Abstract
In plants, excess cellular lysine (Lys) is catabolized into glutamic acid and acetyl-coenzyme A; yet, it is still not clear whether this pathway has other functions in addition to balancing Lys levels. To address this issue, we examined the effects of stress-related hormones, abscisic acid (ABA), and jasmonate, as well as various metabolic signals on the production of the mRNA and polypeptide of the bifunctional Lys-ketoglutarate reductase (LKR)/saccharopine dehydrogenase (SDH) enzyme, which contains the first two linked enzymes of Lys catabolism. The level of LKR/SDH was strongly enhanced by ABA, jasmonate, and sugar starvation, whereas excess sugars and nitrogen starvation reduced its level; thus this pathway appears to fulfill multiple functions in stress-related and carbon/nitrogen metabolism. Treatments with combination of hormones and/or metabolites, as well as use of ABA mutants in conjunction with the tester sugars mannose and 3-O-methyl-glucose further supported the idea that the hormonal and metabolic signals apparently operate through different signal transduction cascades. The stimulation of LKR/SDH protein expression by ABA is regulated by a signal transduction cascade that contains the ABI1-1 and ABI2-1 protein phosphatases. By contrast, the stimulation of LKR/SDH protein expression by sugar starvation is regulated by the hexokinase-signaling cascade in a similar manner to the repression of many photosynthetic genes by sugars. These findings suggest a metabolic and mechanistic link between Lys catabolism and photosynthesis-related metabolism in the regulation of carbon/nitrogen partitioning.
In plants, the essential amino acid Lys is catabolized via the α-amino adipic acid pathway (Fig. 1). The first enzyme in this pathway, Lys-ketoglutarate reductase (LKR), combines Lys and α-ketoglutarate to form saccharopine. Saccharopine is then converted by saccharopine dehydrogenase (SDH) to Glu and α-amino adipic semialdehyde; the latter is further metabolized into acetyl-CoA by several enzymatic reactions. The LKR and SDH enzymes, which play pivotal role in Lys catabolism, are linked on a single bifunctional LKR/SDH polypeptide, encoded by a single LKR/SDH gene (Galili et al., 2001).
Figure 1.
The Lys catabolism pathway and metabolites derived from it. ASD, aminoadipic semialdehyde dehydrogenase. The broken arrow represents several non-specified enzymatic reactions. Glu and acetyl-CoA, the products of this pathway, are boxed.
The regulation and functional significance of the Lys catabolism pathway in plants have only recently begun to unfold. Lys overproduction in seeds of transgenic tobacco plants that expressed a bacterial Lys-insensitive dihydrodipicolinate synthase caused a significant stimulation in LKR activity (Karchi et al., 1994). This implied that the Lys catabolism pathway is an important route for balancing Lys levels, mostly because high Lys is toxic to plants (Jacobs et al., 1987; Falco et al., 1995). Lys-induced stimulation of LKR activity is regulated by calcium as well as by Lys-mediated phosphorylation of the LKR/SDH polypeptide by casein kinase 2 (Karchi et al., 1995; Miron et al., 1997; Arruda et al., 2000). In rapeseed, the level of the LKR/SDH mRNA and the activities of its encoded LKR and SDH polypeptides were found to be stimulated by osmotic stress (Deleu et al., 1999; Moulin et al., 2000). Such stress-stimulated expression was explained by the possibility that Glu, a major end product of Lys catabolism (Fig. 1), is an important precursor for a number of stress-related metabolites (Deleu et al., 1999; Moulin et al., 2000; Galili et al., 2001).
Although not fully elucidated, Lys catabolism apparently serves additional physiological and developmental functions in plants. Under regular growth conditions, expression of the LKR/SDH gene is strongly up-regulated in floral organs and developing seeds (Tang et al., 1997), suggesting an important role in plant reproduction. Moreover, because one of the end products of Lys catabolism is acetyl-CoA (Fig. 1), it is likely that Lys catabolism also participates in the regulation of carbon/nitrogen partitioning, particularly under metabolic starvation and in senescence. Aiming to elucidate the functions of the Lys catabolism pathway, we analyzed here the effects of the stress-related hormones abscisic acid (ABA) and jasmonate, as well as various metabolic signals, on the production of the bifunctional LKR/SDH polypeptide from the Arabidopsis LKR/SDH gene. We show that production of this polypeptide is under concerted regulation by ABA, jasmonate, and a variety of metabolic signals through a number of different signal transduction cascades.
RESULTS
Regulation of LKR/SDH Gene Expression by ABA and Jasmonate
ABA and jasmonate are two likely hormones that regulate the expression of the LKR/SDH gene expression. Besides their strong association with abiotic and biotic stresses, these hormones play a significant role in flower (jasmonate) and seed (ABA) development in normally grown plants (Finkelstein et al., 2002; Turner et al., 2002). Another clue for the potential regulatory role of these two hormones in expression of the Arabidopsis LKR/SDH gene could be deduced from the presence of multiple putative ABA- and jasmonate-responsive elements in the promoter of this gene (data not shown). To test for the regulatory role of these hormones in the production of the LKR/SDH polypeptide, wild-type Arabidopsis plants grown in liquid media were exposed to different concentrations of ABA and methyl jasmonate (MeJa) for 24 h. As shown in the northern blots of Figure 2, A and B, and in the western blots of Figure 3, A and B, these hormones significantly stimulated the production of the LKR/SDH mRNA and polypeptide in a concentration-dependent manner, yielding at the highest hormone concentration approximately 12-fold (ABA) and approximately 5-fold (MeJa) increases in the intensity of the LKR/SDH polypeptide band.
