Abstract
Phosphatidylglycerol (PG) is a ubiquitous component of thylakoid membranes. Experiments with the pgsA mutant of the cyanobacterium Synechocystis sp. PCC6803 defective in biosynthesis of PG have demonstrated an indispensable role of PG in photosynthesis. In the present study, we have investigated the light susceptibility of the pgsA mutant with regard to the maintenance of the photosynthetic machinery. Growth of the mutant cells without PG increased the light susceptibility of the cells and resulted in severe photoinhibition of photosynthesis upon a high-light treatment, whereas the growth in the presence of PG was protected against photoinhibition. Photoinhibition induced by PG deprivation was mainly caused by an impairment of the restoration process. The primary target of the light-induced damage in thylakoid membranes, the D1 protein of photosystem (PS) II was, however, synthesized and degraded with similar rates irrespective of whether the mutant cells were incubated with PG or not. Intriguingly, it was found that instead of the synthesis of the D1 protein, the dimerization of the PSII core monomers was impaired in the PG-deprived mutant cells. Addition of PG to photoinhibited cells restored the dimerization capacity of PSII core monomers. These results suggest that PG plays an important role in the maintenance of the photosynthetic machinery through the dimerization and reactivation of the PSII core complex.
Phosphatidylglycerol (PG) is a ubiquitous phospholipid in almost all organisms. In photosynthetic organisms, the majority of PG is found in thylakoid membranes, which are the sites of the light reactions of photosynthesis. PG is the only phospholipid of thylakoid membranes (Block et al., 1983; Wada and Murata, 1998), which are predominantly composed of glycolipids, monogalactosyldiacylglycerol, digalactosyldiacylglycerol, and sulfoquinovosyldiacylglycerol (Somerville et al., 2000). Some limited information on the role of PG in photosynthesis has previously been obtained using either biochemical or molecular genetic approach. To clarify the role of PG in thylakoid membranes, mutants defective in the biosynthesis of PG are of particular value.
The pgp1 mutant of Arabidopsis has a point mutation in PGP1 gene, encoding a PG phosphate (PGP) synthase targeted to plastids and mitochondria, and has the PG content reduced to about 70% of that found in the wild type. The leaves of pgp1 mutant plants are pale green, and their photosynthetic activity is slightly impaired (Xu et al., 2002). Moreover, a disruption of the PGP1 gene by the insertion of T-DNA resulted in severe inhibition of growth and chloroplast development (Hagio et al., 2002; Babiychuk et al., 2003). These findings demonstrated that PG is essential for growth and chloroplast development. The function of PG has also been studied with the PG mutants of the cyanobacterium Synechocystis sp. PCC6803, in which the pgsA or the cdsA gene, encoding a PGP synthase and a CDP-diacylglycerol synthase, respectively, was disrupted (Hagio et al., 2000; Sato et al., 2000). The growth of these mutants in the growth medium supplemented with PG was comparable with that of the wild type. However, deprivation of PG from the growth medium reduced the PG content of the mutant cells and inhibited the growth. Concomitantly, the photosynthetic activity also decreased.
Deprivation of PG from isolated thylakoid membranes by treatment with phospholipase A2 or C resulted in suppression of photosystem (PS) II electron transport with almost no effect on PSI (Jordan et al., 1983; Droppa et al., 1995). In line with this observation, detailed studies with the pgsA mutant indicated that reoxidation of QA– by QB in PSII reaction centers was impaired after deprivation of PG, suggesting that PG is indispensable for the structural integrity of the QB binding site in PSII (Gombos et al., 2002). The fact that PSII is a major target of various kinds of stresses, particularly the light stress (Aro et al., 1993; Andersson and Aro, 2001), prompted us to study how the pgsA mutant responds to a high-light (HL) stress and maintains its photosynthetic machinery under PG deprivation.
RESULTS
PG Deprivation Increases the Light Susceptibility of Mutant Cells
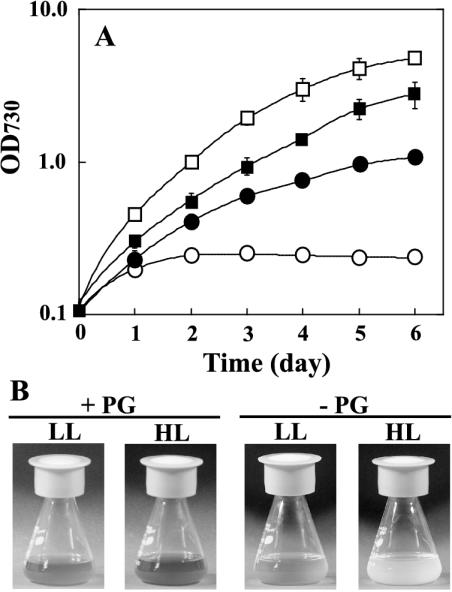
To investigate the light susceptibility of the pgsA mutant cells, the effect of HL treatment on the growth of the mutant cells was first analyzed. The mutant cells grown under low-light (LL) conditions in the presence of PG were transferred to fresh BG-11 medium with or without PG and incubated under LL or HL conditions. As shown in Figure 1A, HL enhanced the growth of the mutant cells in the presence of PG. However, growth of the mutant cells was strictly limited in the absence of PG, and photo-bleaching of the cells was observed 3 or 4 d after the transfer of the cells to HL conditions (Fig. 1B). These results suggest that PG is important for adaptation to HL conditions, and that the decrease in PG content in thylakoid membranes increases the light susceptibility of the cells.
Figure 1.
Effect of light intensity on growth of the pgsA mutant cells. A, The pgsA mutant cells grown in the presence of PG were transferred to fresh BG-11 medium and incubated under HL (white symbols) or LL (black symbols) conditions in the presence (square) or absence (circle) of PG. The values are averages of three or four independent experiments, and the bars represent the sd. B, Cultures of the pgsA mutant cells incubated with or without PG for 5 d under LL or HL conditions.
