Abstract
Perillae fructus (perilla seed) is a traditional medicinal herb used to treat bronchial asthma in Oriental medical clinics. ST36 is one of the most widely used acupuncture points, particularly for immune system regulation. Injection of an herbal extract into an acupuncture point (herbal acupuncture) is a therapeutic technique combining both acupuncture and herbal treatment. Perillae fructus extract was injected subcutaneously (Perillae fructus herbal acupuncture; PF-HA) at acupoint ST36 of OVA-induced asthmatic mice. The lung weight, bronchoalveolar fluid (BALF) cell count, the number of CCR3+, CD11b+, CD4+ and CD3e+/CD69+ cells in the lung, and the level of IgE, IL-4, IL-5 and IL-13 in BALF and serum were then measured. RT-PCR was used to measure the mRNA expression of IL-4, IL-5, IL-13 and TNF-α in the lung. Lung sections were analyzed histologically. PF-HA significantly reduced lung weight, the number of inflammatory cells in the lung and BALF, the levels of IgE and Th2 cytokines in BALF and serum, mRNA expression of Th2 cytokines in the lung, and pathological changes in lung tissue. Our results suggest that PF-HA may have an anti-inflammatory and immune-regulatory effect on bronchial allergic asthma by restoring the Th1/Th2 imbalance in the immune system and suppressing eosinophilic inflammation in airways.
Keywords: herbal acupuncture, OVA-induced asthma, perillae fructus, ST36
Introduction
Asthma is a heterogeneous disease that is broadly defined as a clinical syndrome characterized by altered lung function, peribronchial inflammation and airway responsiveness. It is generally regarded as a T-cell mediated disease that leads to an imbalance of Th1 and Th2 activity (1–3).
Acupuncture has been used to treat various diseases in Asia for thousands of years and several studies have demonstrated that acupuncture is an effective treatment for asthma (4–7). To treat immune diseases, including asthma, acupuncture points that can boost the vital energy and regulate the immune system are used. ST36 is one of the most commonly used acupuncture points to tonify the vital energy and regulate the immune system (8,9).
Perillae fructus (perilla seed, PF) is a traditional medicinal herb that has been used to treat respiratory diseases. Based on Oriental medical theory, PF enters the lung meridian, arrests coughing and wheezing with copious phlegm, and treats exhalation difficulties and stiffness in the chest (10). The leaves of perilla (Perilla frutescens) have shown a suppressive effect on type 1 allergies (11). However, perilla seed has not yet been investigated for use on allergic diseases.
Herbal acupuncture is a traditional Oriental therapeutic technique that combines acupuncture with herbal treatment. This technique involves injecting an herbal extract into certain acupuncture points, according to Oriental medical theory. We hypothesized that combining the immune-strengthening function of ST36 and respiratory disease-treating activity of PF would have a synergistic effect on allergic bronchial asthma.
To investigate perillae fructus herbal acupuncture (PF-HA) for allergic bronchial asthma, we used an OVA-induced asthmatic mouse model.
Methods
Medicinal Substance and Herbal-Acupuncture Solution
Dried PF (5 g) was washed with an ultrasonic cleaner (BRANSON, USA) and ground using a pulverizer. The powder was transferred to a flask containing 500 ml of distilled water, mixed for 3 h at 37°C in a shaking incubator, filtered through 3MM Whatman filter paper and concentrated using a rotary evaporator. Sequentially, 30 ml of 95, 85 and 75% ethanol were then added to the extract, which was maintained at room temperature. After filtering out the sediment, the filtrate was condensed to 20 g. This PF extract was diluted with phosphate buffered saline (PBS) to a total volume of 2 l, and the pH was adjusted to 7.0.
