Abstract
Interstitial pneumonia (IP) frequently occurs in patients with scrub typhus, but its clinical significance is not well known. This study was designed to evaluate interstitial pneumonia as a marker of severity of the disease for patients with scrub typhus. We investigated clinical parameters representing the severity of the disease, and the chest radiographic findings for 101 patients with scrub typhus. We then compared these clinical factors between patients with and without IP. We also studied the relationship between IP and other chest radiographic findings. The chest radiography showed IP (51.4%), pleural effusion (42.6%), cardiomegaly (14.9%), pulmonary alveolar edema (20.8%), hilar lymphadenopathy (13.8%) and focal atelectasis (11.8%), respectively. The patients with IP (n=52) had higher incidences in episode of hypoxia (p=0.030), hypotension (p=0.024), severe thrombocytopenia (p=0.036) and hypoalbuminemia (p=0.013) than the patients without IP (n=49). The patients with IP also had higher incidences of pleural effusion (p<0.001), focal atelectasis (p=0.019), cardiomegaly (p<0.001), pulmonary alveolar edema (p=0.011) and hilar lymphadenopathy (p<0.001) than the patients without IP. Our data suggest that IP frequently occurs for patients with scrub typhus and its presence is closely associated with the disease severity of scrub typhus.
Keywords: Orientia tsutsugamushi; Scrub Typhus; Pneumonia, Rickettsial; Severity of Illness Index
INTRODUCTION
Scrub typhus is a potentially fatal infectious disease caused by the organism Orientia tsutsugamushi. It is common in Asia and the Pacific islands and is sometimes encountered in Western countries (1, 2). Clinical manifestations are fever, skin rash, eschar and varying degree of respiratory distress (2, 3). The spectrum of clinical severity for scrub typhus ranges from undetected to mild, severe or fatal (4). The clinical parameters representing the severity of the disease are known to be hypotension, thrombocytopenia, leukocytosis, hypoxia, acute renal failure, hypoalbuminemia and hepatic dysfunction (2, 3, 5-7).
The central pathophysiologic derangement of scrub typhus is the widespread vasculitis or perivasculitis of multiple organs due to multiplication of the organisms in the endothelial cells lining the small blood vessels (1, 4, 6, 8-10). Such microangiopathies may involve lung (interstitial pneumonia, IP), cardiovascular system (hypotension and myocarditis), brain (meningoencephalitis), kidney (acute renal failure), gastrointestinal tract (gastrointestinal bleeding), liver (hepatic dysfunction and hepatomegaly), spleen (splenomegaly) and lymph node (lymphadenopathy) (1, 4, 6, 8-10).
The chest abnormalities in scrub typhus are IP, cardiomegaly, pulmonary edema, pleural effusion, hilar lymphadenopathy and focal atelectasis (3, 4, 11-13). IP is the most common abnormality in the chest radiographic findings (3, 11-13). However, the impact of IP on the severity of the disease is not well known. When considering the fact that the main pathogenesis of scrub typhus is widespread vasculitis or perivasculitis of multiple organs including the lungs, we hypothesized that IP would be a possible radiologic marker representing the severity of the disease. We studied the relationship between IP and the clinical parameters representing severity of scrub typhus.
MATERIALS AND METHODS
One hundred and one patients with scrub typhus were included in this study, and they were admitted between January 1993 and May 2003 to Uijongbu St. Mary's Hospital, Uijongbu, Korea. The diagnosis of scrub typhus was made on the basis of their clinical manifestations (fever, skin rash, eschar) and the results of an indirect immunofluorescence antibody test against the organism Orientia tsutsugamushi. The patients with chronic liver disease, chronic renal failure, chronic heart disease, hematologic disease and inflammatory bowel disease were excluded. Sixty seven of the patients were female (66.3%) and the mean age of total subjects was 56±15 yr (range: 9-86 yr).
