible combinations. We applied these treatments to plants of Landsberg erecta in a completely randomized experiment. We chose Landsberg erecta because this is the wild-type background for important gibberellin mutants. For details of treatment application, trichome measurements, and statistical analysis, see “Materials and Methods.”
There were strong interactions between jasmonic acid and gibberellin (Table II). In the absence of gibberellin, leaves of plants treated with jasmonic acid exhibited an increase of only 5% in trichome density and number. In the presence of gibberellin, leaves of plants treated with jasmonic acid increased 48.9% in trichome density and 93.1% in trichome number. The greater effect of jasmonic acid in the presence of gibberellin was significant according to the jasmonic acid by gibberellin interaction term in the analysis of variance for trichome density (F1,82 = 3.7, P = 0.056; Table II) and number (F1,82 = 6.9, P = 0.009; Table II).
Table II.
Three-way analysis of variance testing the effects of three eliciting chemicals (JA, GA, and SA) alone and in combination on leaf trichome density (no. / cm2) and number of Landsberg erecta
Significant P values are indicated in bold. df, degrees of freedom; MS, mean square; JA, jasmonic acid; SA, salicylic acid; GA, gibberellin.
| Source
|
df
|
Trichome Density
|
Trichome Number
|
||||
|---|---|---|---|---|---|---|---|
| MS | F | P | MS | F | P | ||
| JA | 1 | 9,052 | 5.3 | 0.022 | 16,300 | 13.2 | 0.001 |
| GA | 1 | 25,820 | 15.3 | 0.001 | 109,013 | 88.3 | 0.001 |
| SA | 1 | 8,293 | 4.9 | 0.029 | 21,856 | 17.7 | 0.001 |
| JA × GA | 1 | 6,295 | 3.7 | 0.056 | 8,623 | 6.9 | 0.009 |
| JA × SA | 1 | 7,970 | 4.7 | 0.032 | 12,591 | 10.2 | 0.002 |
| GA × SA | 1 | 298 | 0.1 | 0.674 | 9,139 | 7.4 | 0.007 |
| JA × GA × SA | 1 | 75 | 0.0 | 0.832 | 1,862 | 1.5 | 0.222 |
| Residual | 82 | 1,680 | 1,233 | ||||
| Total | 89 | ||||||
Additionally, there were strong interactions between jasmonic acid and salicylic acid in the production of trichomes. In the absence of salicylic acid, jasmonic acid caused an increase of 31.4% in trichome density and 35.0% in trichome number. In the presence of salicylic acid, jasmonic acid caused a decrease of 0.9% in trichome density and 0.1% in trichome number. The reduced effect of jasmonic acid in the presence of salicylic acid was significant according to the jasmonic acid by salicylic acid interaction term in the analysis of variance for trichome density (F1,82 = 4.7, P = 0.032; Table II) and number (F1,82 = 10.2, P = 0.002; Table II). This result was consistent with our earlier findings for Columbia and Wassilewskija.
Interestingly, there was also evidence of interaction between gibberellin and salicylic acid. In the absence of salicylic acid, gibberellin caused an increase of 72.0% in trichome number. In the presence of salicylic acid, gibberellin caused only an increase of 29.6% in trichome number. The reduced effect of gibberellin in the presence of salicylic acid was significant according to the gibberellin by salicylic acid interaction term in the analysis of variance for trichome number (F1,82 = 7.4, P = 0.007; Table II). We performed the experiment twice with similar results.
DISCUSSION
Trichomes and thorns are structural traits that protect plants from leaf-damaging insects (Levin, 1973). Studies have shown that individual plants increase or decrease allocation to these physical defenses based on the environmental pressure from herbivores (Pullin and Gilbert, 1989; Baur et al., 1991; Myers and Bazely, 1991), and this study confirms such a response in the model plant, Arabidopsis. Although jasmonic acid has been widely shown to regulate systemic expression of chemical defenses (Karban and Baldwin, 1997), no previous studies have assessed whether the jasmonate-dependent pathway also regulates systemic increases in physical defenses in Arabidopsis. In this study, we observed strong positive effects of jasmonic acid on the trichome density and number of newly produced leaves (Figs. 2, 4, and 5). Our results, therefore, provide the first evidence that jasmonic acid and the jasmonate-dependent pathway regulate trichome induction in Arabidopsis.
