Abstract
Focal adhesion kinase (FAK) is a tyrosine kinase that is found in cellular structures called focal adhesions. FAK appears to be a key element in signal transduction pathways involved in cell adhesion and locomotion. FAK is overexpressed in various tumors, including tumors derived from regions of the head and neck, colon, breast, prostate, and liver. In this study, we investigated immunohistochemically whether FAK expression was increased in thyroid cancers. FAK staining was not seen in any of the 20 normal thyroid tissues or the 6 nodular hyperplasia specimens. In contrast, FAK staining was observed in all of 17 papillary carcinomas, 9 follicular carcinomas, 8 medullary carcinomas, and 2 anaplastic carcinomas. Nine of 17 follicular adenomas showed FAK immunoreactivity. FAK was not expressed in normal tissue and nodular hyperplasia, but was expressed in some of the follicular adenoma, and all of the follicular, papillary, medullary and anaplastic thyroid carcinoma. This result indicates that the up-regulation of FAK may play a role in the development of thyroid carcinogenesis.
Keywords: Focal Adhesion Kinase, Protein-Tyrosine Kinase, Thyroid Nodule, Thyroid Neoplasms, Immunohistochemistry, Focal Adhesions
INTRODUCTION
Nodular thyroid disease is a very common disorder. According to the Framingham database (1), the estimated lifetime risk of having a thyroid nodule is 5-10%. However, thyroid cancer represents just 1-2% of all malignancies and only 5-24% of thyroid nodules treated surgically are malignant. These days, fine-needle aspiration (FNA) is the best tool for the evaluation of thyroid nodules. But misdiagnoses may occur, due to either insufficient or unsuitable aspirated material or sampling error. Furthermore, the main limitation of FNA is the lack of sensitivity in the evaluation of follicular neoplasms, due to the inability to differentiate follicular adenoma from carcinoma. If a more reliable marker for the presence of thyroid cancer were available for preoperative evaluation, we could avoid unnecessary thyroid surgeries.
Invasion and metastasis are one of the main characteristics of cancer. They are a complex process that includes changes in cell adhesion, allowing transformed cells to invade and migrate through the extracellular matrix (ECM) (2). Focal adhesions are considered to be a key role in this change (3). Focal adhesions are cell-ECM contact points containing membrane-associated, cytoskeletal, and intracellular signaling molecules. A number of proteins are found to be preferentially associated with the focal adhesion complex, including focal adhesion kinase (FAK), paxillin, vinculin, and talin. Among these proteins, FAK is a critical mediator of signaling events between cells and the ECM (4). FAK is a cytosolic protein of 125 kDa, first recognized as a major phosphotyrosine-containing protein in v-Src-transformed chicken embryo fibroblasts (5). FAK is associated with the cytoplasmic domain of integrin receptors, and becomes phosphorylated in response to integrin-mediated cell adhesion, integrin clustering, cell migration (6, 7), stimulation by mitogenic neuropeptides such as bombesin (8), and transformation by v-Src, v-Crk and Bcr-Abl (9). This, in turn, allows for the association of mitogenic proteins that contribute to the control of cell growth and differentiation. The potential involvement of FAK in promotion of cell proliferation and migration in several cell types in vitro suggests that FAK could potentially play a role in neoplastic processes in which cell proliferation has escaped control mechanisms.
Evidence suggests that FAK is overexpressed in various tumors, including tumors derived from regions of the head and neck, colon, breast, prostate, liver, cervix, and thyroid (10-19). In this study, we investigated FAK expression in thyroid cancers and the possibility of its usage as a tumor marker.
MATERIALS AND METHODS
Specimens
Fifty-nine paraffin-embedded thyroid specimens were obtained from surgical resections performed due to thyroid nodules. These consisted of 17 papillary carcinomas, 9 follicular carcinomas, 17 follicular adenomas, 6 nodular hyperplasias, 8 medullary thyroid carcinomas (MTC), and 2 anaplastic carcinomas. Twenty normal thyroid tissues were used as controls. Normal tissue samples were taken from histologically normal areas adjacent to the neoplastic lesion.
Immunohistochemistry
Immunohistochemical analysis of FAK was performed using established protocols. Briefly, paraffin-embedded tissue was cut into 5 µm section and dried for 1 hr at 57℃ in oven. After routine deparaffinization and rehydration, tissue sections were microwaved for 20 min in 0.01 M sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 30 min followed by incubation with rabbit antihuman FAK antibody (Upstate Biotechnology, Lake Placid, NY, U.S.A.) at a dilution of 1:100 for 60 min at room temperature. Then, all slides were washed 3 times for 3 min each with phosphate-buffered saline (PBS). Samples were incubated with PicTure-plus bulk kit (Zymed Lab, San Francisco, CA, U.S.A.), that is Zymed's HRP polymer detection system, for 20 min at room temperature, washed and incubated with Liquid DAB substrate kit (Zymed Lab) for 5 min. And then counterstained with Mayer's hematoxylin for 5 min and mounted. For negative controls, incubation with the primary antibody was omitted. Staining was scored as follows: 0 (-)= absent, 1 (+)=weak staining, 2 (++)=moderate staining, and 3 (+++)=strong staining in epithelial cells.
