Summary
This is a review of recently published papers showing that bone-conducted vibration of the head causes linear acceleration stimulation of both inner ears and this linear acceleration is an effective way of selectively activating otolithic afferent neurons. This simple stimulus is used in a new test to evaluate clinically the function of the otoliths of the human inner ear. Single neuron studies in animals have shown that semicircular canal neurons are rarely activated by levels of bone-conducted vibration at 500 Hz which generate vigorous firing in otolithic irregular neurons and which result in a variety of vestibulo-spinal and vestibulo-ocular responses, and the latter is the focus of this review. In humans, 500 Hz bone-conducted vibration, delivered at the midline of the forehead, at the hairline (Fz), causes simultaneous and approximately equal amplitude linear acceleration stimulation at both mastoids and results in ocular-evoked myogenic potentials (oVEMPs) beneath both eyes. The first component of this myogenic potential, at a latency to peak of about 10 ms is a negative potential and is called n10 and, in healthy subjects, is equal in amplitude beneath both eyes, but after unilateral vestibular loss, the n10 potential beneath the eye opposite to the lesioned ear is greatly reduced or totally absent. n10 is a myogenic potential due to a crossed otolith-ocular pathway. In patients with total unilateral superior vestibular neuritis, in whom saccular function is largely intact (as shown by the presence of cervical vestibular evoked myogenic potentials (cVEMPs), but utricular function is probably compromised, there is a reduced n10 response beneath the contralesional eye, strongly indicating that n10 is due to utricular otolithic function.
Keywords: Vestibular disorder, Otolith, Vestibular-evoked myogenic potentials, Ocular vestibular-evoked myogenic potentials, Bone conduction
Riassunto
La vibrazione portata al cranio (bone-conducted vibration) determina una stimolazione pari ad una accelerazione lineare di entrambi gli orecchi interni. Queste accelerazioni lineari sono in realtà un vero e proprio modo per attivare selettivamente i neuroni provenienti dalle macule utricolo sacculari. Lo studio di singoli neuroni nell’animale ha dimostrato che i neuroni dei canali semicircolari sono raramente attivati dai livelli di vibrazione che invece generano una vigorosa scarica nervosa da parte dei neuroni otolitici di tipo irregolare. In tal modo, l’attivazione otolitica indotta dalla vibrazione ossea risulta in una varietà di risposte di tipo vestibolo-spinale e vestibolo-oculare e proprio queste ultime, le vestibolo oculari, sono l’argomento di questa review. La vibrazione portata al cranio portata alla posizione della testa che coincide con la linea mediana in corrispondenza dell’inserzione-attaccatura dei capelli (Fz) causa un’accelerazione lineare simultanea ed approssimativamente uguale in ampiezza in corrispondenza di entrambe le mastoidi e risulta in potenziali evocati miogenici (oVEMPs) registrati sotto gli occhi nei soggetti sani. La prima componente di questo potenziale (n10) è uguale in ampiezza se registrata sotto gli occhi, ma dopo la perdita della funzione vestibolare l’onda potenziale n10 registrata sotto l’occhio opposto all’orecchio sede di lesione è grandemente e fortemente ridotta o del tutto assente. Questo risultato è dovuto all’esistenza di una via crociata otolito-oculare. Nei pazienti con esiti di nevrite del nervo vestibolare superiore nei quali la funzione sacculare è intatta, ma la funzione utricolare è compromessa, c’è una riduzione in ampiezza dell’onda n10 registrata sotto l’occhio opposto al lato leso, ciò sta fortemente a significare che l’onda potenziale n10 è dovuta alla funzione otolitica della macula utricolare.
Introduction
To identify the cause of patients’ reports of dizziness, it is necessary to have information concerning the function of vestibular end-organs of both ears. While semicircular canal function can be tested with calorics or the head impulse test 1, safe simple tests of otolith function are not common. Recently, two important tests of otolith function have been reported – firstly, the cervical vestibular evoked myogenic potential (cVEMP) from contracted sternocleidomastoid (SCM) muscles to an air-conducted sound (ACS) 2 or bone-conducted vibration (BCV) 3 and, secondly, the ocular vestibular evoked myogenic potential (oVEMP) to BCV 4–11. This review focuses on the new oVEMP test of otolithic function 6–9.