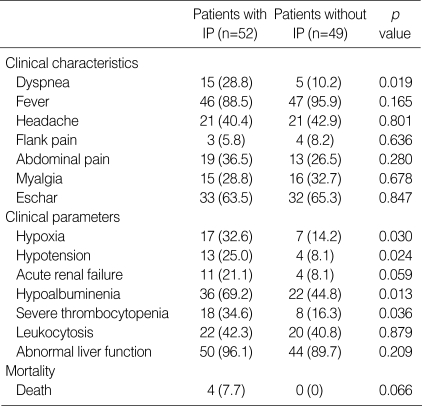
We retrospectively studied medical records for the patients with scrub typhus. We evaluated the following clinical symptoms, signs, and laboratory findings: fever, headache, flank pain, abdominal pain, myalgia, dyspnea, eschar, hemoglobin, hematocrit, platelet count, leukocyte count, blood urea nitrogen (BUN), serum creatinine (Scr), serum albumin, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). We selected 7 clinical factors (hypoxia, hypotension, severe thrombocytopenia, leukocytosis, acute renal failure, hypoalbuminemia and hepatic dysfunction) as the clinical parameters representing the severity of the disease. Hypotension was defined as an arterial systolic blood pressure below 90 mmHg (14). Hypoxia was defined as a partial oxygen pressure of arterial blood (PaO2) below 60 mmHg at room air. Hypoalbuminemia was defined as a serum albumin of less than 3.0 g/dL. Severe thrombocytopenia was defined as a platelet count below 50,000/µL. Leukocytosis was defined as a leukocyte count over 10,000/µL. Acute renal failure was defined as a serum creatinine level over 1.6 mg/dL. Hepatic dysfunction was defined as an AST or ALT over 60 IU/L.
Two radiologists reviewed posteroanterior chest radiographs (n=101) and thin-section computed tomographs (CTs) (n=12) and a final decision was reached by consensus. The radiologists were blinded as to the clinical information and outcomes of the patients. CTs were performed on a Somatom Volume Zoom (Siemens Medical Systems, Forchheim, Germany). All CT images were obtained with 1.0 mm collimation at 10 mm intervals through the thorax. The images were reconstructed with a high spatial-frequency algorithm, and the images were printed with window settings appropriate for viewing the lungs (window width, 1,500 H; window level -600 H). We assessed the presence and zonal predilection for the following parenchymal lung abnormalities: interstitial pneumonia, pulmonary alveolar edema and subsegmental atelectasis. Interstitial pneumonia on plain chest radiographs was defined as reticulonodular opacities, bronchial wall thickening and septal lines. Interstitial pneumonia on CTs was defined as interlobular septal thickening, bronchial wall thickening and centrilobular nodules. We regarded confluent ground glass opacity with or without consolidation as the radiographic signs of pulmonary alveolar edema. We also assessed the presence of cardiomegaly, pleural effusion and the presence and location of hilar lymphadenopathy. We considered the cardiomegaly by scrub typhus as change of cardiac size, on the basis of prior or follow-up chest examinations. If the cardiothoracic ratio of the cardiomegaly was unchanged, as determined on the basis of prior or follow-up chest examinations, we excluded the cases in the positive rate of cardiomegaly by scrub typhus.
The patients were divided into two groups based on presence (n=52) or absence (n=49) of interstitial pneumonia. The 7 clinical parameters representing the severity of the disease (hypoxia, hypotension, severe thrombocytopenia, leukocytosis, acute renal failure, hypoalbuminemia and hepatic dysfunction), duration of hospitalization, and mortality were then compared between the two groups. The following combined chest radiographic abnormalities were compared between the two groups: pulmonary edema, focal atelectasis, cardiomegaly, pleural effusion and hilar lymphadenopathy.
Data were expressed as mean±standard deviation (SD). Statistical analysis was performed using the unpaired Student's t test, the chi-square test or the Fisher's exact test. Statistical significance was defined as p<0.05.
RESULTS
The indirect immunofluorescence antibody tests against the Orientia tsutsugamushi were positive for all patients. Eschar developed in 65 (64.4%) patients. Fever developed in 93 (92.1%) patients, headache in 42 (41.6%), flank pain in 7 (6.9%), abdominal pain in 32 (31.7%), myalgia in 31 (30.7%), and dyspnea in 20 (19.8%) patients. Of the 7 clinical parameters representing the severity of the disease, episode of hypotension occurred in 17 (16.8%) patients, hypoxia in 24 (23.8%), leukocytosis in 42 (41.6%), severe thrombocytopenia in 26 (25.7%), acute renal failure in 15 (14.9%), hypoalbuminemia in 58 (69.2%) and hepatic dysfunction in 94 (93.1%). Laboratory findings upon admission to the hospital are listed in Table 1. The average length of stay in the hospital (n=101) was 10.3±4.7 days (2-33 days). Four (4.0%) patients died from acute respiratory distress syndrome (n=2) and septic shock (n=2).
Table 1.