Figure 2.
Effects of ABA and MeJa on the level of the Arabidopsis bifunctional LKR/SDH mRNA. Nine-day-old Arabidopsis plants were grown in liquid cultures in the absence (control) or presence of increasing concentrations of ABA (A) or MeJa (B) for 24 h. Total RNAs were then reacted in northern blots with the LKR domain of the AtLKR/SDH cDNA as a probe. The different lanes contained comparable amounts of RNA (stained blots in the bottom panels of A and B).
Figure 3.
Effects of ABA and MeJa on the level of the Arabidopsis bifunctional LKR/SDH polypeptide. Nine-day-old Arabidopsis plants were grown in liquid cultures in the absence (control) or presence of increasing concentrations of ABA (A) or MeJa (B) for 24 h. Proteins were then reacted in western blots with monoclonal anti-LKR antibodies (top panels in A and B). The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panels of A and B. The relative intensities of the LKR/SDH bands, normalized to controls treated with no hormones (left lane of each section), are indicated between the top and bottom panels. Arrowheads and asterisks on the right indicate the positions of the LKR/SDH and Rubisco small subunit polypeptides, respectively.
Metabolic Regulation of LKR/SDH Gene Expression by Carbon and Nitrogen
Being a catabolic enzyme, LKR/SDH apparently participates in the regulation of carbon/nitrogen partitioning, and its production is expected to be subject to metabolic regulation. To test the role of sugars in the production of LKR/SDH, wild-type Arabidopsis plants were grown in a medium containing 2% (w/v) Suc (control sugar level) for 9 d and then transferred to a new medium lacking Suc for 72 h (Fig. 4A, lane b). To test the effect of excess Suc, 11-d-old seedlings grown in 2% (w/v) Suc-containing medium were transferred to a new medium containing 5% (w/v) Suc for 24 h (Fig. 4A, lane c). As shown in Figure 4A, lanes a through c, the intensity of the LKR/SDH polypeptide was enhanced by approximately 4-fold upon exposure to sugar starvation, but 5% (w/v) Suc had no significant effect on its level. Next, we tested whether the stimulatory effect of sugar starvation on the production of the LKR/SDH could be reversed by Suc. As shown in Figure 4B, the 6-fold increase in the level of LKR/SDH (compare with lanes a and b) was largely reversed by the addition of 1%, 2%, or 5% (w/v) Suc for 24 h to the starved cells (Fig. 4B, compare with lanes b and c–e). As shown further in Figure 4, C (lanes a–f) and D (lanes a–f), addition of Fru, Glc, or Suc, but not mannitol, to sugar-starved cultures reduced the levels of the LKR/SDH mRNA and polypeptide, indicating that the sugar effect was not specific to Suc and was not related to a change in osmotic conditions.
Figure 4.
Effects of sugars and sugar starvation on the level of the Arabidopsis bifunctional LKR/SDH mRNA and polypeptide. Nine-day-old Arabidopsis plants grown in a liquid culture were transferred to a new medium containing various levels of different sugars and incubated for different durations before harvesting. The LKR/SDH mRNA (C) and polypeptide (A, B, and D) were detected as described in Figures 2 and 3, respectively. The different lanes contained comparable amounts of RNA (stained blot in the bottom panel of C) as well as proteins (stained blots in the bottom panels of A, B, and D, depicted by the region of the Rubisco large subunit). A, Seedlings were transferred to new media as follows: The first group (lane a) was transferred to a medium containing 2% (w/v) Suc (control level) and grown for 72 h before harvesting. The second group (lane b) was transferred to a Suc-free medium for 72 h before harvesting. The third group (lane c) was first transferred to a medium containing control Suc levels for 48 h and then to a medium containing 5% (w/v) Suc for additional 24 h before harvesting. B, Plants were transferred to media containing 2% or 0% (w/v) Suc, incubated for 48 h and harvested (lanes a and b). In addition, plants exposed to 48 h of sugar starvation (lane b) were further transferred to new media containing 1%, 2%, or 5% (w/v) Suc for additional 24 h before harvesting. C and D, Sucstarved plants were transferred to new media containing 2% (w/v) of the indicated sugars and incubated for 24 h before harvesting. The relative intensities of the LKR/SDH bands, normalized to the controls (left lane in each section), are indicated in between the top and bottom panels.