BCV to the midline of the forehead at the hairline (a location called Fz) causes small short-latency negative ocular myogenic potentials from surface electrodes beneath the eyes. The first of these, n10, is enhanced when the subject looks up 4 6 7. In healthy subjects, n10 is almost equal beneath both eyes for Fz BCV stimulation 6 7 (Fig. 1), however if one labyrinth is damaged or diseased, the BCV Fz stimulus causes unequal n10 responses 8 (Fig. 1). The asymmetry of n10 is thus an indicator of otolithic and, indeed, as shown below, utricular function. The n10 is a crossed vestibulo-ocular response: for Fz BCV, it is absent beneath the eye opposite to the affected ear, whereas it is of normal amplitude beneath the eye on the side of the affected ear 6 8.
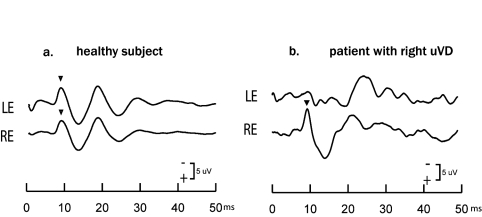
Fig. 1.
Examples of average oVEMPs to Fz BCV stimulation in a healthy subject and a patient with known unilateral vestibular deafferentation (uVL) after vestibular schwannoma surgery. The n10 responses (arrowheads) are the early negative components of the entire ocular vestibular evoked myogenic potential and are approximately equal beneath both eyes in the healthy subject, but in the uVL patient the n10 beneath the contralesional (left) eye is very small or absent, whereas the n10 beneath the ipsilesional eye is of normal amplitude. This asymmetry in n10 simultaneous linear acceleration stimulation of both mastoids is the indicator of otolithic, and, indeed, utricular function.
Measures with linear accelerometers on the mastoids show that 500 Hz bone-conducted vibration to Fz causes equal linear accelerations of both mastoids 7. Recordings from vestibular afferent neurons, in guinea pigs, show that one class of otolith neurons – otolith irregular neurons – are sensitively and selectively activated by such linear accelerations 12. Semicircular canal neurons do not respond to such linear acceleration stimuli 12 at low intensities. This selective otolith activation will result in otolith-ocular and otolith-spinal responses, as Suzuki et al. have shown in cats 13. One otolith-ocular response to selective unilateral utricular stimulation is activation of contralateral inferior oblique (IO) and inferior rectus (IR) muscles. Looking up brings the IO and IR close to the skin surface, so that appropriately placed surface electrodes on the skin surface beneath the eyes in humans record the myogenic activity in these muscles. A simplified schematic illustration of possible neural pathways responsible for the activation of IO and IR, by otolithic stimulation, and thus for the n10 component of the oVEMP is shown in Figure 2, together with examples of oVEMPs and cVEMPs in a normal subject and in an uVD patient.
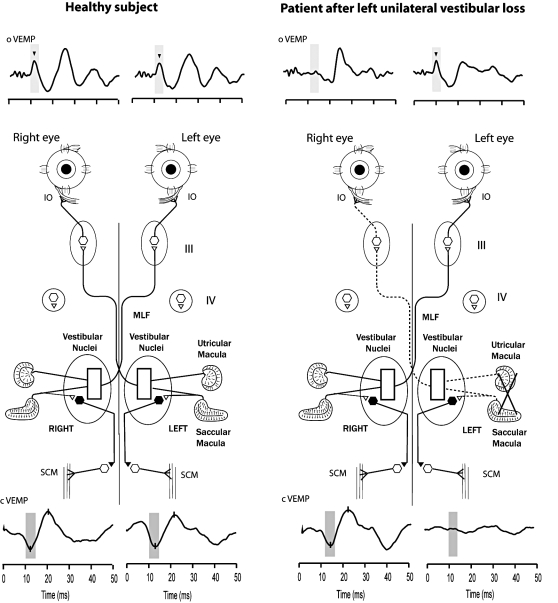
Fig. 2.