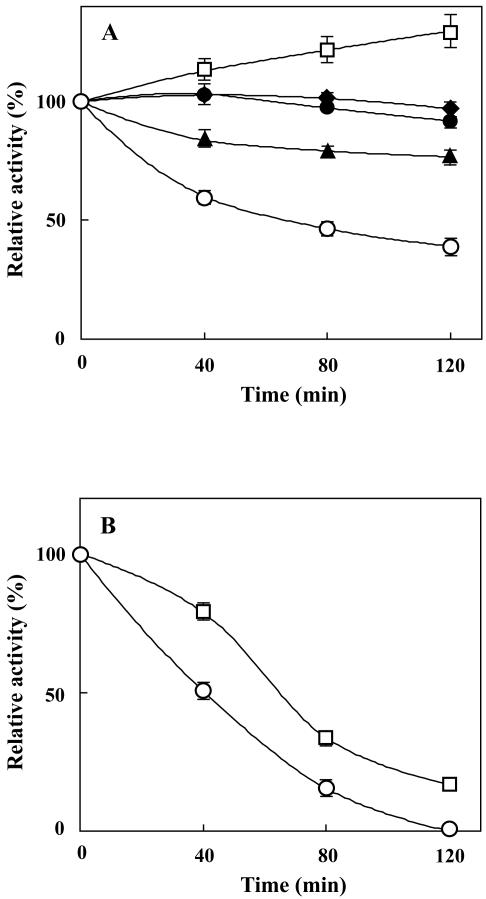
To resolve whether the inhibition of growth of the mutant cells at HL in the absence of PG was caused by photoinhibition of photosynthesis, the photosynthetic activity was monitored in the PG-deprived mutant cells upon transfer to HL conditions. The PG-deprived mutant cells contained 5% of PG in total lipids in contrast to 20% in the wild-type cells. As shown in Figure 2A, the photosynthetic activity decreased to less than 40% of the initial value during the HL treatment for 120 min. Such a decrease in the activity was not observed in the mutant cells incubated with PG. The degree of photoinhibition of photosynthesis was strictly dependent on the light intensity, and the higher light intensity induced more severe photoinhibition.
Figure 2.
Photosynthetic activity of the pgsA mutant cells upon incubation under different light intensity in the presence and absence of protein synthesis. A, Effect of light intensity. The pgsA mutant cells grown under LL conditions for 5 d in the absence of PG were transferred to fresh BG-11 medium without PG, and the photosynthetic activity was measured after incubation in the dark (black diamond) or under LL (black circle), ML (black triangle), or HL (white circle) conditions for designated times. As a control, the cells were incubated under HL conditions with PG (white square). B, Effect of lincomycin (Lin). The pgsA mutant cells grown under LL conditions for 5 d in the absence of PG were transferred to fresh BG-11 medium with (white square) or without (white circle) PG. After addition of Lin, the cells were incubated under HL conditions for designated times, and the photosynthetic activity was measured. Photosynthetic activities are indicated as relative values compared with that before treatments. The oxygen-evolving activity before treatments was 56 μmol O2 h–1 10–7 cells. The values are averages of three independent experiments, and the bars represent the sd.
PG Is Important for the Maintenance of Photosynthetic Machinery
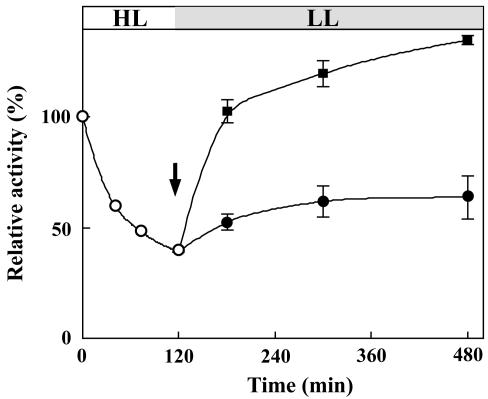
It is considered that photoinhibition of photosynthesis results from imbalance between the inactivation and repair processes of the photosynthetic machinery. To determine which one of these processes is affected by deprivation of PG, we first focused on the inactivation process. PG-deprived mutant cells were incubated under HL conditions in the presence of lincomycin, which inhibits the de novo protein synthesis required for the repair of the photosynthetic machinery. As shown in Figure 2B, inhibition of de novo protein synthesis enhanced the light-induced decrease of photosynthetic activity in the absence of PG as well as in the presence of PG. Although the loss of photosynthetic activity in the presence of PG was less than that in the absence of PG, the difference did not account for the difference in photoinhibition observed in the absence of lincomycin. These results thus suggest that PG does not primarily protect the photosynthetic machinery against photodamage under HL conditions but PG rather plays an important role in the restoration process. The importance of PG for restoration was further tested by transferring the PG-deprived HL-treated (120 min) mutant cells to LL conditions, both in the absence and presence of PG. As shown in Figure 3, some limited restoration of photosynthetic activity occurred in the absence of PG, whereas the restoration was much enhanced in the presence of PG. This result clearly demonstrates that PG plays an important role in the restoration processes of the photosynthetic machinery.
Figure 3.
The restoration of photosynthetic activity. The pgsA mutant cells grown under LL conditions for 5 d without PG were first incubated under HL conditions (white circle) for 120 min, and thereafter, the restoration of photosynthetic activity was monitored under LL conditions in the presence (black square) or absence (black circle) of PG. The arrow indicates the time for the addition of PG to the medium. Photosynthetic activities are indicated as relative values compared with that before treatments. The values are averages of three independent experiments, and the bars represent the sd.