OVA-Induced Asthmatic Mouse Model
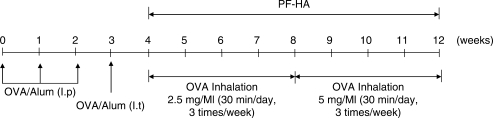
Five-week-old male C57BL/6 mice were purchased from Daehan Biolink (Chungbuk, Ochang, Korea). The mice were maintained under conventional conditions at a constant temperature (22 ± 2°C), humidity and ventilation, on a 12 h light/dark cycle. Mice had access to water and food ad libitum. Chicken egg albumin (OVA; 500 µg/ml; Sigma, USA) in PBS was mixed with an equal volume of 10% (w/v) aluminum potassium sulfate (alum; Sigma) in distilled water and adjusted to pH 6.5 using 10N NaOH. The solution was then incubated for 60 min at room temperature. After centrifugation at 750 × g for 5 min, the OVA/alum pellet was resuspended to the original volume in distilled water. The experimental animals were sensitized by intra-peritoneal injections of OVA/alum (500 µg/ml) in the amounts of 200 µl on day 0 and 100 µl on days 7 and 14. The mice were challenged with an intra-tracheal injection of 100 µl OVA/alum (500 µg/ml) on day 21, and underwent an aerosol challenge with OVA/alum (2.5 mg/ml for 4 weeks from week 4 to week 8, and 5 mg/ml for 4 weeks from week 8 to week 12) for 30 min per day, 3 times a week using an air compressor (Tamiya, Japan; 12; Fig. 1).
1.
OVA-induced asthmatic mouse model.
Experimental Groups and Treatments
The mice were divided into six groups (ten mice per group): normal, normal-PF-HA, OVA-control, OVA-needle prick (OVA-NP), OVA-saline and OVA-PF-HA group. All animals except those in the normal and normal-PF-HA groups underwent OVA exposure. The mice in the OVA-NP group were given a single needle prick (subcutaneously with an empty 1-mL syringe needle). The mice in the OVA-saline group were injected with saline (100 µl) and the mice in the normal-PF-HA and OVA-PF-HA groups were injected subcutaneously with PF extract (100 µl) at ST36, alternating between left and right.
NP and PF-HA treatments were conducted for 8 weeks, three times per week (Fig. 1), using a 1 ml injection syringe. ST36 is located on the stomach meridian, longitudinally three cun below the knee joint, transversely in the middle of the tibialis anterior muscle. For better point location on the mice, we placed a rubber band along a ruler and marked it at 0, 3 and 16 cm. The rubber band was then set on the mouse hind limb, with the 0 cm mark at ST35 (located at the lower border of the patellar, in the depression lateral to the patellar ligament; 8) and the 16 cm mark at ST41 (located on the dorsum of the foot, at the midpoint of the transverse crease of the ankle joint, in the depression between the m. extensor digitorum longus and hallucis longus tendons; 8). The point corresponding to the 3 cm mark on the rubber band was determined to be ST36. While the needle prick, saline injections or PF extract injections were delivered, the mice were restrained by hand.
Bronchoalveolar-lavage Fluid (BALF)
The mouse trachea was cannulated and the lungs were washed 3 times with 1 ml of DMEM. The bronchoalveolar lavage fluid (BALF) was immediately centrifuged and the supernatant was collected for cytokine measurements. Cell pellets were resuspended and cytocentrifuged for total leucocyte measurement after red blood cell lysis.
Photomicrographs
The total BALF was collected and washed with PBS. The BALF cells were cytocentrifuged onto slides using a Cytospin and stained with a Hemacolor rapid staining set (Merck KGaA, Germany). Eosinophils were counted using a bright field microscope (×400; Nikon, Japan).
Histological Analysis
The lungs were removed, washed with PBS and infused with 10% formalin in PBS for fixation. The lung tissue was sectioned and stained with Masson's trichrome reagent.
Fluorescence-Activated Cell Sorter (FACS) Analysis
The lungs were removed and minced in RPMI1640. Lung cells were harvested in a shaking incubator and reacted with either PE or FITC-conjugated antibodies (Pharmingen, USA) for 1 h on ice. After washing, the cells were analyzed using a Flow cytometer (Becton Dickinson, USA). One sample from each group was analyzed as a representative.
Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
Each cytokine was evaluated with an ELISA kit (Biosource, USA) according to the manufacturer's protocol.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
After the lung tissue was homogenized, the total RNA was extracted using RNAzolB (Tel-Test, USA). Three µg of the total RNA were denatured for 10 min at 75°C and mixed with 2.5 µl of 10 mM dNTPs, 1 µl of random sequence hexanucleotides (25 pmole/25 µl), 0.5 µl of RNA inhibitor, 1 µl of 100 mM DTT, 4 µl of 5× RT buffer (250 mM Tris-Cl, pH 8.3, 375 mM KCl, 15 mM MgCl2) and 1 µl of M-MLV RT (200 U/µl). The synthesis of first-strand cDNA was carried out for 1 h in a 37°C water bath. PCR amplification was carried out in 2 µl of PCR buffer (10 mM Tris-HCl, pH 8.3), 1.6 µl of dNTP (2.5 M), 1 µl of sense primer (20 pmole/µl) and antisense primer (20 pmole/µl), 0.15 µl of Taq polymerase, 13.25 µl of distilled water and 2 µl of each cDNA sample. One PCR cycle (29 cycles total) consisted of denaturation at 94°C for 1 min, annealing at 57°C for 1 min and polymerization at 72°C for 1 min. The RT-PCR products were electrophoresed on a 1.2% agarose gel. The primer sequences were as follows: mouse IL-4, 5′-AGCCATATCCACGGATGCGA C-3′ and 5′-GCATGGTGGCTCAGTACTACG-3′; IL-5, 5′-GCTCCTTCAGGAATCTGTTC-3′ and 5′-GGCTCATGTACTTTCATGAG-3′; IL-13, 5′-GCCGGGATGGGCATTCCACGTGTG-3′ and 5′-GGACGCCAAGGTCAAGAACAGTTG-3′; TNF-α, 5′-ACGGCTGACTGTCAGATTGTTAG -3′ and 5′-GTCAC AGTTTTCAGCTGTATAGGG-3′; β-actin, 5′-TGGAATCCTGATCCATGAAC-3′ and 5′-TAAAACGCAGCTCAGTAGTCCG-3′.
Statistical Analyses
Statistical analyses were performed using analysis of variance (ANOVA) multiple t-tests (JAVA, Bonferroni Ver II). The results are expressed as a mean ± SEM. Results were considered statistically significant at P ≤ 0.05.
Results
Lung Weight and BALF Cell Counts Reduced
The lung weight of the OVA-control group was significantly higher than that of the normal group. In contrast, the lung weight of the OVA-PF-HA group was lower than those of the OVA-control and OVA-NP groups. The number of leucocytes and eosinophils in the BALF were significantly higher in the OVA-control group than in the normal group. In contrast, those of the OVA-PF-HA group were significantly lower than the OVA-control and OVA-NP groups (Table 1).
Table 1.
Effect of PF-HA at ST36 on lung weight and BALF cell counts
| Normal | Normal-PF-HA | OVA-Control | OVA-NP | OVA- Saline | OVA-PF-HA | |
|---|---|---|---|---|---|---|
| Lung weight (g) | 0.382 ± 0.048 | 0.327 ± 0.022 | 0.704 ± 0.171*†† | 0.684 ± 0.029† | 0.462 ± 0.05 | 0.478 ± 0.095 |
| Leucocyte (× 105cells) | 17.9 ± 1.7 | 17.39 ± 1.68 | 119 ± 29.5***††† | 98.5 ± 2.4**†† | 55.2 ± 4.2‡‡‡## | 21.9 ± 3.39‡‡## |
| Eosinophil (cells/µl) | 4.43 ± 1.604 | 2.683 ± 0.3286 | 41.67 ± 3.14***††† | 40.2 ± 0.57***††† | 12.9 ± 0.99**††‡‡‡### | 16.7 ± 2.687**††‡‡‡### |
Values are mean ± SEM.
*indicates significantly different versus Normal (P < 0.05); **(P < 0.01); ***(P < 0.001); †versus Normal-PF-HA (P < 0.05); ††(P < 0.01); †††(P < 0.001); ‡‡versus OVA-control (P < 0.01); ‡‡‡(P < 0.001); ##versus OVA-NP (P < 0.01); ###(P < 0.001).