We also tested the effect of nitrogen starvation, either alone or in combination with sugar starvation, on the level of the LKR/SDH polypeptide. To impose nitrogen starvation, wild-type Arabidopsis plants were grown for 9 d in a medium containing 26.4 mm nitrogen (control nitrogen level; see “Materials and Methods”) and then transferred to a new medium lacking nitrogen for additional 3 d. To test the combined effect of the both sugar and nitrogen starvation, plants were pre-incubated in sugar-free medium for 48 h and then transferred to a new medium lacking both sugar and nitrogen for additional 24 h before harvesting. As shown in Figure 5A, lanes a and c, exposure of the Arabidopsis cultures to a nitrogen-free medium for 3 d caused a approximately 70% reduction in the level of the LKR/SDH polypeptide. Exposure to sugar starvation for 3 d caused in this experiment a approximately 4-fold increase in the level of LKR/SDH (Fig. 5A, lane b). Exposure of the Arabidopsis cultures to sugar followed by both sugar and nitrogen starvation caused an approximately 1.6-fold increase in the level of LKR/SDH polypeptide (Fig. 5A, lane d). This value, being between the 70% repression caused by nitrogen starvation and the approximately 4-fold stimulation caused by sugar starvation (compare with lane d with lanes b and c), indicates that sugar and nitrogen starvations regulate LKR/SDH gene expression in an opposite manner.
Figure 5.
Effects of nitrogen, nitrogen starvation, and amide amino acids on the level of the LKR/SDH polypeptide. Nine-day-old Arabidopsis plants grown in a liquid culture were transferred to a new medium containing various levels of different nitrogenous metabolites for different durations before harvesting. Proteins were then reacted in western blots with monoclonal anti-LKR antibodies (top panels in A and B). The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panels of A and B. A, Plants were grown in media containing or lacking the regular levels of nitrogen and Suc, as indicated on top, for 72 h and harvested (lanes a–c). In addition, plants were pre-incubated in sugar-free medium for 48 h and then transferred to a new medium lacking sugar and nitrogen for additional 24 h before harvesting (lane d). B, Plants were transferred to new media containing either no added amino acids (C) or 10 mm of any of the amino acids Glu, Gln, Asp, or Asn for 24 h before harvesting (lanes a–e, respectively). The relative intensities of the LKR/SDH bands, normalized to the controls (left lane in each section), are indicated in between the top and bottom panels.
Considering the repression of LKR/SDH gene expression by nitrogen starvation, we determined the effects of the amino acids Glu, Gln, Asp, and Asn (10 mm each for 24 h) on the level of the LKR/SDH polypeptide. As shown in Figure 5B, lanes a–e, whereas Glu and Gln had no significant effect, Asp and Asn caused a small but consistent increase of approximately 1.5- to approximately 2-fold in the level of LKR/SDH.
The Effects of ABA and Sugar Starvation on LKR/SDH Gene Expression Are Independent of Each Other
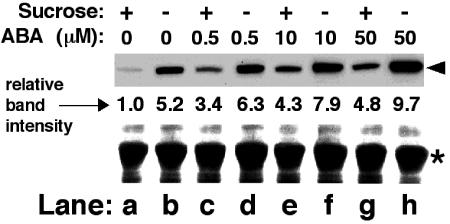
Genetic and molecular studies showed that sugars and ABA cross-interact in the regulation of several developmental and molecular events in germinating Arabidopsis seedlings and often use common signaling cascades (Finkelstein et al., 2002; Rolland et al., 2002). Therefore, we examined whether the response of LKR/SDH gene expression to sugar starvation is dependent on ABA, and vice versa. To address this question, cultured Arabidopsis plants were grown on media containing increasing concentrations of ABA with or without Suc. As shown in Figure 6, at all ABA concentrations, the intensity of the LKR/SDH polypeptide band was higher in media lacking Suc than in media containing Suc (Fig. 6, compare lanes, d, f, and h with lanes, c, e, and g, respectively). This implies that the induction of LKR/SDH gene expression by sugar starvation does not depend on ABA, and vice versa.
Figure 6.
Effects of combined treatments with ABA and Suc starvation on the level of the LKR/SDH polypeptide. Nine-day-old seedlings grown in a liquid culture were transferred to new media containing or lacking 2% (w/v) Suc for 48 h and then exposed to different concentrations of ABA, as indicated on top, for 24 h before harvesting. Top, Proteins were then reacted in western blot with monoclonal anti-LKR antibodies. The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panel. The relative intensities of the LKR/SDH bands, normalized to the control treated with sugar and no ABA (left lane), are indicated in between the top and bottom panels.
The Effects of ABA and Jasmonate on the Expression of the LKR/SDH Gene Are Independent of Each Other
Next, we determined whether the induction of LKR/SDH gene expression by ABA depends on jasmonate, and vice versa. To this end, Arabidopsis cultures were exposed to 0.1 or 0.5 μm ABA or to 0.1 or 0.5 μm MeJa, alone or in combination. As shown in Figure 7, treatment with the two hormones together, at both concentrations, caused a nearly additive increase in the level of the LKR/SDH polypeptide, compared with treatments with each of these hormones individually (Fig. 7, compare lane d with lanes b and c, and lane f with lanes e and g). This suggests that ABA and MeJa are not dependent on each other in the regulation of LKR/SDH gene expression.