Jasmonic acid is either methylated (Seo et al., 2001) or adenylated (Staswick et al., 2002) during the process of induction, with different consequences for the expression of resistance. Staswick et al. (1998) determined that adenylation is particularly important for some jasmonate-dependent induction responses. Our study suggests that adenylated jasmonic acid is not necessary for constitutive trichome production or the induction of trichomes following addition of jasmonic acid. This conclusion is based on the fact that the jar1-1 mutant, which is unable to adenylate jasmonic acid, exhibited trichome induction following the addition of 0.45 mm jasmonic acid that was highly similar to its background, Columbia. Our finding is consistent with recent evidence for JAR1-independent induction of other plant defenses against herbivores (van Poecke and Dicke, 2003).
Salicylic acid or a downstream component reduced trichome density and number on new leaves (Figs. 3, 4, and 5). This is a novel finding for exogenously applied salicylic acid. It is also consistent with observations by Bowling et al. (1997) that the cpr mutant of Arabidopsis, which overexpresses salicylic acid, has reduced trichome densities. In addition to directly inhibiting trichome production, salicylic acid reduced the positive effects of jasmonic acid on trichome induction for all three lines (Tables I and II). Because some plants respond to biotrophic bacteria by rapidly increasing tissue concentrations of salicylic acid (Gaffney et al., 1993; Ryals et al., 1994; Ton et al., 2002), we predict that biotrophic pathogen attack will reduce plant allocation to trichomes and thereby decrease resistance to insect herbivores. Negative effects of biotrophic bacterial infection on plant resistance to an insect herbivore have been shown in Arabidopsis (Cui et al., 2002), but the role of trichome down-regulation via the salicylate-dependent pathway has not been specifically addressed. Interestingly, some herbivores induce both the jasmonate and salicylate-dependent pathways (Stotz et al., 2002; van Poecke and Dicke, 2002), and thus feeding by herbivores may not always lead to a systemic increase in trichome production. Herbivores may even exploit the salicylic acid pathway to reduce expression of jasmonate-dependent responses, but there is little data on this topic (Bostock, 1999; Bostock et al., 2001).
Our results suggest a role of the Npr1/Nim1 gene in the trichome induction response to jasmonic acid but not in the manner suggested for other jasmonatedependent responses (Spoel et al., 2003). Npr1/Nim1 codes for a nuclear protein that is necessary for downstream activity of the salicylate-dependent pathway (Cao et al., 1997; Delaney, 2000). The nim1-1 mutant therefore has increased susceptibility to biotrophic pathogens due to its inability to induce pathogen-related defenses (Delaney, 2000). In our study, the nim1-1 mutant produced normal constitutive trichome densities and responded to salicylic acid with a reduction in trichome production similar to wild-type plants (Fig. 5, B and D), suggesting that the Npr1/Nim1 gene is not downstream of salicylic acid in the negative regulation of trichome production. Interestingly, the nim1-1 mutant had reduced response to 1 mm jasmonic acid relative to its wild-type background, the Wassilewskija ecotype (Fig. 5, A and C). Thus, the Npr1/Nim1 gene appears to increase rather than decrease trichome induction at this concentration of jasmonic acid. In a previous study, we found elevated susceptibility of the nim1-1 mutant to a biotrophic pathogen (Traw et al., 2003) consistent with general expectations with this mutant. Assessment of effects of jasmonic acid on trichome induction in the npr1 mutant, which is in the Columbia background, will be an important follow-up to this research. Application of salicylic acid increased susceptibility of the npr1 mutant to a generalist herbivore (Stotz et al., 2002), which is consistent with our finding that salicylic acid can inhibit the jasmonate-dependent pathway independently of the Npr1/Nim1 gene.
Our results also support a synergistic association between gibberellin and jasmonic acid in the induction of trichomes. Some evidence suggests that jasmonate may up-regulate gibberellin in wounded petunia (Petunia hybrida) corollas (Tamari et al., 1995). Whether the apparent synergism in trichome induction is due to the up-regulation of gibberellin by jasmonic acid is not yet clear.