Eosinophil Recruitment and Collagen Accumulation Reduced in the Lung
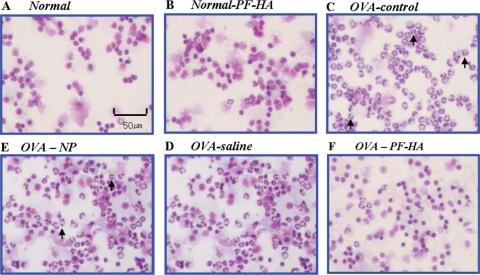
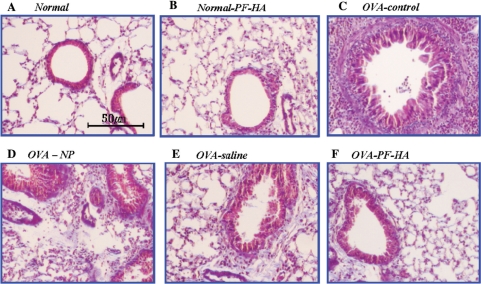
The photomicrographs show that PF-HA reduced eosinophils in BALF (Fig. 2). Histological analysis of mouse lungs in the OVA-control group showed a large increase in eosinophil recruitment in the airways and collagen accumulation compared to the normal group. The lungs of mice treated with PF-HA had significantly fewer eosinophils and less collagen accumulation than in the OVA-control, OVA-NP and OVA-saline groups (Fig. 3).
2.
Photomicrographs of BALF cytospins. The BALF from the OVA-control group had a markedly higher number of eosinophils. BALF from the OVA-PF-HA group exhibited a significantly lower number of eosinophils (arrows) than the OVA-control, OVA-NP and OVA-saline groups.
3.
Histological analysis of mouse lung sections. Mouse lung sections (6 μm) were stained with Masson's trichrome. Lung tissue from the OVA-PF-HA mouse group showed fewer eosinophils and less collagen accumulation than the OVA-control, OVA-NP and OVA-saline groups.
Inflammatory Immune Cell Populations Reduced in the Lung
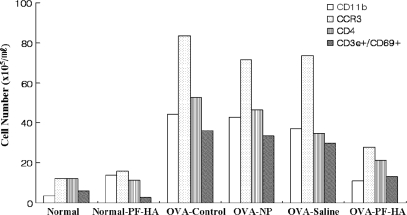
The population of CCR3+ cells in the lungs of the OVA-control group was significantly higher than in the normal group. On the other hand, CCR3+ cells were significantly lower in the PF-HA group than in the OVA-control, OVA-NP and OVA-saline groups (Fig. 4). The number of CD11b+, CD4+ and CD3e+/CD69+ cells in the lung also increased as a result of OVA-exposure and was reduced remarkably by PF-HA. NP and saline injection at ST36 also reduced cell numbers compared to the OVA-control group, but less effectively (Fig. 4).
4.
Populations of CD11b+, CCR3+, CD4+ and CD3e+/CD69+ cells in lungs. The populations of CD11b+, CCR3+, CD4+ and CD3e+/CD69+ cells in lungs were counted using a flow cytometer. One sample from each group was analyzed. CD11b+, CCR3+, CD4+ and CD3e+/CD69+ cell counts were markedly lower in the OVA-PF-HA group than in the OVA-control, OVA-NP and OVA-saline groups.
IgE and Th2 Cytokines in BALF and Serum Lowered
The levels of IgE, IL-4 and IL-5 in BALF and the levels of IL-4, IL-5 and IL-13 in the serum were measured using ELISA. These cytokines and IgE levels were increased remarkably by OVA-exposure and reduced significantly by PF-HA. NP and saline injection at ST36 also reduced these levels, but less effectively (Table 2).
Table 2.
Effect of PF-HA at ST36 on cytokine levels in BALF and serum
| Normal | Normal-PF-HA | OVA-Control | OVA-NP | OVA-Saline | OVA-PF-HA | |
|---|---|---|---|---|---|---|
| BALF | ||||||
| IgE | 0.09 8 ±0.017 | 0.105 ± 0.016 | 0.724 ± 0.151***††† | 0.62 ± 0.132**†† | 0.234 ± 0.029‡‡## | 0.156 ± 0.043‡‡## |
| IL-4 | 0.069 ± 0.008 | 0.105 ± 0.009 | 0.217 ± 0.097* | 0.129 ± 0.03 | 0.2885 ± 0.01**††# | 0.084 ± 0.005§§ |
| IL-5 | 0.164 ± 0.022 | 0.153 ± 0.002 | 0.358 ± 0.032***††† | 0.314 ± 0.03**†† | 0.241 ± 0.03*†‡‡# | 0.176 ± 0.011‡‡‡##§ |
| Serum | ||||||
| IL-4 | 0.05 ± 0.006 | 0.052 ± 0.018 | 0.312 ± 0.025***††† | 0.171 ± 0.048**††‡‡ | 0.08 ± 0.006‡‡‡# | 0.05 ± 0.002‡‡‡## |
| IL-5 | 0.191 ± 0.053 | 0.186 ± 0.006 | 1.026 ± 0.154*† | 1.027 ± 0.101*† | 0.379 ± 0.06 | 0.327 ± 0.037 |
| IL-13 | 0.390 ± 0.024 | 0.471 ± 0.02 | 0.460 ± 0.091**†† | 0.724 ± 0.049**† | 0.682 ± 0.108* | 0.327 ± 0.019‡‡##§§ |
Cytokine levels were determined by measuring absorbance at 450 nm. Values represent mean ± SEM.