Statistical analysis
The correlation between the total score of FAK expression and clinicopathological features (sex, tumor size, and metastasis) was determined by the Spearman's rank correlation. Chi-square test was used for comparison of the FAK expression in thyroid cancer and metastasis. A probability of p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows version 11.0 (Chicago, IL, U.S.A.).
RESULTS
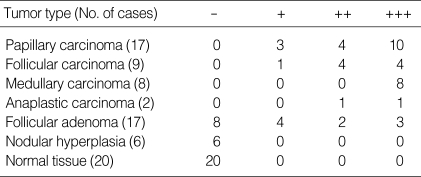
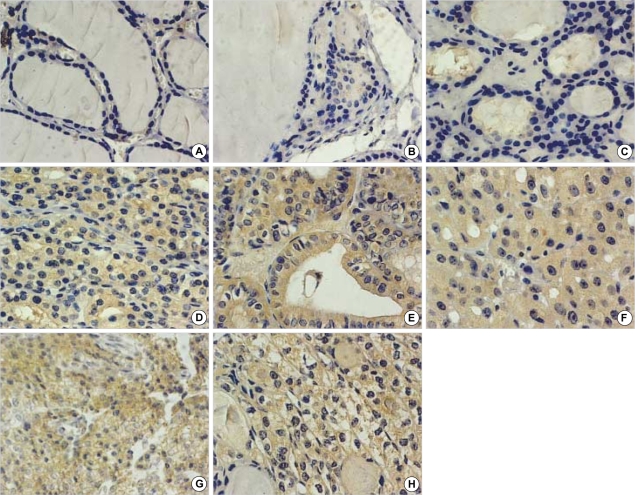
Table 1 summarizes the results obtained for FAK immunostaining in thyroid tissues from 59 patients. FAK, when expressed, was found in the cytoplasm of follicular cells. FAK staining was not seen in any of the 20 normal thyroid tissues (Fig. 1A), and the 6 nodular hyperplasia specimens (Fig. 1B). In contrast, FAK staining was observed in all of 17 papillary carcinomas (Fig. 1E), 9 follicular carcinomas (Fig. 1F), 8 medullary carcinomas (Fig. 1G) and 2 anaplastic carcinomas (Fig. 1H). In follicular adenoma, 8 cases were negative (Fig. 1C), 4 cases were weak, 2 cases were moderate, and 3 cases had strong FAK positivity (Fig. 1D).
Table 1.
Expression of FAK in thyroid lesions
+++, strong staining; ++, moderate staining; +, weak staining; -, no staining.
Fig. 1.
Immunohistochemical expression of FAK in thyroid nodules. (A) normal tissue, (B) nodular hyperplasia, (C) follicular adenoma that shows negative immunostaining, (D) follicular adenoma that shows moderate immunostaining, (E) papillary carcinoma, (F) follicular carcinoma, (G) medullary carcinoma, (H) anaplastic carcinoma (×400); All carcinoma tissues show the strong staining of FAK.
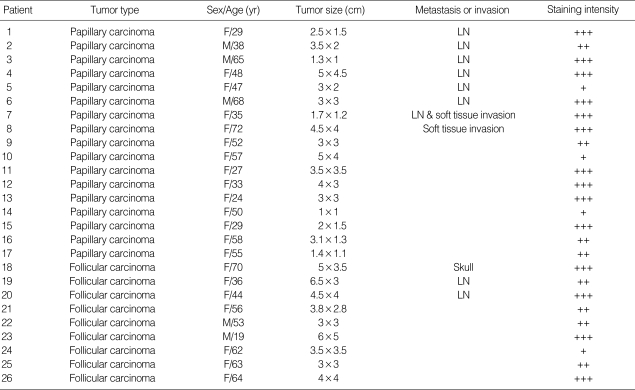
In papillary carcinoma, 7 cases had lymph node (LN) metastasis, 1 case had soft tissue invasion, and 9 cases had neither. In 7 cases with LN metastasis, 5 cases were strong, 1 case was moderate, and 1 case was weak FAK staining. One case with soft tissue invasion had strong FAK positivity. In 9 cases of papillary carcinoma without LN metastasis or soft tissue invasion, 4 cases were strong, 3 cases were moderate, and 2 cases were weak FAK expression. In follicular carcinoma, 2 cases had LN metastasis, which showed strong FAK expression in one and moderate FAK expression in the other. One case had distant metastasis to the skull and showed strong FAK expression. In 6 cases of follicular carcinoma without metastasis, 2 cases were strong, 3 cases were moderate, and 1 case had weak FAK positivity (Table 2). The expression of FAK in thyroid cancer was significantly increased. FAK expression was stronger in thyroid cancer than in benign thyroid tumor (p=0.000), but there were no differences in FAK expression between the types of cancer. Sex, tumor size, and metastasis had no significant correlation with FAK expression.