A simplified schematic version of some of the known neural vestibulo-ocular projections responsible for the asymmetric oVEMP response to Fz BCV after unilateral vestibular loss. It is based on known anatomical projections and physiological results from Suzuki et al. 13 and Uchino et al. 19 that high frequency electrical stimulation of the utricular nerve results in activation of the contralateral inferior oblique muscle, and the ipsilateral superior oblique muscle via some of the pathways shown schematically here. Afferents from the saccular and utricular macula project to the vestibular nuclei, but the exact termination of these afferents is not yet known so this figure represents the present uncertainty about the exact neural connections of these afferents within the vestibular nuclei 19 as an open box. The otolithic projections to other eye muscles are not shown. Fz stimulation is indicated schematically. The afferents from the saccular macula course predominantly in the inferior vestibular nerve, and synapse on inhibitory neurons in the vestibular nucleus (black hexagon), which, in turn, project to spinal motoneurons controlling the sternocleidomastoid muscle (SCM). So the cVEMP indicates predominantly sacculo-collic function.
In recent studies, the n10 of the oVEMP to Fz BCV stimulation has been recorded in a large number of unselected healthy subjects 7 8. The amplitude of n10 varies considerably between subjects but in the same subject it is repeatable from day to day. To examine the within-subject repeatability, oVEMPs, in response to Fz BCV stimulation, were recorded on two occasions in a number of subjects 7 8. Despite considerable differences between subjects, the n10 responses are repeatable: in both the first and the second recording sessions, the n10 responses were similar in shape and there were no major differences in the amplitudes and latencies of the n10s from one occasion to the next, even allowing for the different electrode placements, different stimulator placements and variation in eye muscle tension between tests. The absolute size of n10 is not of great diagnostic value since some patients have very large n10s and some have very small n10s, probably because of differences in skull characteristics, however, the relative size of n10 beneath the two eyes, in response to this Fz BCV stimulus causing simultaneous bilateral otolith stimulation, is of diagnostic value 6 7. From the amplitudes of the n10 potentials to Fz BCV (where the amplitude of n10 is measured as baseline to peak) an asymmetry ratio (AR) is calculated using a version of the standard Jongkees formula for asymmetry calculations in vestibular testing:
Asymmetry Ratio (AR) = ((larger n10 - smaller n10) / (larger n10 + smaller n10 )) x 100
The average AR for healthy subjects is approximately 11.73% ± 8.26 (n = 67) 7 and none of the 67 asymptomatic healthy subjects tested showed ARs > 40%. On the other hand, all patients with known unilateral vestibular loss (uVL), due to schwannoma surgery, show asymmetric n10s and all had ARs > 40% 6 8. In these patients, the average AR is 75.03% ± 16.32 (n = 11), with the n10 beneath the eye, on the healthy side (opposite the affected ear), being absent or greatly reduced (Fig. 1).
It has been demonstrated that n10 to Fz BCV is a vestibular response since n10 is absent bilaterally in patients with bilaterally absent vestibular function following systemic gentamicin treatment 7. This result also shows that n10 is not due to a blink or facial nerve or auditory activation since these patients with bilateral vestibular loss had normal blinks, normal auditory function and normal facial nerve function, but no n10. The n10 is further demonstrated not to be auditory since it was present in other patients who had lost auditory function but still have vestibular function 4 6. Conversely, patients without hearing, but with residual vestibular function, show n10s 4 6.
n10 is a negative myogenic potential and as such is an excitatory myogenic potential 14. This distinguishes n10 from the cervical vestibular-evoked myogenic potential where the p13 potential recorded from the tensed SCM, in response to air-conducted sounds, is a positive inhibitory potential the magnitude of which depends on SCM tension (Fig. 2). Since it is inhibitory, the p13 of the cVEMP requires normalization to correct for muscle tension, whereas the n10 of the oVEMP, being excitatory, does not require normalization.