Figure 7.
Effects of combined treatments with ABA and MeJa on the level of the LKR/SDH polypeptide. Nine-day-old seedlings grown in a liquid culture were transferred to new media containing different concentrations of MeJa, ABA, or MeJa + ABA, as indicated on top, for 24 h before harvesting. Top, Proteins were then reacted in western blots with monoclonal anti-LKR antibodies. The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panel. The relative intensities of the LKR/SDH bands, normalized to the control treated with no hormones (left lane), are indicated in between the top and bottom panels.
The Regulation of LKR/SDH Gene Expression by Jasmonate and Sugar Starvation Does Not Depend on the Presence of ABA
To address this question, we used the ABA-deficient Arabidopsis mutant aba2-1 (Koornneef et al., 1984). These mutant or wild-type plants were exposed to 50 μm ABA or 50 μm MeJa for 24 h or to a sugar-free medium for 72 h. As shown in Figure 8B, these concentrations of ABA and MeJa caused, respectively, an approximately 3.7-fold and approximately 2.7-fold induction in the intensity of the LKR/SDH band in the aba2-1 mutant compared with non-treated aba2-1 plants (Fig. 8B, compare lanes a with c). Similarly, exposure to sugar starvation caused an approximately 3.8-fold induction in the intensity of the LKR/SDH band in the aba2-1 mutant compared with non-treated aba2-1 (compare with lanes a and d), implying that the response of LKR/SDH gene expression to MeJa and sugar starvation is independent of ABA.
Figure 8.
Effects of ABA, MeJa, and sugar starvation on the level of the LKR/SDH polypeptide in different aba mutants. In all experiments, the level of the LKR/SDH polypeptide was monitored by western-blot analysis with monoclonal anti-LKR antibodies. A, Control non-treated nine-day-old seedlings of the wild-type plants (lane a) as well as the aba2-1, abi2-1 and abi1-1 mutants (lanes b–d, respectively) showing the basal levels of the LKR/SDH polypeptide. B, Nine-day-old seedlings of wild type as well as the aba mutants (top to bottom panels) were transferred to new regular media without ABA or MeJa, or with either 50μm ABA or MeJa for 24 h before harvesting (lanes a–c, respectively). Lane d, In addition, plants were transferred to sugar-free medium for 72 h before harvesting. The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panels of A to C. The relative intensities of the LKR/SDH bands, normalized to the controls (left lanes), are indicated in between the top and bottom panels of each section.
Regulation of LKR/SDH Gene Expression by the Signal Transduction Cascade Containing the ABI1-1 and ABI2-1 Protein Phosphatases
The ABI1 and ABI2 genes encode two distinct protein phosphatases that negatively regulate an ABA signal transduction cascade (Finkelstein et al., 2002). Hence, the expression of genes that positively respond to this signal transduction cascade is constitutively up-regulated in the Arabidopsis abi2-1 and abi1-1 mutants (Finkelstein et al., 2002). To test whether this signal transduction cascade participates in the regulation of LKR/SDH gene expression, we first grew plant cultures of the aba2-1, abi2-1, and abi1-2 mutants as well as wild-type Arabidopsis in a regular medium without exposure to any hormone or sugar starvation. As shown in Figure 8A, lanes a and b, the intensity of the LKR/SDH polypeptide was slightly lower in the aba2-1 mutant than in wild-type plants, as expected for a mutant lacking ABA. By contrast, the intensity of the LKR/SDH band in the abi2-1 and abi1-1 mutants was higher by approximately 1.5- and approximately 2-fold, respectively, than in the wild-type plants (Fig. 8A, lanes a, c, and d). This implies that the signal transduction cascade, operating through the ABI1 and ABI2 genes, does play a role in the regulation of LKR/SDH gene expression. Next, we tested the effects of ABA, MeJa, and sugar starvation on the level of the LKR/SDH polypeptide in the abi1-1 and abi2-1 mutants. As shown in Figure 8B, ABA, MeJa, and sugar starvation induced higher levels of the LKR/SDH polypeptide in both mutants, although the relative induction in the abi1-1 and abi2-1 mutants was considerably smaller than that in wild-type plants. This lower induction rate in the abi1-1 and abi2-1 mutants is likely to reflect the higher basic level of LKR/SDH in these mutants than in wild-type plants (Fig. 8A, compare lane c and d with a). Thus, our results suggest that the response of LKR/SDH gene expression to MeJa and sugar starvation operates, at least in part, through an ABI-independent signal transduction cascade. Interestingly, in this experiment, the level of the LKR/SDH polypeptide was stimulated to a higher extent by sugar starvation than by MeJa both in the wild type and in the abi1-1 and abi2-1 mutants. This is in contrast to the experiments presented in Figures 3B and 4 in which the level of the LKR/SDH polypeptide was stimulated roughly to the same extent by MeJa and sugar starvation. Because the experiments in Figures 3B and 4 were conducted on a C24 ecotype, whereas those in Figure 8 were conducted on Landsberg erecta ecotype, it is possible that these variations are ecotype specific.
