Abstract
Plectranthus amboinicus (P. amboinicus) is a folk herb that is used to treat inflammatory diseases or swelling symptoms in Taiwan. We investigated therapeutic efficacy of P. amboinicus in treating Rheumatoid Arthritis (RA) using collagen-induced arthritis animal model. Arthritis was induced in Lewis rats by immunization with bovine type II collagen. Serum anti-collagen IgG, IgM and C-reactive protein (CRP) were analyzed. To understand the inflammation condition of treated animals, production of TNF-α, IL-6 and IL-1β from peritoneal exudates cells (PEC) were also analyzed. P. amboinicus significantly inhibited the footpad swelling and arthritic symptoms in collagen-induced arthritic rats, while the serum anti-collagen IgM and CRP levels were consistently decreased. The production of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were also decreased in the high dosage of P. amboinicus group. Here, we demonstrate the potential anti-arthritic effect of P. amboinicus for treating RA, which might confer its anti-rheumatic activity. This differs the pharmacological action mode of indomethacin.
Keywords: collagen, rheumatoid, arthritis, herbal medicine, inflammation
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation in synovial tissue and joints, which leads to impaired joint function, severe pain and reduced life expectancy (1). This disease affects about 1% of the human population. The etiology and pathogenesis of this disease are not yet fully understood but it seems likely that an autoimmune-mediated attack on joints plays a crucial role in RA (2). In brief, inflammation and bone destruction may occur uncoupled. Therefore, therapeutic agents developed for anti-inflammatory and immunosuppressant activity will be useful and indispensable for RA therapy. Currently, non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin and celecoxib are commonly used to treat RA disease (3,4). However, these drugs produce strong alleviative effect including gastric ulcer and dysfunction or the risk of cardiovascular disease (5,6). Although a panel of drugs has been developed for the treatment of RA, therapies for RA are not satisfied with these marketed drugs.
P. amboinicus belonging to the to Lamiaceae family, is a perennial with a 3- to 10-years life span, and is distributed in Tropical Africa, Asia and Australia, is used as food, additive and fodder, and especially as medicine in treating a wide range of diseases (7). For chemical composition of P. amboinicus as an aqueous extract, the major components are Δ-3-carene, γ-terpinene, camphor and carvacrol (8). The leaf extract of P. amboinicus was externally used in the skin allergy in India (9). In Taiwan, P. amboinicus is used to treat inflammatory disease or swelling symptoms based on historical experiences (10). Furthermore, P. amboinicus is traditionally used treating RA, which involved oral consumption wherein 50–70 ml of P. amboinicus extract was consumed daily. However, there is no scientific evidence to prove the effectiveness of P. amboinicus on RA. For this reason, P. amboinicus was studied for its efficacy on RA treatment employing a collagen-induced rat model, which is commonly used in the validation of anti-rheumatic drugs because it has various similarities to human RA (11). Additionally, the levels of IgG and IgM against self-antigen collagen and production of inflammatory cytokines, TNF-α, IL-6 and IL-1β, from peritoneal exudates cells (PECs) were also determined.
Methods
Sample Preparation and Reagents
Whole plants of P. amboinicus were collected in autumn in Taitung, Taiwan. Briefly, 100 g of P. amboinicus was ground with 100 ml distilled water and filtered using 0.22 μM mesh. The filtrate was lyophilized and 100 g of plant yields about 1.66 g of solid content (1.66%), and stored at −20°C prior to animal experiments. Indomethacin was purchased from Johnson Chemical Pharmaceutical Work LTD (Taiwan, ROC). The bovine type-II collagen from tracheal cartilage and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St Louis, USA), and complete Freund's adjuvant (CFA) was obtained from Difco Laboratories (Detroit, USA). Indomethacin and P. amboinicus extract powder were suspended in distilled water and adjusted to the experimental doses prior to the animal experiment. All test articles were administered to animals using gastric tube.