The Effect of PG Molecular Species on the Restoration of Photosynthetic Activity
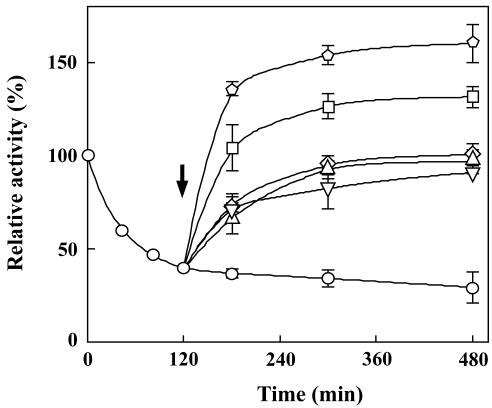
We next determined whether the different acyl groups bound to the glycerol backbone in PG are of importance for the restoration of photosynthetic activity. Although all PG molecular species examined accelerated the restoration of photosynthetic activity under HL conditions (Fig. 4), the PG molecular species containing unsaturated fatty acids (dioleoyl-PG) were far more effective than those containing saturated fatty acids (dimyristoyl-PG, dipalmitoyl-PG, and distearoyl-PG). The similar differences among PG molecular species in the restoration of photosynthetic activity were also observed under LL conditions (data not shown). These results suggested that unsaturated fatty acids have an important role in the function of PG but did not, however, exclude the possibility that the different effects of PG molecular species on the restoration of photosynthetic activity could be due to the difference in the amount of PG molecules incorporated into the thylakoid membranes. Analysis of the incorporation of different PG molecular species into the thylakoid membranes (Table I) revealed each PG molecular species to be equally efficient, the distearoyl-PG (18:0/18:0-PG) being the only exception with about one-half of the incorporation efficiency compared with the other molecular species. Moreover, the fatty acid composition of PG incorporated into thylakoid membranes was almost identical to that of the PG molecular species added to the medium (data not shown). The less incorporation of distearoyl-PG into thylakoid membranes compared with that of other molecular species could affect the restoration of photosynthetic activity. However, the incubation of the cells with a reduced amount of dioleoyl-PG, which resulted in a half incorporation of dioleoyl-PG into thylakoid membranes, gave the similar effect on the restoration (data not shown). This result demonstrates that dioleoyl-PG is more effective than distearoyl-PG on the restoration of photosynthetic activity.
Figure 4.
Effect of PG molecular species on restoration from photoinhibition. The pgsA mutant cells grown under LL conditions for 5 d in the absence of PG were incubated for 120 min under HL conditions without PG and then further incubated under HL conditions without PG (circle) or with dimyristoyl-PG (diamond), dipalmitoyl-PG (inverted triangle), distearoyl-PG (triangle), dioleoyl-PG (square), or PG prepared from the wild-type cells (pentagon) for designated time. The arrow indicates the time for addition of each PG molecular species to the medium. Photosynthetic activities are indicated as relative values compared with that before transfer to HL conditions. The values are averages of three independent experiments, and the bars represent the sd.
Table I.
Lipid composition of thylakoid membranes of the pgsA mutant cells
The pgsA mutant cells grown under LL condition for 5 d in the absence of PG (0 min) were incubated for 120 min under HL condition (120 min), and further incubated with different molecular species of PG for 180 min under HL condition (300 min).
| Time
|
PG molecular species
|
Lipid
|
|||
|---|---|---|---|---|---|
| MGDG | DGDG | SQDG | PG | ||
| min | mol% | ||||
| 0 | 53 | 17 | 25 | 5 | |
| 120 | 54 | 16 | 25 | 5 | |
| 300 | Without PG | 52 | 19 | 24 | 5 |
| 14:0/14:0-PG | 34 | 9 | 15 | 42 | |
| 16:0/16:0-PG | 34 | 9 | 17 | 40 | |
| 18:0/18:0-PG | 45 | 14 | 21 | 20 | |
| 18:1/18:1-PG | 34 | 9 | 21 | 36 | |
The wild-type cells of Synechocystis sp. PCC6803 contain mixed species of PG carrying an unsaturated C18 fatty acid at the sn-1 position and a palmitoyl group at the sn-2 position. Because the PG present in the wild-type cells was expected to be the most effective on the restoration of photosynthetic activity, its effect was compared with other molecular species of PG. As expected, the PG prepared from the wild-type cells was more effective than other molecular species (Fig. 4).
The Effect of PG on D1 Protein Turnover
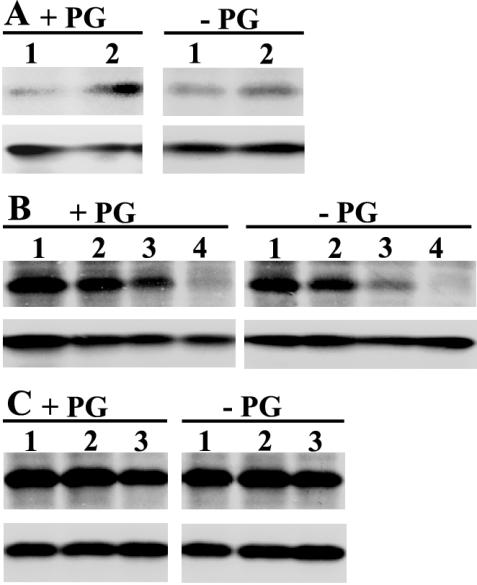
To clarify the molecular background of photoinhibition observed in the PG-deprived mutant cells under HL conditions, we focused on the D1 subunit of PSII because of the following reasons: (a) deprivation of PG was previously shown to inactivate the electron transport in PSII (Hagio et al., 2000; Gombos et al., 2002), and (b) it is known that photoinhibition induces an irreversible damage to the D1 protein of PSII and that the turnover of the D1 protein is a key step to maintain the photosynthetic activity under HL conditions (Aro et al., 1993; Andersson and Aro, 2001). Therefore, the steady-state amount of the D1 protein was determined by western blotting, and [35S]Met labeling analysis was performed to reveal the rate of D1 protein turnover. The steady-state amount of D1 protein did not significantly change during the HL treatment in the presence or absence of PG (Fig. 5, A and B, bottom panels) or during subsequent incubation under LL conditions (Fig. 5C, bottom panels). These results indicated that the photoinhibition observed in the PG-deprived mutant cells did not cause any net changes in the content of the D1 protein, however, it remained to be elucidated whether the accumulation of damaged D1 protein possibly occurs in PSII reaction centers of mutant cells in the absence of PG. To check this possibility, the turnover of the D1 protein was analyzed by pulse and pulse-chase experiments with [35S]Met. The radioactivity incorporated into the D1 protein during the first 10 min of HL treatment did not significantly differ between the mutant cells incubated with PG or without PG (Fig. 5A, lane 1 in top panels). A similar 10-min pulse given after 110 min of acclimation of mutant cells under HL conditions indicated significantly enhanced biosynthesis of the D1 protein, but again, no difference existed whether the pulse was given to cells incubated with PG or without PG (Fig. 5A, lane 2 in top panels). Furthermore, as shown in Figure 5B (top panels), the rate of D1 degradation during the HL treatment did not differ between the mutant cells incubated with PG or without PG. Similarly, no difference existed in the degradation of the D1 protein in the mutant cells transferred to LL conditions after the HL treatment and incubated in the presence or absence of PG (Fig. 5C, top panels).