Regulation of LKR/SDH Gene Expression by the Hexokinase-Dependent Signaling Cascade and by Phosphate Metabolism
Studying mechanisms of sugar-dependent regulation of gene expression are complicated by the fact that sugars act both as nutrients and as signaling molecules (Rolland et al., 2001, 2002). First, we tested whether induction of LKR/SDH gene expression by sugar starvation is mediated by hexokinase. To this end, we analyzed whether 3-O-methyl-Glc (not phosphorylated by hexokinase) or Man (phosphorylated by hexokinase, but not metabolized; also inhibits Glc-6-phoshate and ATP production) has any antagonistic effect on the stimulation of LKR/SDH gene expression by sugar starvation. Whereas Suc and Glc significantly reversed the stimulatory effect of sugar starvation on the level of LKR/SDH (Fig. 9, compare lanes b with a and c), 3-O-methyl-Glc did not reverse this stimulatory effect (Fig. 9, compare lanes b and d). Yet, Man significantly reversed the stimulatory effect of sugar starvation on the production of the LKR/SDH polypeptide in a similar manner to Suc and Glc (Fig. 9, compare lanes b with a, c, and e), implying that the response of LKR/SDH gene expression to sugars and sugar starvation operates via the hexokinase signaling cascade.
Figure 9.
Effects of various metabolized and non-metabolized sugars on the level of the LKR/SDH polypeptide. Nine-day-old seedlings grown in a liquid culture were transferred to new media containing different sugars or no sugar, as indicated on top, for 48 h before harvesting. Top, Proteins were then reacted in western blot with monoclonal anti-LKR antibodies. The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panel. The relative intensities of the LKR/SDH bands, normalized to the control (left lane), are indicated in between the top and bottom panels.
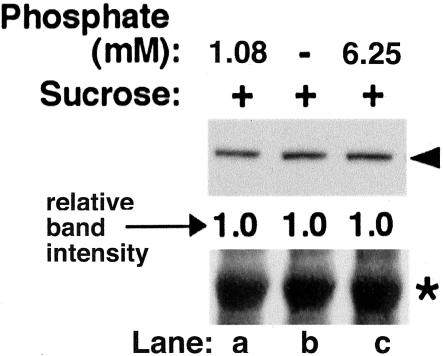
Although hexokinase polypeptides function as signaling molecules per se, their enzymatic reactions also alter the intracellular phosphate pool, a fact that by itself may trigger regulatory responses. The regulation of a number of genes is reciprocally regulated by sugars and phosphate (for example, see Preiss, 1984; Sadka et al., 1994; Zhu-Shimoni and Galili, 1998). We therefore also studied whether the induction of LKR/SDH gene expression by sugar starvation is regulated by phosphate metabolism. To test the effect of phosphate starvation on the production of the LKR/SDH polypeptide, wild-type Arabidopsis seedlings were grown for 9 d in a medium containing 1.08 mm phosphate (control level; see “Materials and Methods”) and then transferred to a new medium lacking phosphate for 72 h. To test the effect of excess phosphate, 11-d-old seedlings were grown in a medium containing control phosphate levels and then transferred to a new medium containing 6.25 mm phosphate for 24 h. As shown in Figure 10, lanes a to c, neither phosphate starvation nor excess phosphate had any effect on the level of the LKR/SDH polypeptide. Together, our results suggest that the response of LKR/SDH gene expression to sugar starvation is regulated by hexokinase as a signaling molecule but not by hexokinase-catalyzed Pi metabolism.
Figure 10.
Effect of phosphate on the LKR/SDH gene expression. Nine-day-old seedlings of wild-type Arabidopsis grown in a liquid culture were transferred to new media as follows: The first group (lane a) was transferred to a medium containing 1.08 mm phosphate (control level) and grown for 72 h before harvesting. The second group (lane b) was transferred to a phosphate-free medium for 72 h before harvesting. The third group (lane c) was transferred to a medium containing 1.08 mm phosphate for 48 h and then transferred to a medium containing 6.25 mm phosphate for additional 24 h before harvesting. Top, Proteins were then reacted in western blot with monoclonal anti-LKR antibodies. The different lanes contained comparable amounts of proteins as depicted by the region of the Rubisco large subunit in the bottom panel. The relative intensities of the LKR/SDH bands, normalized to the control (left lane), are indicated in between the top and bottom panels.
DISCUSSION
The Complex Regulation of LKR/SDH Gene Expression Suggests Multiple Functions
In the present report, we studied several regulatory patterns governing the expression of LKR/SDH gene to gain a deeper insight into the potential functions of Lys catabolism in plants. Using northern-blot as well as western-blot analyses, our study showed that expression of the Arabidopsis LKR/SDH gene is strongly up-regulated, in a concerted manner, by the two hormones ABA and jasmonate, as well by the levels of other cellular metabolites, particularly sugar and nitrogen starvation. Jasmonate and ABA function mainly in flowers (jasmonate) and developing seeds (ABA) as well as in the response of plants to a variety of abiotic and biotic stresses (Finkelstein et al., 2002; Turner et al., 2002). In rapeseed, LKR/SDH was identified as a gene, which is most responsive to osmotic stress (Deleu et al., 1999; Moulin et al., 2000). Thus, the complex regulatory pattern of LKR/SDH gene expression in plants suggests that Lys catabolism participates in multiple metabolic processes in plants.