Collagen-induced Arthritic Rat Animal Experiment
For collagen-induced arthritis (CIA) experiments, Lewis rats, body weight ranging from 155 to 165 g, were purchased from National Laboratory Animal Center (Taiwan, ROC). Rats were maintained under climate-controlled conditions under 12 h light–dark cycle. The animals were fed with standard rodent chow (PMI Nutrition International, USA) and water ad libitum. Twenty-five rats were divided into five groups as follows: (i) Normal; (ii) CIA plus vehicle; (iii) CIA plus Indomethacin (2.5 mg/kg); (iv) CIA plus P. amboinicus, low dosage (75 mg/kg); (v) CIA plus P. amboinicus high dosage (375 mg/kg). The induction of arthritis in animals was performed as described by Campo et al. (12). Briefly, arthritis in rats was induced by immunization with collagen type II that was dissolved in 0.1 M acetic acid (2 mg/ml) at 4°C overnight and emulsified with an equal volume of CFA. Each rat was immunized with a dose of 200 μg collagen type II/0.2 ml emulsion intradermally at the base of the tail. Rats were boosted with the same antigen preparation on day 14. After the second immunization, development of rheumatic symptoms was observed starting from day 18 and rats were administered test article by gavages starting day 25 for 20 consecutive days. Blood was withdrawn from tail vein on days 0, 20 and 35, and rats were sacrificed under CO2 euthanasia on day 45 on which day blood was withdrawn from the heart. All rats received human care, and the study protocol followed guidelines of Institutional Animal Care and Use Committees of the Development Center for Biotechnology.
Assessments of Arthritic Index and Footpad Thickness in CIA Rats
A blinded independent observer with no knowledge of the treatment protocol performed evaluation of joint inflammation. The severity of arthritis in each footpad quantified daily by a clinical score measurement. The level of arthritic inflammation of each paw was graded from 0 to 4 according to Campo et al. (12). The maximum arthritic index (MAI) of four paws was summed for each rat and ranged from 0 to 16 (0, no disease; 16, highest possible score). The degree of footpad swelling was measured using a caliper. The increased thickness of footpad in percentage was compared with normal rats without immunization of collagen.
Ex vivo Determination of Cytokines Releasing from Peritoneal Exudates Cells
Peritoneal exudates cells (PEC) were stimulated by injection with Hank's balanced salt solution (HBSS) into abdominal cavity of rats after sacrifice of the animals and PEC cells were collected by centrifugation. PEC were cultured in DMEM containing 10% fetal bovine serum in a 48-well plate at a density of 106 cells/ml at 37°C, 5% CO2 for 24 h. After incubation, the culture medium was collected and stored at −20°C prior to cytokine determination. Cytokines were measured by using enzyme-linked immunosorbent assay (ELISA). Briefly, the concentrations of inflammatory cytokine TNF-α, IL-6 and IL-1β in PEC cell culture medium were quantified by using commercial cytokine ELISA kit (R&D, DuoSet, MN) and performed in duplicate according to the manufacturer's instructions.
Measurement of Serum-specific Anti-Collagen Type II IgG and IgM Antibody
Blood was allowed to clot for 30 min, and serum was obtained by centrifugation at 800g for 10 min that stored at −20°C prior to analysis of anti-collagen type II specific IgM and IgG antibodies. The method was performed as Jonsson et al. (13) described with a slight modification. Briefly, the collagen was coated onto the 96-well plate and IgM was detected by goat anti-rat IgM conjugated with horse-reddish perxoidase (HRP) for 2 h at room temperature follower by staining with tetramethylbenzidine substrate. The optical density was measured with a Multiskan Ex ELISA reader (Thermo Fisher Scientific, USA) at 450 nm and wells without loading serum sample were regarded as blank. For measurement of IgG, goat anti-rat IgG Fc HRP-conjugated monoclonal antibody was used, and the procedure measurements were similar to those followed for IgM.
Analysis of Serum C-reactive Protein
The level of serum CRP was measured by the commercial Rat CRP ELISA kit (BD™ Pharmingen) and each sample was performed in duplicate according to the manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA to analyze the variances, and significant difference was evaluated by Dunnett's test for multiple comparisons. A P-value less than 0.05 was considered statistically significant.