Figure 5.
Biosynthesis and degradation of the D1 protein. A, Biosynthesis of the D1 protein under HL conditions. The pgsA mutant cells grown under LL conditions for 5 d in the presence (+PG) or absence (–PG) of PG were transferred to HL conditions for 120 min. Cells were pulse labeled with [35S]Met for 10 min at the beginning (lane 1) or at the end (lane 2) of HL treatment. B, Degradation of the D1 protein under HL conditions. The pgsA mutant cells grown under LL conditions for 5 d in the presence (+PG) or absence of PG (–PG) were labeled with [35S]Met under LL conditions for 30 min, and thereafter radioactivity was chased for 0 (lane 1), 30 (lane 2), 60 (lane 3), or 120 min (lane 4) under HL conditions in the presence of nonradioactive Met. C, Degradation of the D1 protein under LL conditions after HL treatment. The pgsA mutant cells grown under LL conditions for 5 d in the absence of PG were preincubated for 100 min under HL conditions and then labeled with [35S]Met for 20 min under HL conditions. Thereafter, these cells were transferred to LL conditions in the presence (+PG) or absence (–PG) of PG, and the radioactivity was chased for 0 (lane 1), 15 (lane 2), or 60 min (lane 3) in the presence of nonradioactive Met. The top panel in each figure depicts an autoradiogram showing the incorporation of radioactive Met into the D1 protein, and the bottom panel shows the steady state of the D1 protein detected by western-blot analysis with the same membranes.
Effect of PG on Dimerization of the PSII Core Complex
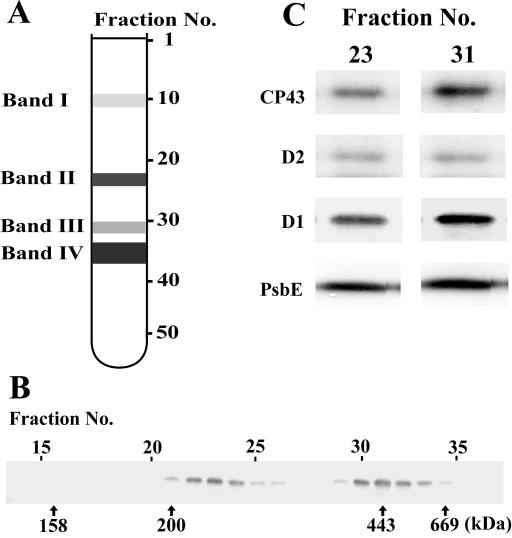
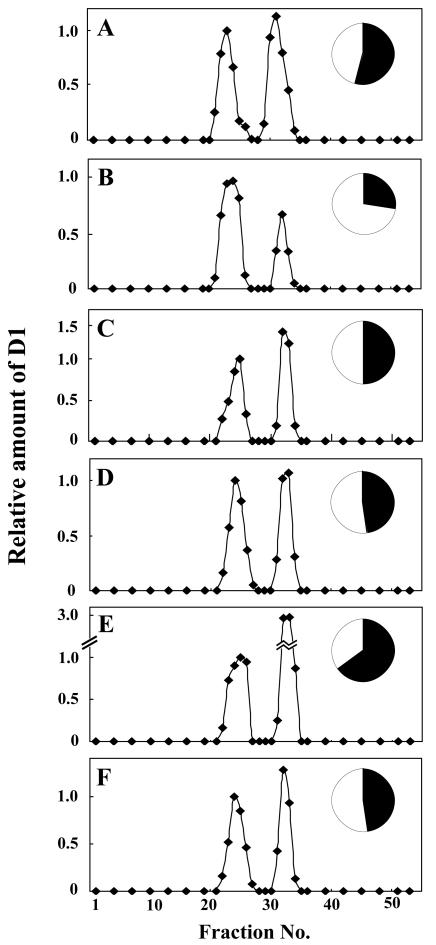
Dimerization of the PSII core monomer is likely to be an essential step in the repair process of PSII. To investigate the effect of PG on dimerization of the PSII core monomers, the thylakoid membranes were solubilized with n-dodecyl-β-d-maltoside (DM) and subjected to Suc density gradient centrifugation. The basic separation patterns of the thylakoid protein complexes in the gradient were very similar in all cells irrespective of growth conditions, as shown in Figure 6A. Four major bands (bands I–IV) were observed from the top to the bottom of the gradient. The color of band I was orange, indicating the presence of carotenoids. The other three bands were green, suggesting the presence of protein complexes containing chlorophylls. As shown in Figure 6B, the D1 protein was detected in two fraction areas corresponding to bands II and III, localizing the PSII core complexes to these bands. The molecular masses of the protein complexes in bands II and III were approximately 230 and 450 kD, corresponding to the masses of the monomeric and dimeric forms of the PSII core complex, respectively (Rögner et al., 1987; Hankamer et al., 1997). Other subunits of the PSII complex, D2, CP43, and PsbE (α-subunit of Cyt b559), were also detected in the same fractions together with the D1 protein (Fig. 6C), confirming that the bands II and III contained the monomeric and dimeric forms of the PSII core complex, respectively. The ratio of the monomeric form to the dimeric form of the PSII core complex was estimated using the distribution of the D1 protein as an indicator. As shown in Figure 7, the dimeric form of the PSII core complex decreased during HL treatment of the PG-deprived mutant cells, but not in the mutant cells incubated with PG. Upon transfer of cells to LL conditions, the dimeric form of the PSII core complex increased slowly even in the absence of PG, whereas the addition of PG strongly enhanced the increase in the dimeric form of the PSII core complex as compared with the monomeric form of PSII core complex. These results suggest that PG plays an important role in the effective dimerization of PSII core complex, but it is not essential for the dimerization.