The n10 probably indicates mainly utricular function since it is small or absent in patients with total superior vestibular neuritis. The afferents from each vestibular sensory region and how they project within the branches of the vestibular nerve 17 are schematically outlined in Figure 3. All afferents from the utricular macula cross in the superior vestibular nerve, whereas most afferents from the saccular macula cross in the inferior vestibular nerve. Some patients have vestibular neuritis which affects both the superior and the inferior branch 15 16. In patients with superior vestibular neuritis, the inferior vestibular nerve is still functional, as shown by preserved cVEMPs to ipsilateral air-conducted clicks. If n10 to Fz BCV were due to saccular activation, we reasoned that it should be present, as are cVEMPs, in such patients. On the other hand, if n10 to Fz BCV is due to utricular function, then superior vestibular neuritis should abolish n10 and there should be a marked asymmetry in n10. The data (Fig. 4) clearly show that the latter is correct: superior vestibular neuritis abolishes or greatly reduces n10 and results in asymmetry ratios which are indistinguishable from the asymmetry ratios of uVLs 9. That result strongly implies that n10 of the oVEMP to Fz BCV is due to utricular activation. It is worthwhile stressing that in these patients the saccular macula was functioning normally, as shown by normal cVEMPs to air-conducted sound; in contrast the n10 of the oVEMP was absent or greatly reduced.
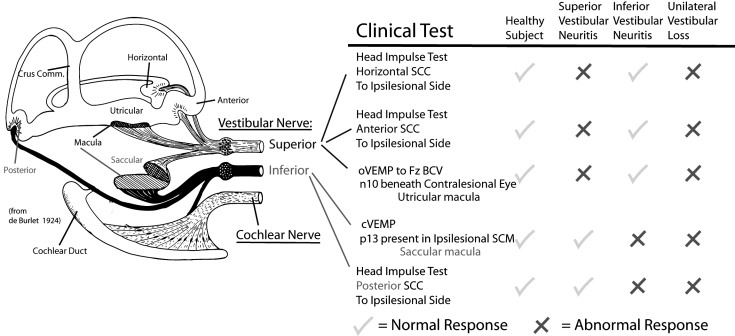
Fig. 3.
The neural innervation of the vestibular sense organs of the labyrinth (after de Burlet) and clinical tests which evaluate the functional status of the various vestibular sensory regions. The columns labelled Response identify the responses associated with clinical tests of each sense organ.
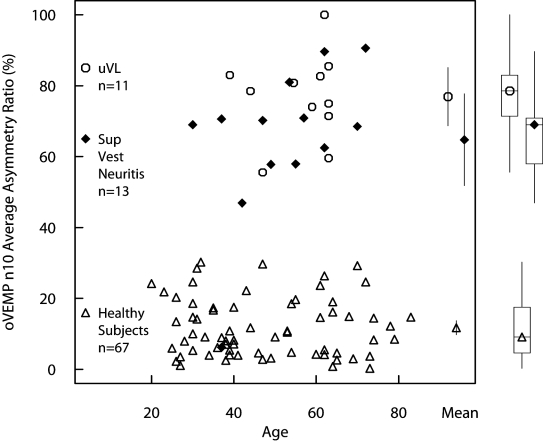
Fig. 4.
The asymmetry ratio of all 13 patients with superior vestibular neuritis (filled diamonds) plotted as a function of age. Also on this graph are the data points from earlier published data from our laboratories 4-6 showing the asymmetry ratios of 67 healthy subjects (open triangles) and 11 patients with complete unilateral vestibular loss (open circles). The mean values and two-tailed 95% confidence intervals for the mean are shown within the square while the boxplots for the medians, quartiles and ranges are shown outside the square. All patients showed an asymmetry ratio greater than any of the normal subjects tested, and the similarity of the AR results for SVN and uVL patients is clear.