Results
P. Amboinicus Prevented Body Weight Loss of CIA Rats
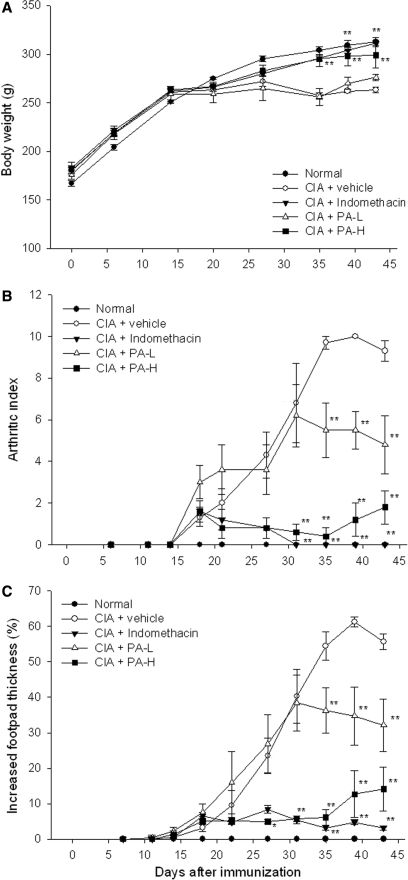
Lewis rats were immunized with the collagen type II on days 1 and 14. The onset of symptoms of arthritis was observed around day 20. In collagen-induced arthritic rats (vehicle group), body weight was not increased in that the inflammation was induced by self-antigen. In the group of collagen-induced arthritic rats treated with indomethacin and high dosage (375 mg/kg) of P. amboinicus, the body weight was normally increased as in the normal group but decreased in the group treated with low dosage of P. amboinicus (75 mg/kg) and vehicle control (Fig. 1A).
1.
Assessment of body weight, arthritic index and footpad thickness in CIA rats with P. amboinicus aqueous extract. Effects on body weight (A), arthritic index (B) and footpad swelling (C) of indomethacin and P. amboinicus aqueous extracts (L: 75 mg/kg, H: 375 mg/kg) in Lewis rats subjected to collagen-induced arthritis (CIA). **P < 0.01 vs. vehicle group (see text).
Ankylosis Significantly Improved in CIA Rats
The arthritic index represents the grade of arthritis that was used to assess efficacy of P. amboinicus. In the vehicle group, diseased rats without any treatment showed an increased arthritic index starting on day 18 to a plateau on day 35. Indomethacin appears to have the ability to inhibit progression of disease, and the high dosage of P. amboinicus was also observed to have significantly strong activity in preventing progression of arthritic disease (Fig. 1B). However, the low dosage of P. amboinicus showed moderate efficacy in inhibiting an increase in arthritic index.
Footpad Swelling Significantly Suppressed in CIA Rats
The swelling of footpad in collagen-induced rat model also represents the severity of arthritis. In the vehicle group, thickness of footpad was increased to a maximum of 61%. By the end of this experiment, swelling of footpad thickness was inhibited by indomethacin, high dosage and low dosage of P. amboinicus by 93.2%, 70.7% and 41.8%, respectively, and the scale of footpad thickness was set as 100% between the vehicle group and normal group (Fig. 1C). Indomethacin and the high dosage of P. amboinicus showed significant inhibitory activity in footpad swelling. The low dosage of P. amboinicus also had the ability to inhibit footpad swelling with moderate effectiveness. These results were coincident with the observation in arthritic index. According to these data, the arthritic rats were established and responded to the indomethacin.