Figure 6.
Separation of DM-solubilized protein complexes by Suc density gradient centrifugation. A, The typical pattern of protein complexes after Suc density gradient centrifugation. The Suc density gradients were fractionated into 53 equal volume fractions from top to bottom. B, Distribution of the D1 protein in the Suc density gradient fractions prepared from the mutant cells grown under LL conditions for 5 d in the absence of PG. Fraction numbers and molecular masses are indicated above and below the immunoblot, respectively. C, Immunoblots showing distribution of PSII core proteins CP43, D2, D1, and PsbE (α-subunit of Cyt b559) in fractions 23 and 31 prepared from the mutant cells grown under LL conditions for 5 d in the absence of PG.
Figure 7.
Change in distribution of the D1 protein in Suc density gradients. The pgsA mutant cells grown under LL conditions for 5 d in the absence of PG (A) were transferred to HL conditions and incubated for 120 min in the absence (B) or in the presence (C) of PG. The cells treated under HL conditions for 120 min in the absence of PG were incubated further in the presence of PG for 60 (D) and 180 (E) min or the absence of PG for 180 min (F) under LL conditions. Inset figures represent the ratio of the monomeric to the dimeric form of the PSII core complex as estimated by the D1 protein content. The amount of D1 protein contained in the monomeric (white area) or the dimeric (black area) form of the PSII core complex was calculated as the sum of the values of the quantified signal intensity of the D1 protein.
DISCUSSION
In the present study, we have studied the function of PG in the maintenance of the photosynthetic machinery of Synechocystis sp. PCC6803 by using the pgsA mutant, which is defective in the biosynthesis of PG. PG deprivation induced a severe photoinhibition of photosynthesis when mutant cells were subjected to HL conditions (Fig. 2). Such enhanced photoinhibition was primarily due to an impairment of the restoration process of the photosynthetic machinery. The molecular background of photoinhibition has been studied in many previous studies, and it has been generally accepted that the PSII complex, in particular the D1 protein, is the primary target for photodamage (Aro et al., 1993; Andersson and Aro, 2001). PG deprivation, however, was observed to have no detrimental effect on D1 turnover in the mutant cells; neither the synthesis nor the degradation of the D1 protein was impaired. Intriguingly, a later step, the dimerization of the PSII core monomer complexes was impaired in the PG-deprived mutant cells. Upon PG deprivation, a distinct accumulation of the monomeric form of the PSII core complex occurred under HL conditions. Incubation of these photoinhibited cells with PG restored the dimerization of PSII core complexes and increased the ratio of the dimeric to monomeric form of the PSII core complex. Our finding on PG having an important role in effective reactivation and dimerization of the PSII core monomer thus corroborates with previous in vitro studies with spinach (Spinacia oleracea) thylakoids (Kruse et al., 2000). In line with these observations, PG has earlier been shown to be bound to the D1 protein (Kruse and Schmid, 1995) and to be abundant in the PSII core complex preparations (Murata et al., 1990).
The PG molecular species efficient in the restoration of the photosynthetic activity were those containing unsaturated fatty acids. This is in accordance with these molecular species being the most prominent ones in the thylakoid membranes (Joyard et al., 1998; Wada and Murata, 1998), and PG prepared from the wild-type cells was the most effective on the restoration of photosynthetic activity. The importance of unsaturated molecular species of PG in the tolerance against low-temperature photoinhibition was previously demonstrated with tobacco (Nicotiana tabacum) plants transformed with genes for glycerol-3-phosphate acyltransferase (Murata et al., 1992; Moon et al., 1995). The content of unsaturated molecular species in PG correlated with the tolerance of transgenic plants to low-temperature photoinhibition. It is likely that unsaturated molecular species of PG are more efficient than the saturated ones also in the restoration of the photosynthetic activity at low temperature. The low-temperature photoinhibition and turnover of D1 protein were also studied with Synechocystis sp. PCC6803 mutants defective in fatty acid desaturases (Gombos et al., 1992, 1994; Kanervo et al., 1995). The results obtained by these studies provided the evidence that membrane lipids with polyunsaturated fatty acids accelerated the recovery of photosynthetic activity similar to the results presented in the present study. However, the effect was attributed to a stimulation of the synthesis of D1 protein or its assembly into the protein complex. These findings demonstrate that membrane lipids play important roles in the recovery process of photosynthesis although the step affected in the desaturase mutants is different from that affected in the pgsA mutant. The difference in the affected steps might be caused by the differences of membrane lipids that were manipulated in the mutants. In the desaturase mutants, unsaturation of all membrane lipids was manipulated, whereas PG was only manipulated in the pgsA mutant.