In summary, our study provides the first evidence that artificial damage and jasmonic acid cause the induction of trichomes in Arabidopsis. The effect of jasmonic acid reflected an increase in the proportion of cells that became trichomes, rather than an increase in the overall epidermal cell number of the leaves. Adenylation of jasmonic acid was not necessary for the induction response. Salicylic acid down-regulated trichome production and exhibited negative cross-talk with the jasmonate-dependent pathway. This is the first observation of negative cross-talk between these two major induction pathways in their effects on a physical/structural plant defense. Gibberellin and jasmonic acid exhibited a synergistic effect on trichome production, suggesting an important link between these two pathways in Arabidopsis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Columbia (Col-0) and Wassilewskija (Ws-0) ecotypes, as well as the jar1-1 mutant, were obtained from the Arabidopsis Biological Resource Center. The nim1-1 mutant and Landsberg erecta ecotype were provided by Novartis Agribusiness Biotechnology (Basel, Switzerland) and Lehle Seeds (Round Rock, TX), respectively. Seeds were sown on wet Promix-BX (Premier Horticulture, Red Hill, PA) in 36-celled flats. Flats were placed in the dark at 4°C for 3 d and moved 3 d later to an environmental growth chamber with a temperature of 20°C and 12 h of artificial light at 500 μmol m–2 s–1 photosynthetically active radiation from halogen arc lamps. The day of germination was considered d 1 of the experiment. Plants were watered daily and fertilized twice per week with 30 mL of 200 1 g L–1 Peter's 15:16:17 NPK solution. Flats were thinned to one plant per cell on d 10.
Application of Treatments
Artificial wounding consisted of one or two pinches with serrated forceps to each of the three largest leaves of plants on d 19 of plant growth, when plants typically had six to eight leaves on their main stem. Solutions of jasmonic acid (J-2500, Sigma-Aldrich, St. Louis), salicylic acid (S-7401, Sigma-Aldrich), and gibberellin (A3; #G-1025, Sigma-Aldrich) were neutralized to pH 7 through the addition of NaOH. Jasmonic acid was added from a concentrated stock solution containing 100% (v/v) ethanol. Each treatment consisted of 0.6 mL of the appropriate solution, applied by pipettor to the top surface of all leaves greater than 1 cm in length. Controls for the application of salicylic acid and gibberellin received 0.6 mL of water, whereas controls for the application of jasmonic acid received 0.6 mL of water and a minute amount of ethanol, to control for the presence of ethanol in the stock solution. Jasmonic acid and gibberellin were applied on d 19, whereas salicylic acid was applied on d 20 to allow the other compounds sufficient time to be taken up by the plant. After the application of salicylic acid, the youngest visible leaf was marked with black ink to facilitate later selection of a target leaf for trichome measurement.
Measurement of Trichome Density and Number
We measured trichome density and number on the second new leaf produced following the application of treatments. This leaf was chosen because, at the time of damage, it was the leaf most likely to be in the developmental stage at which trichome fates are assigned to cells (Larkin et al., 1996; Traw and Dawson, 2002a). Arabidopsis exhibits 2/5 phyllotaxy, which means that from a given leaf, two revolutions and five leaves are required to return to the same position. Thus, leaves are spaced by roughly 140° around the stem (Hamada et al., 2000), and we were able to be very accurate in determining the second new leaf above the leaf marked at the time of treatment. Additionally, we used this same phyllotaxy principle to determine the position of our target leaves relative to the first true leaf. Our target leaf upon which trichomes were measured was typically the tenth or eleventh leaf on the main stem.
The target leaf was first removed from the plant and traced. A leaf disc (area = 0.29 cm2) was then removed by hole punch from the center of the leaf blade and counted for adaxial trichome number under a dissecting microscope (Zeiss, Jena, Germany). Trichome density was calculated as the trichome number per disc divided by the disc area. Leaf tracings were scanned and digitized for area determination using a graphic analysis program (ImageJ for Windows v1.29). Leaf trichome number was calculated as the trichome density multiplied by the leaf area. Because trichomes are distributed uniformly across the upper surface of mature leaves of Arabidopsis (Larkin et al., 1996), these discs were probably fairly accurate in estimating the total number of trichomes on the adaxial surface of the leaf and allowed us to measure a sufficient number of leaves for statistical analysis.