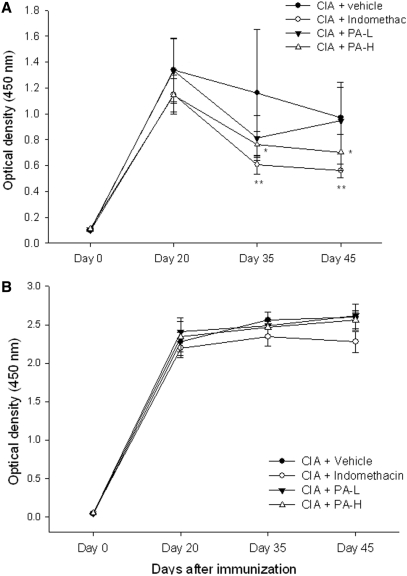
The Production of Serum Anti-Collagen IgM was Inhibited by P. Amboinicus in CIA Rats
The serum IgM and IgG levels against self-antigen collagen are important biomarkers in collagen-induced arthritis rat model. In this study, we analyzed the serum IgG and IgM levels in collagen-induced arthritic rats. Results revealed that serum IgM levels dramatically increased and achieved a maximum level on day 20 (Fig. 2A). The IgM levels were elevated in collagen-induced arthritic rat on day 20, and their production were inhibited up to 51% by indomethacin and to 39% by the high dosage of PA using the end-point analysis (day 45). The levels of serum anti-collagen IgG antibodies were also monitored on day 20, 35 and 45, respectively (Fig. 2B). Results indicated that the levels of serum anti-collgen IgG antibodies significantly increased in the collagen-induced arthritic rats, whereas neither indomethacinn nor P. amboinicus had an effect on the reduction of anti-collagen IgG antibody levels.
2.
Assessment of anti-collagen type II antibody IgM and IgG in CIA rats with P. amboinicus aqueous extract. Effects on anti-collagen type II antibody IgM and IgG of indomethacin and P. amboinicus aqueous extracts (L: 75 mg/kg, H: 375 mg/kg) in Lewis rats subjected to collagen-induced arthritis (CIA). **P < 0.01 vs. Vehicle group (see text).
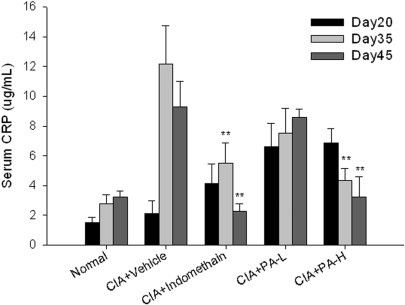
The Levels of C-Reactive Protein were Suppressed by P. Amboinicus in CIA Rats
Serum CRP, a marker of systemic inflammation, is produced by liver, which is one of the most responsive acute phase serum reactants. In this study, the high levels of CRP were induced in the CIA-vehicle group, which were maintained on a level of 9–12 μg/ml. For indomethacin treatment, the CRP levels were significantly suppressed to 2.5 μg/ml at day 45. In the high dosage of P. amboinicus treatment, the levels of serum CRP were significantly suppressed and gradually decreased (Fig. 3). These data showed that P. amboinicus had the activity to decrease the systemic inflammation.
3.
Assessment of serum CRP in CIA rats with P. amboinicus aqueous extract. Effects on CRP of indometacin and P. amboinicus aqueous extracts (L: 75 mg/kg, H: 375 mg/kg) in Lewis rats subjected to collagen-induced arthritis (CIA). **P < 0.01 vs. Vehicle group (see text).
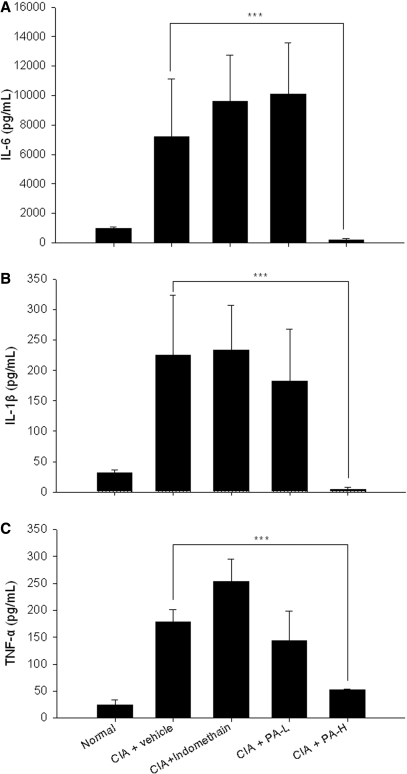
The Secretion of Pro-inflammatory Cytkines in PEC was Suppressed by P. Amboinicus
The PEC cells were washed out from the abdominal cavity of rats for the assessment of inflammatory TNF-α, IL-6 and IL-1β production. Results revealed that inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, were increased around 7-fold in collagen-induced arthritic rats, whereas this elevated cytokine production was not inhibited by treatment with indomethacin (Fig. 4A, B and C). Notably, a significant inhibition in TNF-α, IL-6 and IL-1β production was observed in the high-dosage group of P. amboinicus, but not in treatment with indomethacin. It was suggested that P. amboinicus conferred its ability in treatment of RA via the inhibition of inflammatory cytokines, which might differ from effects of indomethacin.