*indicates significantly different versus Normal (P < 0.05); **(P < 0.01); ***(P < 0.001); †versus Normal-PF-HA (P < 0.05); ††(P < 0.01); †††(P < 0.001); ‡‡versus OVA-control (P < 0.01); ‡‡‡(P < 0.001); ##versus OVA-NP (P < 0.01); ###(P < 0.001).
mRNA Expression of Inflammatory Cytokines Lessened
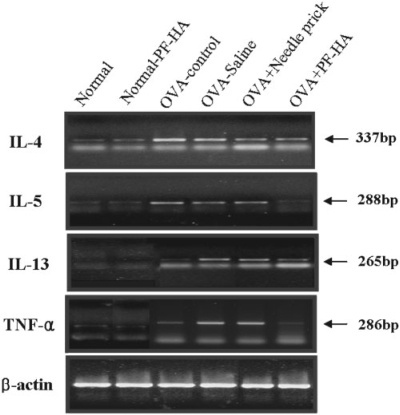
The mRNA expression levels of TNF-α, IL-4, IL-5 and IL-13 in the lung were analyzed by RT-PCR. Expression increased remarkably after OVA exposure. PF-HA administration at ST36 remarkably reduced mRNA expression of these cytokines (Fig. 5).
5.
mRNA expression of cytokines in the lung. The mRNA expression of IL-4, IL-5, IL-13 and TNF-α in the lung was analyzed by RT-PCR. Note that the mRNA expression of IL-4, IL-5 and TNF-α was lower in the OVA-PF-HA group than in the OVA-control, OVA-NP and OVA-saline groups. mRNA expression of IL-13 was significantly lower in the OVA-PF-HA group than in the OVA-NP and OVA-saline groups.
Discussion
In Oriental medicine, acupuncture and herbs are widely used to treat bronchial asthma (11,13–16), theoretically by clearing the pathogenic factors and reinforcing the body's resistance (immune system; 8). We combined acupuncture with herbal treatment, to achieve herbal acupuncture. PF was used for the herbal treatment, and acupoint ST36 was selected for the acupuncture treatment. According to Oriental medical theory, PF enters the lung meridian and arrests coughing and wheezing. ST36 is on the foot Yangming stomach meridian. This acupoint is known to strengthen the vital energy (‘qi’ in Oriental medical terminology). The ‘vital energy’ here means not only stomach qi, even though this acupoint belongs to the stomach meridian, but also the general qi in the whole body. For this reason, ST36 is used to treat various diseases in different parts of the body or general symptoms in the whole body, including deficiency and weakness (8,9,17,18). We hypothesized that ST36 could strengthen the general immune energy as well as lung qi in the case of bronchial asthma. To investigate the effect of PF-HA at ST36 on allergic bronchial asthma, we used the OVA-induced asthmatic mouse model (12).
PF-HA at ST36 reduced the lung weight of OVA-induced asthmatic mice, suggesting it may inhibit asthmatic lung edema and cellular infiltration into the lungs (Table 1). The cellular infiltrate that characterizes asthma consists of mononuclear cells and eosinophils. The ability to control leukocyte infiltration into the lungs is viewed as the key to regulating disease severity (19). PF-HA at ST36 reduced the total number of leukocytes and eosinophils in the BALF of OVA-induced asthmatic mice (Table 1). In addition, photomicrographs of the BALF and histological analysis of lung tissue showed that PF-HA at ST36 inhibited eosinophil infiltration and collagen accumulation in the lung (Figs 2 and 3). From these results, we infer that PF-HA may have therapeutic activity against airway obstruction and eosinophilic inflammation in allergic asthma via inhibiting cellular infiltration and collagen accumulation in the lungs.