Method of stimulation and recording oVEMPs
In the oVEMP studies on normal subjects and patients reviewed here, the subject or patient was lying supine on a bed with their head supported by a pillow but positioned so that the head was horizontal or pitched slightly nose down, with the chin close to the chest. The skin below the eyes was very carefully cleaned with alcohol wipes (with the patient’s eyes closed), and surface electromiography (EMG) electrodes (Cleartrace disk electrocardiography – ECG electrodes (1700-030), Conmed Corp, New York, NY, USA) were applied to record the surface potentials from beneath both eyes (Fig. 5). The self-adhesive pads around each electrode were cut to allow close electrode placement, taking care that no electrical bridge was formed between the conductive gel of the two closely juxtaposed electrodes beneath the eyes. To record the myogenic potentials, the active (+) recording electrodes were located on the infra-orbital ridge 1 cm below the lower eyelid, and the reference (-) electrodes were placed about 2 cm below the first electrodes, as shown. The electrodes were aligned with the centre of the pupil as the subject looked up at a distant target exactly in the midline (i.e., the eye position in the orbit was elevated). The ground electrode was on the chin or sternum.
Fig. 5.
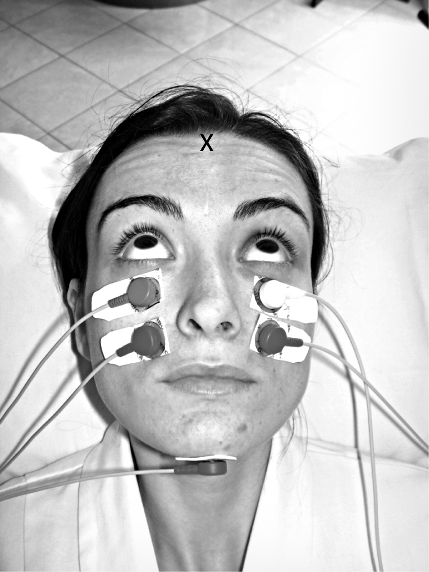
The electrode configuration for optimum recording of oVEMPs. N.B. the subject is looking upwards in the median plane. The point marked X indicates the location of Fz.
The EMG signals were amplified by two independent differential amplifiers (filtre cut-offs: 20 Hz to 500 Hz), and the unrectified signals were averaged (n = 50 presentations) and simultaneously acquired from the two eyes using a Medelec Sapphire II Averager (Oxford Instruments, Surrey, UK) or an AMPLAID MK12 averager (Amplifon, Milan, Italy) with a sampling rate 20 kHz. It is important to emphasize that n10 is a very small potential, only 8-10 μV (or less) and the peak is about 10 ms from onset of the stimulus, therefore, care is needed in order to minimize electrical noise and interference, stimulus artifact. The electrical convention adopted was that a negative potential, at the active electrode (the electrode closest to the eye) caused an upward (negative) trace deflection, so that, as the subject looked up towards the top of their head, the traces deflected upward. The electrode impedance was maintained below 5 kΩ in all trials. To optimize oVEMPs, particular care was taken to make sure that the subject’s jaw muscles were relaxed and, of crucial importance, that during the Fz BCV stimulation the person was looking straight up (towards the top of their head) at approximately 25 degrees above their visual straight ahead, and they were required to maintain fixation on a small dot approximately 60-70 cm from their eyes (on the wall behind and above the patients head) during stimulation. The elevated position of the eye, in the orbit, is crucial since the size of n10 decreases substantially as gaze is lowered 3 4 and n10 is almost undetectable when the gaze is straight ahead. The elevated fixation point maintains the eye position elevated in the orbit during the brief test time (20 sec), but it is important that the fixation be aligned with the midline since eccentric fixation alone can produce asymmetric n10 potentials in the two eyes. For most subjects, the n10, with gaze straight ahead, was only just detectable above the noise level, but by changing gaze position to looking up, a clear n10 response was detected.