4.
Ex vivo assessments of TNF-α, IL-1β and IL-6 cytokine secretion from PEC in CIA rats with P. amboinicus aqueous extract. Effects on TNF-α, IL-1β and IL-6 of indomethacin and P. amboinicus aqueous extracts (L: 75 mg/kg, H: 375 mg/kg) in Lewis rats subjected to collagen-induced arthritis (CIA). ***P < 0.001 vs. Vehicle group (see text).
Discussion
When compared with the other arthritic animal models, arthritis induced by immunization with collagen is a promising model for validation of the efficacy of anti-rheumatic drugs (14). The most important index for evaluation of arthritis is to measure the footpad swelling with calipers and visual inspection, and that is not restricted through different ways to induce arthritis in animals (15). In this article, P. amboinicus showed the ability to treat collagen-induced arthritis in rats. The reduction of CRP was also relevant to inhibiting arthritic progression. These findings were positively consistent with the results of histological examinations, which reduced inflammation lesion and bone erosion in the high dose of P. amboinicus and indomethacin groups (data not shown).
According to the production of cytokines from PEC cells, P. amboinicus might confer its activity differing from indomethacin that inhibits the cyclooxygenase enzyme. Recently, Patten and his colleagues (16) reported that TNF-α, IL-6 and IL-1β were persistently up-regulated in arthritic joints of the pristane-induced arthritis, whereas indomethacin failed to reduce the expression of these pro-inflammatory cytokines. In their study, only prednisoline significantly reduced these cytokines and revealed the most effective joint protection. P. amboinicus might have elicited their anti-RA activity with different pharmacological action mode to nonsteroidal anti-inflammatory drugs.
In autoimmune disease, pro-inflammatory cytokines play an important role in RA and scientists intend to design drugs for suppression of this disease via anti-inflammation (4). Currently, therapeutically controlling inflammation is essential for the clinical management of many high-prevalence human diseases (17). Drugs that block pro-inflammatory cytokines, such as TNF-α and IL-1β, can improve outcomes for RA, and Kineret and Enbrel have been developed to block IL-1β receptor and neutralize the TNF-α (18,19); however, these protein drugs are costly in the treatment of RA (20). Indomethacin is a cyclooxygenase inhibitor that inhibits the production of prostaglandins, which this kind of NSAIDs may cause undesired side-effects, such as gastrointestinal damages (21). P. amboinicus might improve the therapeutic efficacy of NSAID or lessen the use of NSAID for combining with two different pharmacological action drugs. In addition, the active ingredients for treatment of RA should be identified and characterized. For safety, P. amboinicus is used as food additive and an unpublished data reported that acute phase toxicity was not observed in rats with one dosage of 5000 mg/kg. In our study, we demonstrated that the feasibility of P. amboinicus in treating RA and P. amboinicus might be developed as a disease-modifying anti-rheumatic drug.
Acknowledgement
This study is supported by a grant from Ministry of Economic Affairs, Taiwan, ROC (94-EC-17-A-20-R7-0722).