Our observation that the responses of LKR/SDH gene expression to ABA, MeJa, and sugar starvation occur already at the RNA level suggests that these responses are regulated at the transcriptional level, although additional posttranscriptional and posttranslational control mechanisms may also operate. Such a transcriptional control of AtLKR/SDH gene expression is in agreement with the fact that the promoter upstream the LKR/SDH-coding region contains an number of putative elements that are similar to elements that are responsive to ABA, MeJa, and sugar starvation in other genes (data not shown). This promoter region also contains putative elements of other signals, such as light, drought, and wounding (data not shown), suggesting that the regulation of AtLKR/SDH gene expression is not restricted to the signals studied in the present report.
Association of the Lys Catabolism Pathway with the Regulation of Carbon/Nitrogen Partitioning
Our results showed that expression of the Arabidopsis LKR/SDH gene was stimulated by carbon starvation but repressed by nitrogen starvation. These findings are in agreement of the expected regulatory function of an amino acid catabolic enzyme in carbon/nitrogen partitioning. Under sugar starvation, plant cells generally convert amino acids to sugars to balance the sugar levels, whereas under nitrogen starvation such conversion must be blocked. One of the major catabolic products of the Lys catabolism is acetyl-CoA (Galili et al., 2001), which is readily converted to various sugars (Owen et al., 2002).
The small, but consistent approximately 2-fold increase in the level of LKR/SDH polypeptide upon exposure to Asp and Asn, but not to Glu and Gln, is also consistent with the function of LKR/SDH in the regulation of carbon/nitrogen balance. Glu and Gln are known to stimulate nitrogen assimilation and its incorporation into amino acids in actively growing tissues of light-grown plants, a process that also stimulates the conversion of sugars to amino acids (Stitt, 1999, 2002). By contrast, Asn, which is synthesized from Asp, accumulates in the dark and functions as a nitrogen storage compound when amino acids are catabolized to sugars (Lam et al., 1995).
Relationship of the Lys Catabolism with Stress-Associated Metabolism
The response of LKR/SDH gene expression to ABA and MeJa (Figs. 2 and 3) as well as to biotic and abiotic stresses (Deleu et al., 1999; Moulin et al., 2000) implies that the Lys catabolism pathway participates in metabolic networks that help plants withstand such stresses. A dominant characteristic in the response of plants to stresses, particularly abiotic stresses, is the fast and extensive accumulation of a number of protecting metabolites including Pro, Arg, polyamines, γ-amino butyric acid (GABA), and nitric oxide (Galili et al., 2001). Glu, a dominant product of the Lys catabolism pathway (Fig. 1), serves as a major precursor for the synthesis of these stress-associated metabolites (Galili et al., 2001). The contribution of Lys catabolism to the overall stress-associated metabolism awaits to be assessed.
Functional Role of Lys Catabolism during Flower and Seed Development
ABA and jasmonate are important hormones for seed and flower development, respectively (Finkelstein et al., 2002; Turner et al., 2002). Accordingly, the stimulation of LKR/SDH gene expression by these hormones is consistent with the up-regulation of LKR/SDH mRNA in these organs (Tang et al., 1997). The functional role of Lys catabolism in flower and seed development is not clear. Yet, a potential function of this pathway in developing seeds is to balance Lys levels as well as to participate in metabolite partitioning between amino acids (proteins), carbohydrates, and lipids.
Mechanisms and Signal Transduction Cascades That Are Responsible for Regulating LKR/SDH Gene Expression by ABA, Jasmonate, and Sugar Starvation
Hormonal and metabolic regulation of gene expression in plants may operate either independently or in a concerted, interactive manner (Finkelstein et al., 2002; Rolland et al., 2002). Our present results imply that ABA, MeJa, and sugar starvation operate in the regulation of LKR/SDH gene expression through distinct signal transduction cascades. Moreover, analysis of the Arabidopsis aba2-1 mutant shows that the function of jasmonate and sugar starvation is not dependent on ABA. The enhanced level of the LKR/SDH polypeptide in the abi1-1 and abi2-1 mutants relative to wild-type plants implies that the signal transduction cascade that is negatively regulated by the ABI1-1 and ABI2-1 protein phosphatases (Finkelstein et al., 2002) participates in the regulation of LKR/SDH gene expression. This signal transduction cascade is likely to regulate the response of LKR/SDH gene expression to ABA. Notwithstanding, these mutants showed comparable induction of LKR/SDH gene expression by MeJa and sugar starvation with that in wild-type plants, suggesting that MeJa and sugar starvation operate via other signal transduction cascades. This conclusion is in agreement with previous genetic and molecular studies, showing that both ABA and sugars can operate via common or different signal transduction cascades, whereas ABA and jasmonate operate via different cascades (Finkelstein et al., 2002; Turner et al., 2002).