Table 2.
Clinicopathological features and FAK expression in thyroid cancer
+++, strong staining; ++, moderate staining; +, weak staining; -, no staining.
DISCUSSION
FAK plays an important role in cell proliferation. Zhao et al. (20) demonstrated that overexpression of wild-type FAK resulted in an accelerated G1 to S transition in Chinese hamster ovary (CHO) cells, suggesting a role for FAK in the promotion of cell proliferation. Several evidences have showed that FAK promotes cell survival. Antibody or peptide blocking of FAK binding to integrins resulted in rapid cell death of fibroblasts (21), and antisense oligonucleotides directed toward FAK mRNA resulted in apoptosis of cancer cells (22). Overexpression of FAK can rescue normal, suspended epithelial cells from anoikis, a process by which adherent cells become apoptotic after they are detached from their underlying extracellular matrix as a result of the loss of integrin adhesion-mediated FAK signaling (23, 24). FAK also promotes cell migration. Ilic et al. (25) suggested that fibroblasts isolated from FAK-knockout mice were shown to exhibit reduced cell motility in culture, and Owen et al. (26) demonstrated that FAK re-expression in FAK null cells restored cell migration. Furthermore, ectopic expression of FRNK causes dephosphorylation of FAK at Tyr397 (27) and blocks FAK-mediated fibroblast migration (28), and overexpression of wild-type FAK in CHO and African monkey kidney cell line COS cells resulted in increased cell migration (29, 30). Recent reports suggest that p53 may be associated in function with the FAK. Loss of cell adhesion deactivates FAK, causing anoikis through the p53-mediated cell death pathway (23). Inhibition of FAK induces apoptosis in normal cells and tumor cells in a p53 dependent manner (21, 31-33). In normal cells, FAK might be a sensor of cell adhesion, limiting growth in anchorage-dependent manner, whereas in transformed cells, overexpression of FAK may override this regulation and allow anchorage-independent growth in the absence of cell adhesion.
There are a lot of reports that FAK is overexpressed in various tumors, including tumors derived from the head and neck, colon, breast, prostate, liver, cervix, and thyroid (10-19). Owens et al. (14) assessed the level of FAK expression in 30 human thyroid tissue samples from 27 patients that included paired normal and malignant specimens by using Western blot. The levels of FAK expression were directly correlated with the most aggressive phenotype of thyroid carcinomas. The highest levels of FAK were seen in follicular carcinomas and tumors associated with distant metastatic foci. In contrast, neoplastic thyroid tissues with limited invasive potential, such as papillary carcinomas, follicular adenomas, and other nonmalignant thyroid lesions, showed minimal FAK expression. So they suggested that FAK might be useful as a marker of invasive potential in differentiated thyroid cancer. These results were somewhat different from our results. In our study, metastasis was not significantly correlated with FAK expression. The expression of FAK may be not related to invasion or metastasis, and clinical stage. In ovarian (34) and prostate carcinoma (35), there also was no association of FAK expression with the grade or stage of tumor. Some reports argue that the invasiveness and metastatic potential of human tumors is directly proportional to the level of FAK expression (12, 22, 36). But Kahana et al. (37) suggested that the mechanism by which FAK may be involved in tumorigenesis is not by overexpression but rather by FAK constitutive activation. In our study, FAK was expressed in all of the follicular and papillary carcinoma, and some of the follicular adenoma, but was not expressed in normal tissue and nodular hyperplasia. So, FAK overexpression may play a role in the early event of development of thyroid carcinogenesis.
In this study, FAK was overexpressed in all of the medullary thyroid carcinoma specimens. The RET gene is mutated in more than 95% of patients with hereditary MTC (39) and in up to 70% of DNA from sporadic tumors (40). RET signaling has been shown to lead to phosphorylation of the adhesion-dependent signaling molecules FAK, paxillin, and p130CAS (41). Kim et al. (38) reported that FAK was constitutively phosphorylated in MTC cells. But, they did not observe the degree of FAK expression. Our study is the first report that shows the overexpression of FAK in MTC. Therefore, constitutive activation and overexpression of FAK may contribute to MTC pathogenesis.