Before each test, the electrodes, amplifiers and averaging system were checked by requesting the subject to make vertical saccades through ± 5° directly above and below the vertically located fixation point (i.e., to points about 30° and 20° above the subject’s visual straight-ahead). The examiner ensured that these saccades produced symmetrical potentials (steps) for both eyes of about 50 μV before proceeding. Care was also taken to ensure that the polarities were correct so that an upward eye movement produced an upward (negative) voltage and that the signals during these saccades were close to being equal amplitude for both eyes and showed a good signal to noise ratio. If these conditions were not met, the electrodes were removed, the skin re-cleaned and new electrodes introduced. Checking the systems was a mandatory criterion before delivering the stimuli since it shows that:
The electrodes are correctly placed and that the electrodes, the leads and the amplifiers are working correctly and also the polarities are correct.
The amplitude of the potentials produced by the saccades, in the two eyes, is approximate, therefore, any asymmetry on averaging the EMG responses is not due to asymmetrical electrode placement or electronic problem but indicate asymmetrical vestibulo-ocular function. The clinical indicator is the asymmetry ratio (AR) of the n10 to Fz BCV stimulation which, our stimulus measures show, stimulates both ears equally: in patients in whom only one ear is affected, the n10 beneath the eye opposite the affected ear shows a smaller potential to Fz BCV stimulation.
In some healthy subjects and patients, the absolute size of the n10 potential may be very small – for example, in patients with very large heads, and/or very thick lower eyelids. In such circumstances, the surface electrodes are unable to adequately record the myogenic potential from the underlying eye muscles even when the patient is looking up. In these cases, it is unwise to overestimate n10 asymmetry because with very small n10 potentials, measurement error may suggest marked asymmetry. In such patients, a 250 Hz stimulus, rather than 500 Hz, may provide a clearer n10 and averaging more stimulus presentations may be an advantage.
Bone conducted vibration was delivered using a hand-held, Bruel and Kjaer (Naerum, Denmark), Mini-shaker 4810, fitted with a short bolt (2 cm long, M5) terminated in a bakelite cap 1.5 cm in diameter. The flat end of this cap was the contact point for the stimulator on the subject’s forehead at Fz. Excellent electrical isolation between the 4810 and the subject is mandatory in order to avoid artifacts from the Mini-shaker contaminating the recordings of the small myogenic potentials, and the use of a bakelite cap, cemented on the head of the M5 bolt in the 4810, ensures that electrical isolation. The 4810 was driven by computer generated signals, usually, consisting of 50 repetitions of a 500 Hz tone burst lasting a total of 7 ms (including a 1 ms rise and 1 ms fall with a zero crossing start) and 5 ms duration or a square wave of 1 ms. Most of the testing time is devoted to applying electrodes and, since the data acquisition epoch is so short, it is wise to repeat each test twice – at 3 stimuli per second, the data is acquired within 20 seconds. However, we found that a rate of 3/s is comfortable for the patient and the stimuli are spaced well apart, therefore, with minimal carry-over from one stimulus to another. If patients have difficulty maintaining the elevated fixation for the brief 20-second stimulation time, a faster repetition rate can be used – we found that even a rate of 21 per second has no substantial effect on n10 characteristics.
The 4810 Mini-shaker weights approximately 1 kg and the weight of the shaker was used to standardize the force used in all subjects. The Mini-shaker was hand-held but the task of the operator is to hold the Mini-shaker so that the bakelite cap lightly touches the skin of the forehead at Fz and to maintain the Mini-shaker close to vertical but not to use force to push the bakelite cap against Fz. Fz is the junction of the midline at the hairline. The exact location of Fz is not critical since small position changes (± 1 cm) have little effect on the symmetry of n10. The actual force delivered to Fz is of the order of 24 N (calibrated by a Bruel and Kjaer artificial mastoid (4930, Bruel and Kjaer, Naerum, Denmark)), resulting in accelerations, at the mastoids, of approximately 0.2-0.4 g.