Our results also show that the induction of LKR/SDH gene expression by sugar starvation is reversed by Man in a similar manner to Suc, Glc, and Fru, but not reversed by 3-O-methyl-Glc or by phosphate (Figs. 4, 9, and 10). This observation implies that the induction of LKR/SDH gene expression by sugar starvation and the antagonistic effects of Suc, Glc and Fru on this induction is regulated by hexokinase as a signaling molecule, and not as an enzyme(s) of phosphate metabolism (for review, see Rolland et al., 2002). A similar hexokinase-dependent regulatory effect of sugars on photosynthetic gene expression was previously reported (see Rolland et al., 2002, and refs. therein). Thus, our results also suggest a coordinated regulation of Lys catabolism and photosynthesis by sugars via the same machinery. When cellular sugar levels are high, there is no need for enhanced photosynthesis nor for catabolism of amino acids to sugars and, hence, sugars repress the expression of both photosynthetic and amino acid catabolism genes via the hexokinase signaling cascade. Under sugar starvation, the hexokinase-signaling cascade triggers, in a concerted manner, enhanced expression of both photosynthetic and amino acid catabolism genes to synthesize new sugars.
MATERIALS AND METHODS
Materials
ABA, MeJa, 3-O-methyl-Glc, Man, MES buffer, phenylmethylsulfonyl fluoride, the protease inhibitor cocktail for plant cell and tissue extracts, and the TRI reagent were purchased from Sigma-Aldrich (Rehovot, Israel). Gamborg B5 and Murashige and Skoog media were purchased from Dushefa (Haarlem The Netherlands). Rediprime II (random prime labeling system) and Rapid-Hyb buffer for probe hybridization were obtained from Amersham Biosciences (Buckinghamshire, UK).
Plant Material and Growth Conditions
Arabidopsis ecotype C-24 was used in most experiments. The Arabidopsis ecotypes Columbia and Landsberg erecta were used as wild-type controls for the ABA-deficient mutant aba2-1 (CS156) and the ABA-insensitive mutants abi1-1 (CS22) and abi2-1 (CS23), kindly obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Arabidopsis seeds were germinated in a growth chamber with a 16-h-light/8-h-dark cycle in flasks containing liquid Gamborg B5 medium. This medium is composed of B5 salts, 100 mg L–1 myo-inisitol, 1 mg L–1 nicotinic acid, 10 mg L–1 thiamine-HCl, and 1 mg L–1 pyridoxine-HCl and supplemented with 20 g L–1 Suc and 20 mm MES, pH 6. Nine days after culturing, seedlings were transferred to new media in which various hormonal and metabolic treatments were performed for different time periods, as described in “Results” and in the figure legends. For nitrogen or phosphate starvation treatments, ammonium sulfate and potassium nitrate or sodium dihydrogen orthophosphate were omitted. After the treatments, seedlings were harvested, immediately frozen in liquid nitrogen, and stored at –70°C.
The Arabidopsis ABA mutants and their control wild-type seeds were germinated in flasks containing modified liquid Murashige and Skoog medium. This medium contained one-half Murashige and Skoog salts, 100 mg L–1 myo-inisitol, 1 mg L–1 thiamine-HCl, 0.5 mg L–1 nicotinic acid, and 0.5 mg L–1 pyridoxine-HCl and was supplemented with 1% (w/v) Suc and 2.5 mm MES, pH 6, and subjected to hormonal and metabolic treatments as described above.
Stock solutions of ABA and MeJa were prepared in ethanol and added to the media aseptically at the desired concentrations. Equal volumes of ethanol were added to the control flasks of related treatments.
Protein Extraction and Western-Blot Analysis
Plants were homogenized in 25 mm potassium phosphate buffer, pH 7.5, containing 1 mm EDTA, 1 mm dithiothreitol, 5 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail for plant cell and tissue extracts diluted 100 times, at 4°C. The homogenate was centrifuged at 14,000g for 15 min, and the supernatant was collected. Protein levels were determined by the Bradford method (Bradford, 1976) and samples containing approximately 30 μg of protein were fractionated on 10% (w/v) polyacrylamide-SDS gels (Laemmli, 1970) and transferred to a polyvinylidene difluoride membrane (Pall Gelman, Ann Arbor, MI) using a Protein Trans-Blot apparatus (Bio-Rad Laboratories, Hercules, CA). The membranes were completely dried, stained with Coomassie Blue R-250, and then reacted with monoclonal anti-Arabidopsis LKR antibodies as previously described (Zhu et al., 2001), using a chemiluminescence immunodetection kit (Pierce, Rockford, IL). The relative intensities of the LKR/SDH bands were measured by a fluor-S multiImager (Bio-Rad Laboratories).