Several lines of evidence suggest that PG molecules are crucial not only for electron transfer properties but also for construction and oligomerization of protein complexes in the thylakoid membranes. In chloroplasts, the light-harvesting chlorophyll a/b-binding protein complexes (LHCII) are enriched in PG (Nussberger et al., 1993; Trémolières et al., 1994), and an interaction between the N-terminal region of the LHCII protein and PG is required for trimerization of LHCII (Hobe et al., 1994, 1995; Trémolières and Siegenthaler, 1998). It has also been demonstrated that PG molecular species containing Δ3-trans-hexadecenoic acid are required for dimerization of PSII core complexes in vitro (Kruse et al., 2000). Recently, Jordan et al. (2001) reported that three PG molecules are bound to a reaction center of PSI core complex. Two of these PG molecules are bound to the periphery, and one to the center core of the PSI core complex. Although the function of PG in PSI has not been clarified, the binding of PG to PSI core complex suggests that PG has an important function not only in PSII but also in PSI, presumably in the assembly of the PSI core complex. In the present and previous studies, however, we detected the severe defect only in PSII after the deprivation of PG. The reason why PSII was only affected might be due to the PG that was still present in the PG-deprived mutant cells. The mutant cells still contained 5% of PG in total lipids after the deprivation of PG for 5 d in the absence of PG. This remaining PG might be high enough for maintenance of functional PSI complex. To check this possibility, we are currently investigating whether PSI activity decreases with further decrease of PG content when the cells are incubated without PG for longer periods.
With the findings obtained in the present and previous studies, the turnover of the PSII core complex in the pgsA mutant cells could be considered as described below. Inactivation of the PSII core complex and damage to the D1 protein are the first events in the photoinhibition-repair cycle of PSII. It is conceivable that the active dimeric form of the PSII core complex undergoes monomerization upon damage to the D1 protein (Barbato et al., 1992; Beana-González et al., 1999). The inactive monomeric form of PSII core complex is subsequently repaired via the replacement of damaged D1 protein with de novo synthesized D1 copy. Reassembly and reactivation of the monomeric PSII are followed by dimerization of two monomer complexes. Because the dimeric form of PSII possesses higher activity than the monomeric form (Hankamer et al., 1997), the HL susceptibility of PG-deprived mutant cells is likely to result from impaired reactivation and dimerization of PSII core monomers during the later phases of PSII repair processes, manifested as an increase in the ratio of the monomeric to the dimeric form of the PSII core complex. To prove this interpretation, the activities of the monomeric and the dimeric forms of the PSII core complexes should be measured separately. However, after solubilization of thylakoid membranes with DM, the oxygen-evolving activity of PSII core complexes could no more be measured in PG-deprived cells with artificial quinones, presumably due to the inhibition of the activity with the quinones as previously reported for the intact cells or for thylakoid membranes of the mutant (Hagio et al., 2000).
In conclusion, the present results indicate that (a) the decrease in PG content in thylakoid membranes increases the light susceptibility and leads to photoinhibition of photosynthesis, and (b) PG is not essential for the turnover cycle of the D1 protein as such, but (c) PG is required for effective dimerization and reactivation of the PSII complex, suggesting an important role for PG in the structural maintenance of the photosynthetic machinery by facilitating the dimerization of the PSII core monomers.
MATERIALS AND METHODS
Organism and Growth Conditions
The pgsA mutant cells of Synechocystis sp. PCC6803 were grown photo-autotrophically in BG-11 medium (Allen, 1968) supplemented with 4 mm HEPES-NaOH (pH 7.5), 20 μg mL–1 kanamycin, and 20 μm dioleoyl-PG (18:1/18:1-PG; P9664, Sigma-Aldrich, St. Louis) at 30°C under continuous illumination of 25 μmol photons m–2 s–1. Cultures were aerated on a rotational shaker (NR-3, TAITEC, Saitama, Japan) at 120 rpm. The growth of the cells was monitored by determination of the optical density at 730 nm.
Light and Chemical Treatments of the Cells
The lights provided by a fluorescent lamp at the intensities of 25, 75, and 215 μmol photons m–2 s–1 were used as LL, middle light (ML), and HL, respectively. To test the light susceptibility of the cells, the PG-deprived mutant cells grown for 5 d under LL conditions in the absence of PG were diluted to the fresh BG-11 medium at the concentration of 4 × 107 cells mL–1, and photosynthetic activity was measured after incubation for 0, 40, 80, and 120 min under each light conditions. To follow photodamaging process, a protein synthesis inhibitor, lincomycin, was added at the beginning of HL treatment at final concentration of 100 μg mL–1.
Restoration of photosynthetic activity after the HL treatment was followed as described below. The pgsA mutant cells grown for 5 d under LL conditions in the absence of PG were treated under HL conditions for 120 min. The photodamaged mutant cells were transferred to LL conditions with or without PG, and photosynthetic activity was measured after incubation for 60, 180, and 360 min. The effect of PG molecular species on the restoration of photosynthetic activity was checked with dimyristoyl-PG (14:0/14:0-PG), dipalmitoyl-PG (16:0/16:0-PG), and distearoyl-PG (18:0/18: 0-PG) in addition to dioleoyl-PG at the concentration of 20 μm.
Measurement of Photosynthetic Activity
Photosynthetic activity was measured by a Clark-type oxygen electrode according to Gombos et al. (1991). For measurement of photosynthetic activity, cells were collected by centrifugation and resuspended in fresh BG-11 medium. Light from an incandescent lamp combined with a red optical filter (2–61, Corning, Corning, NY) was provided for all measurements of photosynthetic activity.
Lipid Analysis
Lipids were extracted from intact cells and thylakoid membranes by the method of Bligh and Dyer (1959). Lipids extract and fatty acids were analyzed as described previously (Wada and Murata, 1989).
Pulse and Pulse-Chase Experiments
The pgsA mutant cells grown for 5 d under LL conditions with or without PG were adjusted at the concentration of 1.2 × 108 cells mL–1, and lights provided by a incandescent lamp at the light intensity of 1,100 μmol photons m–2 s–1 or 45 μmol photons m–2 s–1 were used as HL or LL, respectively. For pulse labeling experiments to check the biosysnthesis of D1 protein, the cell suspension was incubated with [l-35S]Met (in vivo cell labeling grade, >1,000 Ci mmol–1; Amersham Biosciences, Buckinghamshire, UK) at a final concentration of 3 μCi mL–1, and excess nonradioactive Met (100 mm) was used to stop the labeling. The labeling was performed under HL conditions for 10 min immediately after the cells were transferred to HL conditions; or the cells were first preincubated for 110 min under HL conditions, and thereafter radioactive Met was added and the HL irradiation was continued for 10 min.