Measurement of Epidermal Cell Number
Top-surface epidermal cell number was scaled up from cell counts of a small rectangular area randomly selected on each leaf disc (area = 0.091 cm2) as seen under the 40× oil immersion objective of a compound microscope. Cell density was multiplied by leaf area to provide an estimate of top-surface epidermal cell number per leaf. Because cell counts were highly time consuming, only two replicate plants per treatment were measured.
Statistical Analysis
Unless otherwise noted, each mean represents the average of five replicate plants. We analyzed each experiment by ANOVA. In several of the analyses, we removed outliers so that the residuals satisfied the assumption of normality, but this did not alter the conclusions drawn. In tables and figures, all P values survived sequential Bonferroni corrections to correct table and figure-wide confidence to α = 0.05 (Rice, 1989). We used planned pair wise contrasts within each ANOVA to compare treatment and control means.
Acknowledgments
We thank J. Coswell, S. Suwanski, and J. Zdenek for plant care and M. Goto for assistance with measurements. We thank J. Larkin, J. Greenberg, and J. Lu for helpful discussions and C. Ballare, A. Agrawal, and two anonymous reviewers for comments on the manuscript. We are grateful to Novartis Agribusiness Biotechnology for providing the nim1-1 mutant. We thank the Arabidopsis Biological Resource Center for the jar1-1 mutant.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027086.
This work was supported by the National Science Foundation (Doctoral Dissertation Improvement grant no. IBN 0073095 to M.B.T.) and by the National Institutes of Health (grant no. GM 62504 to J.B.). M.B.T. was supported in part by a V. Dropkin Foundation postdoctoral fellowship.
References
- Agrawal AA (1998) Induced responses to herbivory and increased plant performance. Science 279: 1201–1202 [DOI] [PubMed] [Google Scholar]
- Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80: 1713–1723 [Google Scholar]
- Agrawal AA (2000) Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 81: 1804–1813 [Google Scholar]
- Agren J, Schemske DW (1994) Evolution of trichome number in a naturalized population of Brassica rapa. Am Nat 143: 1–13 [Google Scholar]
- Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP (1997) Signaling in plant-microbe interactions. Science 276: 726–733 [DOI] [PubMed] [Google Scholar]
- Baur R, Binder S, Benz G (1991) Nonglandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L), against the chrysomelid beetle, Agelastica alni L. Oecologia 87: 219–226 [DOI] [PubMed] [Google Scholar]
- Bostock RM (1999) Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol 55: 99–109 [Google Scholar]
- Bostock RM, Karban R, Thaler JS, Weyman PD, Gilchrist D (2001) Signal interactions in induced resistance to pathogens and insect herbivores. Eur J Plant Pathol 107: 103–111 [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Chien JC, Sussex IM (1996) Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol 111: 1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ (1983) Herbivory: The Dynamics of Animal-Plant Interactions. University of California Press, Berkeley
- Cui J, Jander G, Racki LR, Kim P, Pierce NE, Ausubel FM (2002) Signals involved in Arabidopsis resistance to Trichoplusia ni caterpillars induced by virulent and avirulent strains of the phytopathogen Pseudomonas syringae. Plant Physiol 129: 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP (2000) New mutants provide clues into regulation of systemic acquired resistance. Trends Plant Sci 5: 49–51 [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92: 6602–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes GW (1994) Plant mechanical defenses against insect herbivory. Rev Bras Entomol 38: 421–433 [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2001) Genes controlling expression of defense responses in Arabidopsis: 2001 status. Curr Opin Plant Biol 4: 301–308 [DOI] [PubMed] [Google Scholar]
- Hamada S, Onouchi H, Tanaka H, Kudo M, Liu Y, Shibata D, Machida C, Machida Y (2000) Mutations in the WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves. Plant J 24: 91–101 [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville CR (1988) Genetic control of morphogenesis in Arabidopsis. Dev Genet 9: 73–89 [Google Scholar]