Laboratory and radiographic findings in 101 patients with scrub typhus
Hb, hemoglobin; Hct, hematocrit; WBC, white blood cell; Plt, platelet; BUN, blood urea nitrogen; Scr, serum creatinine; AST, aspartate aminotransferase; ALT, alanine aminotrasferase; Alb, serum albumin.
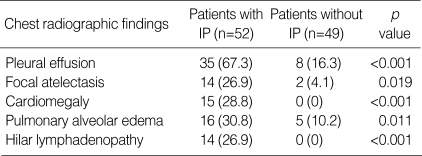
Radiography showed abnormalities in 60/101 (59.4%) patients. The most frequent findings were parenchymal lung lesions (59/60, 98.3%). Among the 59 patients with parenchymal lung abnormalities, 52 (88.1%) patients showed interstitial pneumonia, 21 (35.6%) had pulmonary alveolar edema and 12 (20.3%) patients had focal atelectasis. Among the 52 patients with interstitial pneumonia, 31 (59.6%) patients showed only interstitial pneumonia without evidence of cardiomegaly or pulmonary alveolar edema (Fig. 1), 15 patients (28.8%) had combined cardiomegaly and 16 (30.8%) had combined pulmonary alveolar edema. Interstitial pneumonia (n=52) on the chest radiographs showed reticulonodular opacities (n=51, 98.1%), bronchial wall thickening (n=39, 75.0%) and septal lines (n=26, 50.0%). Interstitial pneumonia (n=11) on CTs (n=12) showed interlobular septal thickening (n=11, 91.7%), bronchial wall thickening (n=7, 58.3%) and centrilobular nodules (n=2, 16.7%). Pulmonary alveolar edema (n=21) showed diffuse ground glass opacity without consolidation (n=12, 57.1%), diffuse ground glass opacity with consolidation (n=5, 23.8%) (Fig. 2), ground glass opacity of the mid-lower zone (n=3, 14.3%) and ground glass opacity of the lower zone (n=1, 4.8%). A lower lung zone predilection or bilateral diffuse distribution of the parenchymal lung abnormalities (n=59) was found in 58 (98.3%) patients. The one remaining patient showed consolidation of peripheral and upper zone predilection, and this suggested acute respiratory distress syndrome (ARDS) with clinical manifestations (Fig. 3). Focal or subsegmental atelectasis was found in 12/101 (11.8%) patients. One patient showed the only abnormal parenchymal lesion of focal atelectasis. Cardiomegaly developed in 15/101 (14.9%) patients. Pleural effusion developed in 43/101 (42.6%) patients. Fourteen of 101 (13.8%) patients showed hilar lymph node enlargement, and among them, 10 were right sided, one was left sided and three patients had enlarged lymph nodes on both sides.
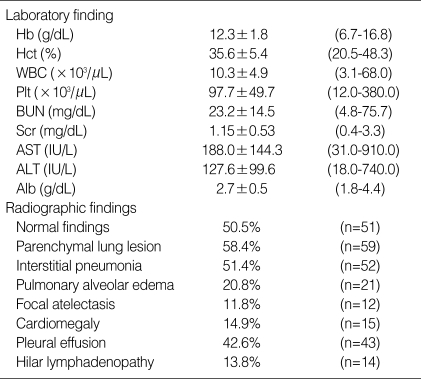
Fig. 1.
Thin-section CT through the lower lung zones in a 43-yr-old woman with scrub typhus. There is thickening of the interlobular septa and small bilateral pleural effusions.
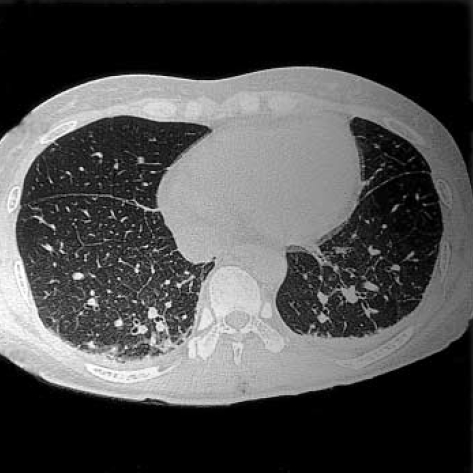
Fig. 2.
Chest radiograph and thin-section CT in a 58-yr-old woman with scrub typhus. (A) Chest radiograph shows diffuse bronchial wall thickening, diffuse ground glass opacities, mild cardiomegaly, bilateral pleural effusions and subsegmental atelectasis. (B) CT of lower zones shows interlobular septal thickening, bronchial wall thickening, diffuse ground glass opacities and patchy consolidations in the dependent lung zones, increased vascular diameter, mild cardiomegaly, bilateral pleural effusions and subsegmental atelectasis.