Degradation of the D1 protein under HL or LL conditions was studied by pulse-chase labeling technique. The pgsA mutant cells grown under LL conditions for 5 d in the presence or absence of PG were pulse labeled for 30 min under LL conditions. Thereafter, the cells were collected, resuspended in fresh BG-11 medium with or without PG containing nonradioactive Met, and incubated under HL conditions for 0, 30, 60, and 120 min. To analyze the degradation of D1 protein in photodamaged mutant cells after transfer to LL conditions, radioactive Met was added to the PG-deprived cell suspension after 100 min of treatment under HL conditions, and HL irradiation was continued for 20 min. Thereafter, the cells were collected and resuspended in fresh BG-11 containing 100 mm nonradioactive Met and 20 μm PG or without PG, and the radioactivity was chased for 0, 15, and 60 min under LL conditions.
The cells treated above were collected by centrifugation at 5,000g for 10 min, and the cell pellet was immediately frozen in liquid nitrogen. Thylakoid membranes were isolated according to Tyystjärvi et al. (1994). Proteins of thylakoid membranes were solubilized and subjected to SDS-PAGE with a 12% (w/v) polyacrylamide gel containing 4 m urea. Separated proteins were blotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore, Billerica, MA). The membranes were exposed to x-ray film for autoradiography and then used for immunodetection of D1 protein.
Separation of Protein Complexes by Suc Density Gradient Centrifugation
Thylakoid membranes were isolated according to Nishiyama et al. (1993). The isolated thylakoid membranes were washed with 1 mL of wash buffer (5 mM MgCl2, 10 mM NaCl, and 25 mM MES-NaOH [pH 6.0]) containing 1 μM antipain, 1 μM leupeptin, and 1 μM pefabloc SC as proteinase inhibitors and collected at 20,000g for 2 min. The washed thylakoid membranes were resuspended in 80 μL of wash buffer, and equal volume of wash buffer containing 4% (w/v) DM (D4641, Sigma-Aldrich) was added to the suspension. After vortexing for 5 s, the suspension of thylakoid membranes was incubated in the dark for 15 min on ice. After centrifugation at 20,000g for 2 min, the supernatant was loaded onto a linear Suc gradient, 5% to 35% (w/v) Suc in 5 mM MgCl2, 10 mM NaCl, 0.5 M Gly betaine, 0.03% (w/v) DM, and 25 mM MES-NaOH (pH 5.7), which was made with a Gradient Master (model 107ip, Biocomp Inc., New Brunswick, Canada). The gradient was centrifuged at 180,000g for 20 h at 4°C (P40ST rotor, Hitachi, Yokohama, Japan). After the centrifugation, the Suc gradient was fractionated into 53 fractions of equal volume using a Piston Gradient Fractionator (model 152, Biocomp Inc.). Fifty microliters of each fraction was used for SDS-PAGE, and separated proteins were blotted onto a nitrocellulose membrane (Hybond-ELC, Amersham Biosciences) for immunodetection. D1 protein was detected by standard color developing method using goat anti-rabbit IgG-alkali phosphatase secondary antibody (170–6518, Bio-Rad Laboratories, Hercules, CA). CP43, D2, and PsbE (α-subunit of Cyt b559) were detected by the chemiluminescence method using CDP-star reagent (N7001S, New England Biolabs, Beverly, MA) and goat anti-rabbit IgG-alkali phosphatase secondary antibody (81-6122, Zymed Laboratories, South San Francisco) according to the protocol supplied by the manufacturer. The signal intensity of D1 protein in each fraction was quantified with NIH Image 1.62 software (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image) on a Macintosh computer and normalized with the strongest signal of D1 protein in the fraction of the monomeric form of the PSII core complex. The molecular masses of each protein complex were determined by comparison of the location of bands formed in Suc density gradient with that of molecular mass standards (MW-GF-1000, Sigma-Aldrich).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026955.
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science, by the Program for Promotion of Basic Research Activities for Innovative Biosciences, and by the Academy of Finland.