- Hülskamp M, Schnittger A (1998) Spatial regulation of trichome formation Arabidopsis thaliana. Semin Cell Dev Biol 9: 213–220 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks MD (1996) The control of trichome spacing and number in Arabidopsis. Development 122: 997–1005 [DOI] [PubMed] [Google Scholar]
- Levin DA (1973) The role of trichomes in plant defense. Q Rev Biol 48: 3–15 [Google Scholar]
- Marks MD (1997) Molecular genetic analysis of trichome development in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 48: 137–163 [DOI] [PubMed] [Google Scholar]
- Marquis RJ (1992) The selective impact of herbivores. In RS Fritz, EL Simms, eds, Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics. University of Chicago Press, Chicago, pp 301–325
- Mauricio R, Rausher MD (1997) Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51: 1435–1444 [DOI] [PubMed] [Google Scholar]
- Myers JH, Bazely D (1991) Thorns, spines, prickles, and hairs: Are they stimulated by herbivory and do they deter herbivores? In DW Tallamy, MJ Raupp, eds, Phytochemical Induction by Herbivores. Wiley, New York, pp 325–344
- Perazza D, Vachon G, Herzog M (1998) Gibberellins promote trichome formation by up-regulating GLABROUS1 in Arabidopsis. Plant Physiol 117: 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullin AS, Gilbert JE (1989) The stinging nettle, Urtica dioica, increases trichome density after herbivore and mechanical damage. Oikos 54: 275–280 [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR (1989) Analyzing tables of statistical tests. Evolution 43: 223–225 [DOI] [PubMed] [Google Scholar]
- Roy BA, Stanton ML, Eppley SM (1999) Effects of environmental stress on leaf hair density and consequences for selection. J Evol Biol 12: 1089–1103 [Google Scholar]
- Ryals J, Uknes S, Ward E (1994) Systemic acquired resistance. Plant Physiol 104: 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong J, Lee Y, Lee Y, Hwang I, Lee JS, Choi YD (2001) Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA 98: 4788–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, van Pelt JA, Mueller MJ, Buchala AJ, Metraux J, Brown R, Kazan K et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15: 747–754 [DOI] [PubMed] [Google Scholar]
- Stotz HU, Koch T, Biedermann A, Weniger K, Boland W, Mitchell-Olds T (2002) Evidence for regulation of resistance in Arabidopsis to Egyptian cotton worm by salicylic and jasmonic acid signaling pathways. Planta 214: 648–652 [DOI] [PubMed] [Google Scholar]
- Tamari G, Borochov A, Atzorn R, Weiss D (1995) Methyl jasmonate induces pigmentation and flavinoid gene expression in petunia corollas: a possible role in wound response. Physiol Plant 94: 45–50 [Google Scholar]
- Telfer A, Bollman KM, Poethig RS (1997) Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124: 645–654 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Tradeoffs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol 25: 1597–1609 [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131: 227–235 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Penninckx IAMA, Broekaert WF, Cammue BPA (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Ton J, De Vos M, Robben C, Buchala A, Metraux JP, van Loon LC, Pieterse CMJ (2002) Characterization of Arabidopsis enhanced disease susceptibility mutants that are affected in systemically induced resistance. Plant J 29: 11–21 [DOI] [PubMed] [Google Scholar]
- Traw MB (2002) Is induction response negatively correlated with constitutive resistance in black mustard? Evolution 56: 2196–2205 [DOI] [PubMed] [Google Scholar]
- Traw MB, Dawson TE (2002a) Differential induction of trichomes by three herbivores of black mustard. Oecologia 131: 526–532 [DOI] [PubMed] [Google Scholar]
- Traw MB, Dawson TE (2002b) Reduced performance of two specialist herbivores (Lepidoptera: Pieridae, Coleoptera: Chrysomelidae) on new leaves of damaged black mustard plants. Environ Entomol 31: 714–722 [Google Scholar]
- Traw MB, Kim J, Enright S, Cipollini DF, Bergelson J (2003) Negative cross-talk between salicylate and jasmonate-mediated pathways in the Wassilewskija ecotype of Arabidopsis thaliana. Mol Ecol 12: 1125–1135 [DOI] [PubMed] [Google Scholar]
- van Poecke RMP, Dicke M (2002) Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J Exp Biol 53: 1793–1799 [DOI] [PubMed] [Google Scholar]
- van Poecke RMP, Dicke M (2003) Signal transduction downstream of salicylic and jasmonic acid in herbivory-induced parasitoid attraction by Arabidopsis is independent of JAR1 and NPR1. Plant Cell Environ 26: 1541–1548 [Google Scholar]