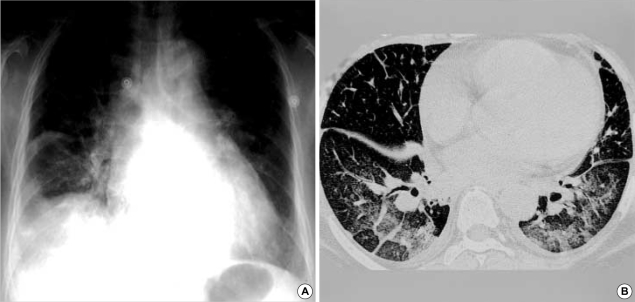
Fig. 3.
Chest radiograph in a 70-yr-old man with scrub typhus. There are bilateral multiple patchy ground glass opacities and minimal right pleural effusion. The consolidations in the upper and peripheral lung zones are suggestive of ARDS.
The patients were divided into 2 groups based on the presence (n=52) or absence (n=49) of IP. There were no differences in age (56±16 vs. 54±16 yr, p=0.635) and sex (female %; 63.4% vs. 69.3%, p=0.529). The patient with IP (n=52) had higher incidence of dyspnea (28.8% vs. 10.2%, p=0.019) than the patients without IP (n=49). There were no differences in clinical signs and symptoms such as fever, headache, flank pain, abdominal pain, myalgia and eschar. We compared the 7 clinical parameters, duration of hospitalization and mortality between the 2 groups. The patients with IP (n=52) had higher incidences in episode of hypoxia (32.6% vs. 14.2%, p=0.030), hypotension (25.0% vs. 8.1%, p=0.024), severe thrombocytopenia (34.6% vs. 16.3%, p=0.036) and hypoalbuminemia (69.2% vs. 44.8, p=0.013) than the patients without IP (n=49) (Table 2). The patients with IP tended to have a higher incidence of acute renal failure than those patients without IP (21.1% vs. 8.1%, p=0.059). However, there was no statistical difference in leukocytosis and abnormal liver function between the two groups. The hospitalization duration of the patients with IP was longer than that of the patients without IP (11.0±5.6 vs. 9.4±3.3 days, p=0.023). The four patients who died all had IP. The cause of death was septic shock (n=2) and ARDS (n=2). The mortality tended to be higher in the patients with IP than for the patients without IP (7.7% vs. 0%, p=0.066).
Table 2.
The comparison of clinical factors between the two groups
IP, interstitial pneumonia.
The patients with IP had a higher incidence of pleural effusion (67.3% vs. 16.3%, p<0.001), focal atelectasis (26.9% vs. 4.1%, p=0.019), cardiomegaly (28.8% vs. 0%, p<0.001), pulmonary alveolar edema (30.8% vs. 10.2%, p=0.011) and hilar lymphadenopathy (26.9% vs. 0%, p<0.001) as compared to the patients without IP (Table 3).
Table 3.
The comparison of other chest radiographic findings between the two groups
DISCUSSION
IP frequently occurs in the acute stage of scrub typhus, but the impact of IP on severity of the disease has not yet been reported. To evaluate the clinical role of IP as a marker of severity of the disease, we studied 101 patients with scrub typhus in a single center over 10 yr. Our data demonstrated that IP was a common finding in the acute stage of scrub typhus, and it was closely associated with severity of the disease. To our knowledge, this is the first report in the medical literature demonstrating the relationship between IP and severity of the disease among patients with scrub typhus.
The characteristic pathophysiologic findings in scrub typhus are well known. Multiplication of the organisms in the endothelial cells lining the small blood vessels causes an endothelial proliferation and perivascular inflammatory cell infiltration, and it results in rash, hemorrhage, and microthrombi. The result is a widespread infectious vasculitis or perivasculitis (1, 4, 6, 8-10). Such microangiopathies may involve the heart, lungs, brain, kidneys, gastrointestinal tract, liver, spleen and lymph nodes (1, 4, 6, 8-10). The clinical parameters representing the severity of the disease are known to be hypotension, thrombocytopenia, leukocytosis, hypoxia, acute renal failure, hypoalbuminemia and hepatic dysfunction (3, 5-7).