References
- Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4: 1–4 [DOI] [PubMed] [Google Scholar]
- Andersson B, Aro E-M (2001) Photodamage and D1 protein turnover in photosystem II. In E-M Aro, B Andersson, eds, Regulation of Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 377–393
- Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Babiychuk E, Müller F, Eubel H, Braun H-P, Frentzen M, Kushnir S (2003) Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J 33: 899–909 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Barbato R, Aro E-M (1999) Role of phosphorylation in the repair cycle and oligomeric structure of photosystem II. Planta 203: 196–204 [Google Scholar]
- Barbato R, Frizzo A, Friso G, Rigoni F, Giacometti GM (1992) Photoinduced degradation of D1 protein in isolated thylakoid and various photosystem II particles after donor-side inactivations: detection of a C-terminal 16 kDa fragment. FEBS Lett 304: 136–140 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Block MA, Dorne A-J, Joyard J, Douce R (1983) Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts: II. Biochemical characterization. J Biol Chem 258: 13281–13286 [PubMed] [Google Scholar]
- Droppa M, Horváth G, Hideg E, Farkas T (1995) The role of phospholipids in regulating photosynthetic electron transport activities: treatment of thylakoids with phospholipase C. Photosynth Res 46: 287–293 [DOI] [PubMed] [Google Scholar]
- Gombos Z, Várkonyi Z, Hagio M, Iwaki M, Kovács L, Masamoto K, Itoh S, Wada H (2002) Phosphatidylglycerol requirement for the function of electron acceptor plastoquinone QB in the photosystem II reaction center. Biochemistry 41: 3796–3802 [DOI] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N (1991) Direct evaluation of effects of fatty-acid unsaturation on the thermal properties of photosynthetic activities, as studied by mutation and transformation of Synechocystis PCC6803. Plant Cell Physiol 32: 205–211 [Google Scholar]
- Gombos Z, Wada H, Murata N (1992) Unsaturation of fatty acids in membrane lipids enhances the tolerance of the cyanobacterium Synechocystis PCC6803 to low-temperature photoinhibition. Proc Natl Acad Sci USA 89: 9959–9963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N (1994) The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: a mechanism of chilling tolerance. Proc Natl Acad Sci USA 91: 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagio M, Gombos Z, Várkonyi Z, Masamoto K, Sato N, Tsuzuki M, Wada H (2000) Direct evidence for requirement of phosphatidylglycerol in photosystem II of photosynthesis. Plant Physiol 124: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagio M, Sakurai I, Sato S, Kato T, Tabata S, Wada H (2002) Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol 43: 1456–1464 [DOI] [PubMed] [Google Scholar]
- Hankamer B, Nield J, Zheleva D, Boekema E, Jansson S, Barber J (1997) Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur J Biochem 243: 422–429 [DOI] [PubMed] [Google Scholar]
- Hobe S, Förster R, Klingler J, Paulsen H (1995) N-proximal sequence motif in light-harvesting chlorophyll a/b-binding protein is essential for the trimerization of light-harvesting chlorophyll a/b complex. Biochemistry 34: 10224–10228 [DOI] [PubMed] [Google Scholar]
- Hobe S, Prytulla S, Kühlbrandt W, Paulsen H (1994) Trimerization and crystallization of reconstituted light-harvesting chlorophyll a/b complex. EMBO J 13: 3423–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BR, Chow W-S, Baker AJ (1983) The role of phospholipids in the molecular organisation of pea chloroplast membranes: effect of phospholipid depletion on photosynthetic activities. Biochim Biophys Acta 725: 77–86 [Google Scholar]
- Jordan P, Formme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I as 2.5 Å resolution. Nature 411: 909–917 [DOI] [PubMed] [Google Scholar]
- Joyard J, Maréchal E, Miege C, Block MA, Dorne A-J, Douce R (1998) Structure, distribution and biosynthesis of glycerolipids from higher plant chloroplasts. In P-A Siegenthaler, N Murata, eds, Lipids in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 21–52
- Kanervo E, Aro E-M, Murata N (1995) Low unsaturation level of thylakoid membrane lipids limits turnover of the D1 protein of photosystem II at high irradiance. FEBS Lett 364: 239–242 [DOI] [PubMed] [Google Scholar]
- Kruse O, Hankamer B, Konczak C, Gerle C, Morris E, Radunz A, Schmid GH, Barber J (2000) Phosphatidylglycerol is involved in the dimerization of photosystem II. J Biol Chem 275: 6509–6514 [DOI] [PubMed] [Google Scholar]
- Kruse O, Schmid GH (1995) The role of phosphatidylglycerol as a functional effector and membrane anchor of the D1-core peptide from photosystem II-particles of the cyanobacterium Oscillatoria chalybea. Z Naturforsch 50c: 380–390 [PubMed] [Google Scholar]
- Moon BY, Higashi S, Gombos Z, Murata N (1995) Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA 92: 6219–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Higashi S, Fujimura Y (1990) Glycerolipids in various preparations of photosystem II from spinach chloroplasts. Biochim Biophys Acta 1019: 261–268 [Google Scholar]
- Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I (1992) Genetically engineered alteration in the chilling sensitivity of plants. Nature 356: 710–713 [Google Scholar]
- Nishiyama Y, Kovács E, Lee CB, Hayashi H, Watanabe T, Murata N (1993) Photosynthetic adaptation to high temperature associated with thylakoid membranes of Synechococcus PCC7002. Plant Cell Physiol 34: 337–343 [Google Scholar]
- Nussberger S, Dörr K, Wang DN, Kühlbrandt W (1993) Lipid-protein interactions in crystals of plant light-harvesting complex. J Mol Biol 234: 347–356 [DOI] [PubMed] [Google Scholar]
- Rögner M, Dekker JP, Boekema EJ, Witt HT (1987) Size, shape and mass of the oxygen-evolving photosystem II complex from the thermophilic cyanobacterium Synechococcus sp. FEBS Lett 219: 207–211 [Google Scholar]
- Sato N, Hagio M, Wada H, Tuzuki M (2000) Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci USA 97: 10655–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Browse J, Joworski JG, Ohlrogge JB (2000) Lipids. In BB Buchanan, W Gruissem RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 456–527
- Trémolières A, Dainese P, Bassi R (1994) Heterogenous lipid distribution among chlorophyll-binding proteins of photosystem II in maize mesophyll chloroplasts. Eur J Biochem 221: 721–730 [DOI] [PubMed] [Google Scholar]
- Trémolières A, Siegenthaler P-A (1998) Reconstitution with lipids. In P-A Siegenthaler, N Murata, eds, Lipids in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 175–189
- Tyystjärvi T, Aro E-M, Jansson C, Mäenpää P (1994) Change of amino acid sequence in PEST-like area and QEEET motif affect degradation rate of D1 polypeptide in photosystem II. Plant Mol Biol 25: 517–526 [DOI] [PubMed] [Google Scholar]
- Wada H, Murata N (1989) Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol 30: 971–978 [Google Scholar]
- Wada H, Murata N (1998) Membrane lipids in cyanobacteria. In P-A Siegenthaler, N Murata, eds, Lipids in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 65–81
- Xu C, Härtel H, Wada H, Hagio M, Yu B, Eakin C, Benning C (2002) The pgp1 mutant locus of Arabidopsis encodes a phosphatidylglycerolphosphate synthase with impaired activity. Plant Physiol 129: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]