Previous studies have suggested that CCR3 is involved in the activation and degranulation of eosinophils, as well as in the primary migration of eosinophils (19,20). In addition, CCR3 is believed to be an important target in the treatment of eosinophilic inflammation (20). In our investigation, CCR3+ cells in the mouse lung were reduced remarkably by PF-HA at ST36, suggesting it may be effective in controlling the activation, degranulation and migration of eosinophils in allergic asthma (Fig. 4). We also observed that the number of CD11b+ cells and mRNA expression of TNF-α in asthmatic lungs were markedly reduced by PF-HA at ST36 (Figs 4 and 5). Based on these results, we speculate that PF-HA may have an anti-inflammatory effect on allergic asthma via inhibiting leukocyte migration into the lungs and suppressing eosinophil activation and degranulation.
Asthma is generally regarded as a T-cell mediated disease. Allergens cause the differentiation of naive T- cells into Th2 cells, which then secrete cytokines such as IL-4, IL-5 and IL-13 (3). IL-4, which is pivotal in the pathogenesis of allergic disorders, acts on B cells to facilitate IgE production (3,21). Increased IgE production in response to common environmental antigens is the hallmark of atopic diseases such as bronchial asthma (22). IL-4 also induces the rolling and adhesion of circulating eosinophils to endothelial cells. Therefore, inhibitors of the IL-4 signaling pathway have been suggested as therapeutic targets (23,24).
Our results showed that PF-HA at ST36 significantly decreased IL-4 in BALF and serum, and mRNA expression of IL-4 in the lung (Table 2, Fig. 5). The lung histology observed here (Fig. 3) showed that PF-HA at ST36 reduced eosinophils in lung tissue, and we speculate that the reduction in eosinophils may be due to a decrease in IL-4 by PF-HA at ST36. Based on these results, it is inferred that PF-HA may have an anti-allergic effect on allergic bronchial asthma by suppressing IL-4 secretion and, in consequence, inhibiting IgE secretion from B cells. Further, reduced IL-4 may reduce eosinophil infiltration into the lungs by reducing the adhesion of circulating eosinophils to endothelial cells.
IL-5 is a Th2-type cytokine that promotes the recruitment and activation of eosinophils. IL-5 stimulates the release of chemical mediators from eosinophils (25). Airway eosinophilic inflammation is one of the characteristics of bronchial allergic asthma. Therefore, IL-5 has been implicated in the pathogenesis of allergic diseases (26,27), and many investigations focused on IL-5 as a viable target for the treatment of asthma and allergic diseases (28–30). In this study, PF-HA at ST36 significantly reduced IL-5 in BALF and serum, as well as mRNA expression of IL-5 in the lung. As mentioned earlier, PF-HA at ST36 reduced eosinophil infiltration and activation in OVA-induced asthmatic mice. We speculate that this result may be due to the inhibition of IL-5 secretion from Th2 cells.
The number of CD4+ cells and CD3e+/CD69+ cells in asthmatic mouse lungs were remarkably reduced by PF-HA at ST36 (Fig. 4). Also, Th2 cytokines such as IL-4, IL-5 and IL-13 were reduced significantly by PF-HA at ST36. These results indicate that PF-HA may suppress the excess activity of T- cells and Th2 cytokines, which are implicated in the pathogenesis of allergic asthma, and consequently restore the Th1/Th2 imbalance of the immune system. From these results, we hypothesize that PF-HA has an immuno-regulatory function for Th1 and Th2, and a suppressive effect on excessive Th2 cytokines. This Th1/Th2 balancing effect may lead to anti-allergic and anti-inflammatory effects by way of (i) reducing IL-4 secretion from Th2 cells to decrease IgE production from B cells and to reduce eosinophil infiltration into the lungs by inhibiting adhesion of eosinophils to endothelial cells and (ii) reducing IL-5 to decrease eosinophil recruitment and activation.
Th2 cytokines such as IL-13, IL-4 and IL-5 and elevated IgE levels are associated with the development of airway hyper-reactivity. In particular, IL-13 mediates airway hyper-responsiveness in allergic lungs (31–33). Here, PF-HA at ST36 reduced IL-4, IL-5, IL-13 and IgE levels in the BALF and serum (Table 2). Furthermore, mRNA expression of these cytokines in the lung was reduced remarkably by PF-HA at ST36 (Fig. 5). From these results, we conclude that PF-HA may suppress airway hyper-responsiveness by reducing these cytokines.