Although IP frequently occurs in the patients with scrub typhus, its exact pathophysiologic mechanism is not well known. Hwang et al. (1) reported that the marked dilatation and congestion of septal capillaries, extravasation of red blood cells into the alveoli, and septal widening by the lymphocytes, histiocytes and a few polymorphonuclear leukocytes were found in the lung of mice infected with the rickettsia. When considering this pathologic study and the fact that the main pathogenesis of scrub typhus is widespread microangiopathy, we suggested that this microangiopathic lesion could be associated with the causes of IP. Therefore, we assumed that IP would be associated with other clinical parameters that represent the severity of the disease.
As the clinical parameters representing severity of the disease, we selected already known 7 clinical factors (hypoxia, hypotension, severe thrombocytopenia, leukocytosis, acute renal failure, hypoalbuminemia and hepatic dysfunction). The patients with IP had greater incidences in episode of hypoxia, hypotension, severe thrombocytopenia, and hypoalbuminemia than the patients without IP. The patients with IP tended to have a greater incidence of acute renal failure than for those patients without IP. Furthermore, the hospitalization duration of the patients with IP was longer than that of the patients without IP, and mortality tended to be higher in the patients with IP than for the patients without IP. Therefore, we believe that IP is associated with severity of the disease of scrub typhus. We also compared other radiographic findings between the patients with or without IP. The patients with IP also had higher incidences of pleural effusion, focal atelectasis, cardiomegaly, pulmonary alveolar edema and hilar lymphadenopathy than for the patients without IP. Therefore, we think that IP is associated with severity of the disease of scrub typhus.
The incidence of the chest radiographic abnormalities for patients with scrub typhus varies from 67.5-78% in the previous reports (11-13), and the percentage in this study was 59.4%. The chest abnormalities of scrub typhus reported in the literature are IP, cardiomegaly, pulmonary edema, pleural effusion, hilar adenopathy, and focal atelectasis (4, 11-13, 15). In our study, the chest radiographic abnormalities were IP (51.4%), pleural effusion (42.6%), cardiomegaly (37.6%), pulmonary alveolar edema (20.8%), hilar adenopathy (13.8%) and focal atelectasis (11.8%). The CT findings of interlobular septal thickening, bronchial wall thickening and centrilobular nodules imply that the basic pathology is interstitial (11, 16). The posteroanterior chest radiographic findings of reticulonodular opacities, bronchial wall thickening and septal lines, and the CT findings of interlobular septal thickening, axial interstitial thickening, intralobular septal thickening, centrilobular nodules, fissural thickening and subpleural nodules may reflect the IP with cellular infiltration. However, it could also reflect the interstitial edema and hemorrhage that is due to vasculitis (11).
The radiographic findings of IP may have a component of pulmonary interstitial edema that occurs secondary to cardiac dysfunction (11). Among the 52 patients with IP, 15 (28.8%) patients showed a combined cardiomegaly and 16 (30.8%) patients had a combined pulmonary alveolar edema. Therefore, there might be a much smaller component of pulmonary interstitial edema in those cases with radiographic findings of IP. Scrub typhus is generally an interstitial lesion in the majority of cases, with hilar lymph node enlargement having a rather slower resolution than the pulmonary edema (11). In this study, hilar lymphadenopathy was detected in 26.9% of IP patients and in contrast, hilar lymphadenopathy was not detected in any patients without IP. These results are suggestive that those cases with radiographic findings of IP might have a much smaller component of pulmonary interstitial edema, although no pathologic specimen was obtained.
We assessed the pulmonary alveolar edema secondary to cardiac dysfunction. The findings of pulmonary fluid retention with consolidation, the dependent distribution of ground glass opacity, increased vascular diameter, and cardiomegaly all are suggestive of concomitant cardiac involvement (11). The cardiomegaly is due to myocardial or pericardial involvement by the organism (17-19). The incidence of cardiomegaly was 23.1% in a previous report (12), and it was 14.9% in our report.
In conclusion, our data suggest that interstitial pneumonia is the most frequent chest manifestation of scrub typhus. The presence of interstitial pneumonia is closely associated with morbidity and severity of disease for patients with scrub typhus. The physician should recognize the presence of interstitial pneumonia as being the important determining factor for predicting the clinical course and prognosis for patients with scrub typhus.