If a square wave is used as the command voltage, the polarity of the stimulus is important – reversing the polarity causes a long delay before the stimulus is actually delivered to the head as the armature in the Mini-shaker takes time to withdraw and then direct back to the head. The Mini-shaker is not like a headphone where the inertial delay in the movement of the diaphragm is very slight and reversing polarity does not cause a long mechanical delay. With a Mini-shaker, a simple polarity reversal may cause an apparent difference in latency of n10 of up to 3 ms but that is merely a mechanical artefact due to the inertia of the armature.
The location of the BCV stimulus at Fz is important as it guarantees almost equal and simultaneous stimulation of both labyrinths. Other devices can generate effective BCV e.g. gentle taps at Fz with a tendon hammer (which can trigger the averager) are also a very effective way of generating n10, at a fraction of the cost. However, a weak bone conduction stimulator, such as a standard clinical bone oscillator (e.g. a Radioear B-71 (Radioear Corp. New Eagle, PA, USA) at Fz is ineffective since the magnitude of the linear acceleration, generated at the mastoids by such a weak stimulator, is so small that, in most subjects, it is incapable of activating otolith afferents 6 7. Attempting to give equal stimulation to both ears, by a standard clinical bone oscillator (such as a B-71) placed on each mastoid successively, is not clinically practical since small changes in B-71 location, direction or force, on the mastoid, cause substantial changes in the applied linear accelerations and, therefore, change the measured n10 response and thus resulting in an artificial AR, simply due to unequal linear acceleration stimuli.
The very fast n10 responses reported here are a different category of responses from the slowly developing nystagmus induced by vibration applied to the mastoid or neck. Long duration of 100 Hz vibration around the mastoid process has been found to evoke predominantly horizontal nystagmus beating on the opposite side with respect to the lesion in subjects with unilateral vestibular hypofunction (e.g. Dumas, et al. 18). It is now generally accepted that horizontal nystagmus reflects asymmetrical vestibular activation, although still remain to be elucidated how exactly this occurs and which receptor structure(s) are responsible. Albeit, these effects are slow, long-latency phenomena which take many hundreds of milliseconds or even seconds to develop, whereas the n10 is a very fast response to the very onset of the 500 Hz vibration stimulus and occurs at a latency of about 10 ms.
Conclusions
It is worthwhile emphasizing that bone conducted vibration at Fz is a modest stimulus which is not painful and which is present for only a very brief time and requires little effort on the part of the patient who is lying supine – they just maintain upward fixation for 20 seconds (or less if a higher repetition rate is used). The procedure is quite acceptable even to senior patients.
The asymmetry of n10 in response to BCV at Fz is a new way of assessing otolithic (utricular) function but care is needed in interpretation. Just as the reduction, or absence, of a cVEMP response to ACS does not necessarily indicate saccular dysfunction, so the reduction, or the absence, of a contralateral n10 response to BCV, at Fz, does not necessarily indicate utricular dysfunction. For example, asymmetry of n10 may occur for many different reasons:
unilateral utricular loss, or superior vestibular nerve loss, or total unilateral vestibular nerve loss. The utricular loss may be caused by hair-cell receptor malfunction due to disease or damage;
asymmetric eye muscle function – e.g. differential functional status of the two inferior oblique muscles;
differential effects of (e.g,) multiple sclerosis on transmission pathways;
visual fixation may not be in the midline;
the electrodes may be inappropriately placed;
the gain of the amplifiers for each eye may not be identical.
The diagnosis of utricular dysfunction from asymmetric n10 responses to Fz BCV must take all these considerations into account.
cVEMPs and oVEMPs give complementary information concerning peripheral vestibular function as otolithic receptors and afferents in the superior division of the vestibular nerve are responsible for the generation of the n10 component of the oVEMP response to Fz BCV, whereas the receptors and afferents in the inferior vestibular nerve are responsible for the ipsilateral cVEMP (Fig. 2). cVEMPs to ACS test primarily saccular and inferior vestibular nerve function whereas oVEMPs to BCV at Fz test primarily utricular and superior vestibular nerve function. However, these two indicators, combined with other standard tests of peripheral vestibular function such as the head impulse test 3, allow clinical testing of all of the vestibular end-organs in each inner ear (Fig. 3).