References
- 1.Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498–507. doi: 10.1128/AAC.48.9.3498-3507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawaheer D, Thomson W, MacGregor AJ, Carthy D, Davidson J, Dyer PA, et al. “Homozygosity” for the HLA-DR shared epitope contributes the highest risk for rheumatoid arthritis concordance in identical twins. Arthritis Rheum. 1994;37:681–6. doi: 10.1002/art.1780370511. [DOI] [PubMed] [Google Scholar]
- 3.Waller ES. Evaluation of new indomethacin dosage forms. Pharmacotherapy. 1983;3:324–33. doi: 10.1002/j.1875-9114.1983.tb03290.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg MM. Celecoxib, a selective cyclooxygenase-2 inhibitor for the treatment of rheumatoid arthritis and osteoarthritis. Clin Ther. 1999;21:1497–513. doi: 10.1016/s0149-2918(00)80005-3. discussion 1427–1498. [DOI] [PubMed] [Google Scholar]
- 5.Bertolini A, Ottani A, Sandrini M. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res. 2001;44:437–50. doi: 10.1006/phrs.2001.0872. [DOI] [PubMed] [Google Scholar]
- 6.White WB, West CR, Borer JS, Gorelick PB, Lavange L, Pan SX, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. 2007;99:91–8. doi: 10.1016/j.amjcard.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 7.Lukhoba CW, Simmonds MS, Paton AJ. Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol. 2006;103:1–24. doi: 10.1016/j.jep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Vera R, Mondon JM, Pieribattesti JC. Chemical Composition of The Essential Oil and Aqueous Extract of Plectranthus amboinicus. Planta Med. 1993;59:182–3. doi: 10.1055/s-2006-959641. [DOI] [PubMed] [Google Scholar]
- 9.Harsha VH, Hebbar SS, Shripathi V, Hegde GR. Ethnomedicobotany of Uttara Kannada District in Karnataka, India–plants in treatment of skin diseases. J Ethnopharmacol. 2003;84:37–40. doi: 10.1016/s0378-8741(02)00261-1. [DOI] [PubMed] [Google Scholar]
- 10.Chang SL, Chang YC, Yang CH, Hong HS. Allergic contact dermatitis to Plectranthus amboinicus masquerading as chronic leg ulcer. Contact Dermatitis. 2005;53:356–7. doi: 10.1111/j.0105-1873.2005.0592f.x. [DOI] [PubMed] [Google Scholar]
- 11.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 12.Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5:R122–31. doi: 10.1186/ar748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson T, Arnason JA, Valdimarsson H. Enzyme-linked immunosorbent assay (ELISA) screening test for detection of rheumatoid factor. Rheumatol Int. 1986;6:199–204. doi: 10.1007/BF00541367. [DOI] [PubMed] [Google Scholar]
- 14.Shou J, Bull CM, Li L, Qian HR, Wei T, Luo S, et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Arthritis Res Ther. 2006;8:R28. doi: 10.1186/ar1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mindrescu C, Thorbecke GJ, Klein MJ, Vilcek J, Wisniewski HG. Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 2000;43:2668–77. doi: 10.1002/1529-0131(200012)43:12<2668::AID-ANR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Patten C, Bush K, Rioja I, Morgan R, Wooley P, Trill J, et al. Characterization of pristane-induced arthritis, a murine model of chronic disease: response to antirheumatic agents, expression of joint cytokines, and immunopathology. Arthritis Rheum. 2004;50:3334–45. doi: 10.1002/art.20507. [DOI] [PubMed] [Google Scholar]
- 17.Vierboom MP, Zavodny PJ, Chou CC, Tagat JR, Pugliese-Sivo C, Strizki J, et al. Inhibition of the development of collagen-induced arthritis in rhesus monkeys by a small molecular weight antagonist of CCR5. Arthritis Rheum. 2005;52:627–36. doi: 10.1002/art.20850. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese LH. Molecular differences in anticytokine therapies. Clin Exp Rheumatol. 2003;21:241–8. [PubMed] [Google Scholar]
- 19.Louie SG, Park B, Yoon H. Biological response modifiers in the management of rheumatoid arthritis. Am J Health Syst Pharm. 2003;60:346–55. doi: 10.1093/ajhp/60.4.346. [DOI] [PubMed] [Google Scholar]
- 20.Lubeck DP. A review of the direct costs of rheumatoid arthritis: managed care versus fee-for-service settings. Pharmacoeconomics. 2001;19:811–18. doi: 10.2165/00019053-200119080-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed AH, Salena BJ, Hunt RH. NSAID–induced gastroduodenal ulcers: exploring the silent dilemma. J Gastroenterol. 1994;29(Suppl 7):34–8. [PubMed] [Google Scholar]