RNA Extraction and Northern-Blot Analysis
Tissue samples were freshly collected and frozen immediately in liquid nitrogen. Total RNA was obtained as indicated by the manufacturer using TRI reagent. Approximately 20 μg of total RNA was loaded on 2% (w/v) agarose gel (in 100 mL: 20 mm MOPS, 5 mm sodium acetate, 1 mm EDTA, and 10 mL of 37% [12.3 m] formaldehyde). For probe, LKR region was amplified by PCR (oligonucleotide primers 5′-ATGATTCAAATGGCCATGAGG-3′ and 5′-TACTCGAGTCATTCTGCCTTCTCCATCAG-3′). Probe labeling generated by a commercial kit Rediprime II according to the manufacturer's instructions. After prehybridization (30 min) and hybridization (2 h) at 65°C in Rapid-Hyb buffer, blots were washed (once in 2× SSC, 0.1% [w/v] SDS and twice in 0.2× SSC, 0.1% [w/v] SDS at 65°C) as indicated by the manufacturer and exposed to x-ray film (Eastman Kodak, Rochester, NY).
Acknowledgments
We thank Xiaohong Zhu and Guiliang Tang for the monoclonal anti-LKR antibodies and Yigal Avivi for scientific editing of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026294.
This work was supported by the FrameWork Program of the Commission of The European Communities, and by The Israel Academy of Sciences and Humanities, National Council for Research and Development. G.G. is an incumbent of the Bronfman Chair in Plant Sciences.
References
- Arruda P, Kemper EL, Papes F, Leite A (2000) Regulation of lysine catabolism in higher plants. Trends Plant Sci 5: 324–330 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Deleu C, Coustaut M, Niogert M-F, Larher F (1999) Three new osmotic stress-regulated cDNAs identified by differential display polymerase chain reaction in rapeseed leaf discs. Plant Cell Environ 22: 979–988 [Google Scholar]
- Falco SC, Guida T, Locke M, Mauvais J, Sandres C, Ward RT, Webber P (1995) Transgenic canola and soybean seeds with increased lysine. Bio/Technology 13: 577–582 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G, Tang G, Zhu X, Gakiere B (2001) Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr Opin Plant Biol 4: 261–266 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Negrutiu I, Dirks R, Cammaerts D (1987) Selection programs for isolation and analysis of mutants in plant cell cultures. In CE Green, DA Somers, WP Hackett, DD Biesboer, eds, Plant Biology, Vol 3. Alan R. Liss, New York, pp 243–264 [Google Scholar]
- Karchi H, Miron D, Ben-Yaacov S, Galili G (1995) The lysine-dependent stimulation of lysine catabolism in tobacco seeds requires calcium and protein phosphorylation. Plant Cell 7: 1963–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchi H, Shaul O, Galili G (1994) Lysine synthesis and catabolism are coordinately regulated during tobacco seed development. Proc Natl Acad Sci USA 91: 2577–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 337–383 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lam H-M, Coschigano K, Schultz C, Melo-Oliveira R, Tjagen G, Oliveira I, Ngai N, Hsieh M-H, Coruzzi GM (1995) Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell 7: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron D, Ben-Yaacov S, Karchi H, Galili G (1997) In vitro dephosphorylation inhibits the activity of soybean lysine-ketoglutarate reductase in a lysine-regulated manner. Plant J 12: 1453–1458 [Google Scholar]
- Moulin M, Deleu C, Larher F (2000) l-Lysine catabolism is osmo-regulated at the level of lysine-ketoglutarate reductase and saccharopine dehydrogenase in rapeseed leaf discs. Plant Physiol Biochem 38: 577–585 [DOI] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277: 30409–30412 [DOI] [PubMed] [Google Scholar]
- Preiss J (1984) Starch, sucrose biosynthesis and partition of carbon in plants are regulated by othophosphate and triose-phosphates. Trends Biochem Sci 9: 24–27 [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14: S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein JM (2001) Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci 26: 310–317 [DOI] [PubMed] [Google Scholar]
- Sadka A, DeWald DB, May GD, Park WD, Mullet JE (1994) Phosphate modulates transcription of soybean VspB and other sugar-inducible genes. Plant Cell 6: 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M (1999) Nitrate and the regulation of primary metabolism, allocation and growth. Curr Opin Plant Sci 2: 178–186 [DOI] [PubMed] [Google Scholar]
- Stitt M, Muller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible WR, Krapp A (2002) Steps towards an integrated view of nitrogen metabolism. J Exp Bot 53: 959–970 [DOI] [PubMed] [Google Scholar]
- Tang G, Miron D, Zhu-Shimoni JX, Galili G (1997) Regulation of lysine catabolism through lysine-ketoglutarate reductase and saccharopine dehydrogenase in Arabidopsis. Plant Cell 9: 1305–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Tang G, Granier F, Bouchez D, Galili G (2001) A T-DNA insertion knockout of the bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase gene elevates lysine levels in Arabidopsis seeds. Plant Physiol 126: 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Shimoni XJ, Galili G (1998) Expression of an Arabidopsis aspartate kinase/homoserine dehydrogenase gene is metabolically regulated by photosynthesis-related signals, but not by nitrogenous compounds. Plant Physiol 116: 1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]