Acknowledgments
Authors are grateful for the support of NH & MRC of Australia and the Garnett Passe and Rodney Williams Memorial Foundation. We thank Anna Rita Tedesco for critically reading this manuscript.
Abbreviations
- AR:
asymmetry ratio for the relative size of the n10 of the oVEMPs for the two eyes
- ACS:
air-conducted sound
- BCV:
bone-conducted vibration
- EMG:
electromyogram
- Fz:
the location on the forehead in the midline at the hairline
- Fz BCV:
bone-conducted vibration delivered to Fz
- B-71:
the standard clinical bone conduction oscillator (Radioear B71)
- 4810:
the Bruel and Kjaer Mini-shaker
- CVEMPs:
cervical vestibular-evoked myogenic potentials
- oVEMPs:
ocular vestibular-evoked myogenic potentials
- n10:
the initial negative potential in the oVEMP response at latency of around 10 ms
- SCM:
Sternocleidomastoid muscle
- TH:
tendon hammer (or reflex hammer)
- uVL:
unilateral vestibular loss
References
- 1.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 1988;45:737-9. [DOI] [PubMed] [Google Scholar]
- 2.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 1994;57:190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology 1995;45:1927-9. [DOI] [PubMed] [Google Scholar]
- 4.Rosengren SM, Todd NPM, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 2005;116:1938-48. [DOI] [PubMed] [Google Scholar]
- 5.Todd NPM, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol 2007;118:381-90. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colebatch JG, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology 2007;68:1227-9. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, MacDougall HG, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 2008;119:2135-47. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, MacDougall HG, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at Fz. A new indicator of unilateral otolithic loss. Audiol Neurotol 2008;13:396-404. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki S, Chihara Y, Smulders Y, Burgess AM, Halmagyi GM, Curthoys IS, et al. The role of the utricular macula and the superior vestibular nerve in the generation of ocular vestibular-evoked myogenic potentials to bone-conducted vibration at Fz. Clin Neurophysiol 2009;120:588-93. [DOI] [PubMed] [Google Scholar]
- 10.Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG. Vestibular activation by bone-conducted sound. J Neurol Neurosurg Psychiatry 2003;74:771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welgampola MS. Evoked potential testing in neuro-otology. Curr Opin Neurol 2008;21:29-35. [DOI] [PubMed] [Google Scholar]
- 12.Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res 2006;175:256-67. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol 1969;68:350-62. [DOI] [PubMed] [Google Scholar]
- 14.Colebatch JG, Rothwell JC. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol 2004;115:2567-73. [DOI] [PubMed] [Google Scholar]
- 15.Aw ST, Fetter M, Cremer PD, Karlberg M, Halmagyi GM. Individual semicircular canal function in superior and inferior vestibular neuritis. Neurology 2001;57:768-74. [DOI] [PubMed] [Google Scholar]
- 16.Fetter M, Dichgans J. Vestibular neuritis spares the inferior division of the vestibular nerve. Brain 1996;119:755-63. [DOI] [PubMed] [Google Scholar]
- 17.de Burlet HM. Zur Innervation der Macula sacculi bei Säugetieren. Anat Anzeig 1924;58:26-32. [Google Scholar]
- 18.Dumas G, Perrin P, Schmerber S. Nystagmus induced by high frequency vibrations of the skull in total peripheral vestibular lesions. Acta Otolaryngol 2008;128:255-62. [DOI] [PubMed] [Google Scholar]
- 19.Uchino Y, Sasaki M, Sato H, Bai R, Kawamoto E. Otolith and canal integration on single vestibular neurons in cats. Exp Brain Res 2005;164:271-85. [DOI] [PubMed] [Google Scholar]