References
- 1.Hwang TS, Chu YC, Kim YB, Lim BU, Kang JS. Pathologic study of mice infected with Rickettsia tsutsugamushi R19 strain. J Korean Med Sci. 1993;8:437–445. doi: 10.3346/jkms.1993.8.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31:240–244. [PubMed] [Google Scholar]
- 4.Chi WC, Huang JJ, Sung JM, Lan RR, Ko WC, Chen FF. Scrub typhus associated with multiorgan failure: a case report. Scand J Infect Dis. 1997;29:634–635. doi: 10.3109/00365549709035911. [DOI] [PubMed] [Google Scholar]
- 5.Kantipong P, Watt G, Jongsakul K, Choenchitra C. Infection with human immunodeficiency virus does not influence the clinical severity of scrub typhus. Clin Infect Dis. 1996;23:1168–1170. doi: 10.1093/clinids/23.5.1168. [DOI] [PubMed] [Google Scholar]
- 6.Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001;3:11–21. doi: 10.1016/s1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- 7.Thap LC, Supanaranond W, Treeprasertsuk S, Kitvatanachai S, Chinprasatsak S, Phonrat B. Septic shock secondary to scrub typhus: characteristics and complications. Southeast Asian J Trop Med Public Health. 2002;33:780–786. [PubMed] [Google Scholar]
- 8.Strickman D, Smith CD, Corcoran KD, Ngampochjana M, Watcharapichat P, Phulsuksombati D, Tanskul P, Dasch GA, Kelly DJ. Pathology of Rickettsia tsutsugamushi infection in Bandicota savilei, a natural host in Thailand. Am J Trop Med Hyg. 1994;51:416–423. [PubMed] [Google Scholar]
- 9.Kim SJ, Chung IK, Chung TS, Song DH, Park SH, Kim HS, Lee MH. The clinical significance of upper gastrointestinal endoscopy in gastrointestinal vasculitis related to scrub typhus. Endoscopy. 2000;32:950–955. doi: 10.1055/s-2000-9621. [DOI] [PubMed] [Google Scholar]
- 10.Sirisanthana V, Puthanakit T, Sirisanthana T. Epidemiologic, clinical and laboratory features of scrub typhus in thirty Thai children. Pediatr Infect Dis J. 2003;22:341–345. doi: 10.1097/01.inf.0000059400.23448.57. [DOI] [PubMed] [Google Scholar]
- 11.Choi YH, Kim SJ, Lee JY, Pai HJ, Lee KY, Lee YS. Scrub typhus; radiological and clinical findings. Clin Radiol. 2000;55:140–144. doi: 10.1053/crad.1999.0336. [DOI] [PubMed] [Google Scholar]
- 12.Kim OH, Oh DH, Kim KS, Woo JH, Kwon JH. Chest radiographic findings of scrub typhus: an analysis of 160 cases occurred in Ulsan area. J Korean Radiol Soc. 1993;29:205–210. [Google Scholar]
- 13.Im JG, Lee KS, Kim JH, Lee WJ. Pulmonary manifestations of tsutsugamushi disease. J Korean Radiol Soc. 1988;24:750–755. [Google Scholar]
- 14.Kim YO, Yoon SA, Ku YM, Yang CW, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. Serum albumin level correlates with disease severity in patients with hemorrhagic fever with renal syndrome. J Korean Med Sci. 2003;18:696–700. doi: 10.3346/jkms.2003.18.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chayakul P, Panich V, Silpapojakul K. Scrub typhus pneumonitis: an entity which is frequently missed. Q J Med. 1988;68:595–602. [PubMed] [Google Scholar]
- 16.Murata K, Itoh H, Todo G, Kanaoka M, Noma S, Itoh T, Furuta M, Asamoto H, Torizuka K. Centrilobular lesions of the lung: demonstration by high resolution CT and pathologic correlation. Radiology. 1986;161:641–645. doi: 10.1148/radiology.161.3.3786710. [DOI] [PubMed] [Google Scholar]
- 17.Levine HD. Pathologic study of thirty-one cases of scrub typhus fever with especial reference to the cardiovascular system. Am Heart J. 1946;31:314–328. doi: 10.1016/0002-8703(46)90313-4. [DOI] [PubMed] [Google Scholar]
- 18.Yotsukura M, Aoki N, Fukuzumi N, Ishikawa K. Review of a case of tsutsugamushi disease showing myocarditis and confirmation of rickettsia by endomyocardial biopsy. Jpn Circ J. 1991;55:149–153. doi: 10.1253/jcj.55.149. [DOI] [PubMed] [Google Scholar]
- 19.Kundin WD, Liu C, Harmon P, Rodina P. Pathogenesis of scrub typhus infection (Rickettsia tsutsugamushi) as studied by immunofluorescence. J Immunol. 1964;93:772–781. [PubMed] [Google Scholar]