In summary, these results are the first immunohistochemical evidence that FAK expression is up-regulated in thyroid cancers. This overexpression of FAK in tumors, combined with the relative lack of expression in normal thyroid tissues, suggests that FAK is a rational target for thyroid cancer therapeutics. Further investigation of FAK as a clinical tumor marker appears to be warranted.
Footnotes
This study was supported by a grant from the research fund of Soonchunhyang University (2003-0148).
References
- 1.Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules: final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 3.Zetter BR. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993;4:219–229. [PubMed] [Google Scholar]
- 4.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 5.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinnett-Smith J, Zachary I, Valverde AM, Rozengurt E. Bombesin stimulation of p125 focal adhesion kinase tyrosine phosphorylation. J Biol Chem. 1993;268:14261–14268. [PubMed] [Google Scholar]
- 9.Zachary I, Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- 10.Kornberg LJ. Focal adhesion kinase expression in oral cancers. Head Neck. 1998;20:634–639. doi: 10.1002/(sici)1097-0347(199810)20:7<634::aid-hed10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 12.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 13.Glukhova M, Koteliansky V, Sastre X, Thiery JP. Adhesion systems in normal breast and in invasive breast carcinoma. Am J Pathol. 1995;146:706–716. [PMC free article] [PubMed] [Google Scholar]
- 14.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol. 1996;3:100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50csk in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Han NM, Fleming RY, Curley SA, Gallick GE. Overexpression of focal adhesion kinase (p125FAK) in human colorectal carcinoma liver metastases: independence from c-src or c-yes activation. Ann Surg Oncol. 1997;4:264–268. doi: 10.1007/BF02306620. [DOI] [PubMed] [Google Scholar]
- 17.Withers BE, Hanks SK, Fry DW. Correlations between the expression, phosphotyrosine content and enzymatic activity of focal adhesion kinase, pp125FAK, in tumor and nontransformed cells. Cancer Biochem Biophys. 1996;15:127–139. [PubMed] [Google Scholar]
- 18.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 19.Moon HS, Park WI, Choi EA, Chung HW, Kim SC. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer. 2003;13:640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu LH, Owens LV, Sturge GC, Yang X, Lui ET, Craven RJ, Cance WG. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413–418. [PubMed] [Google Scholar]
- 23.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisch S, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 26.Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson A, Parsons JT. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 28.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 29.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overepression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 30.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a molecular switch for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Jr, Craven RJ, Cance WG. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem. 2000;275:30597–30604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 32.Xu LH, Yang X, Craven RJ, Cance WG. The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ. 1998;9:999–1005. [PubMed] [Google Scholar]
- 33.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Shlaepfer DD, Damsky CM. Matrix survival signaling from fibronectin via focal adhesion kinase to c-jun nh (2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Judson PL, He X, Cance WG, Le LV. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86:1551–1556. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 36.Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, Frame MC. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–5653. doi: 10.1038/sj.onc.1202957. [DOI] [PubMed] [Google Scholar]
- 37.Kahana O, Micksche M, Witz IP, Yron I. The focal adhesion kinase (p125FAK) is constitutively active in human malignant melanoma. Oncogene. 2002;21:3969–3977. doi: 10.1038/sj.onc.1205472. [DOI] [PubMed] [Google Scholar]
- 38.Kim LT, Fleming JB, Lopez-Guzman C, Nwariaku F. Focal adhesions and associated proteins in medullary thyroid carcinoma cells. J Surg Res. 2003;111:177–184. doi: 10.1016/s0022-4804(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 39.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, van Amster HK, Lips CJ, Nishisho I, Takai SI, Marsh DJ, Robinson BG, Frank-Raue K, Raue F, Xue F, Noll WW, Romei C, Pacini F, Fink M, Niederle B, Zedenius J, Nordenskjold M, Komminoth P, Heudy GN, Mulligan LM. The relationship between specific RET proto-oncogene mutation and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 40.Eng C, Mulligan LM. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes, related sporadic tumours, and Hirschsprung disease. Hum Mutat. 1997;9:97–109. doi: 10.1002/(SICI)1098-1004(1997)9:2<97::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Murakami H, Iwashita T, Asai N, Iwata Y, Narumiya S, Takahashi M. Rho-dependent and -independent tyrosine phosphorylation of focal adhesion kinase, paxillin and p130 Cas mediated by Ret kinase. Oncogene. 1999;18:1975–1982. doi: 10.1038/sj.onc.1202514. [DOI] [PubMed] [Google Scholar]
- 42.Gimm O. Thyroid cancer. Cancer Lett. 2001;163:143–156. doi: 10.1016/s0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]