In most of the measurements discussedearlier, the normal-PF-HA group did not show any considerable differences from the normal group. This result indicates that PF-HA does not cause any changes in normal conditions and, furthermore, it does not interrupt the homeostasis of healthy subjects.
In our study, NP at ST36 appeared to work in a positive direction to suppress OVA-induced asthma, although it was not as effective as PF-HA. This result suggests that the mechanical stimulation of ST36 has a beneficial effect on bronchial allergic asthma, even though it is not as effective as PF-HA at ST36. Saline injection at ST36 also had positive effects on OVA-induced asthma in most measurements; however, PF-HA showed more beneficial effects than saline injections. Thus, we speculate that PF extract has therapeutic activity on OVA-induced asthma in addition to the mechanical stimulation provided by PF-HA. It is regrettable that we do not have experimental data on PF-HA at a non-acupoint to compare to the effect of PF-HA at ST36 because we cannot discuss the role of ST36 based on our results. This may be investigated in future studies.
In summary, we hypothesize that PF-HA may be applicable to bronchial allergic asthma to reduce airway obstruction by inhibiting cellular infiltration and collagen accumulation in the lung, and to relieve airway hyper-responsiveness by reducing IL-4, IL-5, IL-13 and IgE. Our data further suggest that PF-HA has a therapeutic effect on bronchial allergic asthma by regulating the immune system (suppressing excess Th2 activity and rebalancing Th1/Th2 in the immune system) and controlling eosinophilic inflammation in the airway. The anti-inflammatory effect of PF-HA may result from its immuno-regulatory effect on Th1 and Th2 activity. Further study is needed to determine the mechanisms of action and application to human subjects.
References
- 1.Kon OM, Kay AB. T cells and chronic asthma. Int Arch Allergy Immunol. 1999;118:133–5. doi: 10.1159/000024049. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Pathology of mild, severe, and fatal asthma. Am J Respir Crit Care Med. 1996;154:S66–9. doi: 10.1164/ajrccm/154.2_Pt_2.S66. [DOI] [PubMed] [Google Scholar]
- 3.Ramshaw HS, Woodcock JM, Bagley CJ, McClure BJ, Hercus TR, Loperz AR. New approaches in the treatment of asthma. Immuol Cell Biol. 2001;79:154–9. doi: 10.1046/j.1440-1711.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Bresler DE, Kroening RJ, Kerschner H, Katz RL, Coulson A. Comparison of real and simulated acupuncture and isoproterenol in methacholine-induced asthma. Ann Allergy. 1977;39:379–87. [PubMed] [Google Scholar]
- 5.Virsik K, Kristufek P, Bangha O. The effect of acupuncture on pulmonary function in bronchial asthma. Prog Respir Res. 1980;14:271–5. [Google Scholar]
- 6.Takishima T, Mue S, Tamura G, Ishihara T, Watanabe K. The bronchodilating effect of acupuncture in patients with acute asthma. Ann Allergy. 1982;48:44–9. [PubMed] [Google Scholar]
- 7.Yu DY, Lee SP. Effect of acupuncture on bronchial asthma. Clin Sci Mol Med. 1976;51:503–9. doi: 10.1042/cs0510503. [DOI] [PubMed] [Google Scholar]
- 8.Deng L, Gan Y, He S, Ji X, Li Y, Wang R, et al. Chines Acupuncture and Moxibustion. Beiging: foreign languages press; 1997. pp. 145385–8. [Google Scholar]
- 9.Kim DH. Oriental Medicine Series, Volume Two; Acupuncture & Moxibustion. Seoul: The Research Institute of Oriental Medicine, Inc.; 1987. pp. 194–5. [Google Scholar]
- 10.Bensky D, Gamble A. Materia Medica. Washington: Eastland press; 1993. p. 201. [Google Scholar]
- 11.Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–3. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 12.Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999;104:301–8. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockert K, Schneider B, Porenta G, Rath R, Nissel H, Eichler I. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of Traditional Chinese Medicine. Pediatr Allergy Immunol. 2007;18:160–6. doi: 10.1111/j.1399-3038.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehl-Madrona L, Kligler B, Silverman S, Lynton H, Merrell W. The impact of acupuncture and craniosacral therapy interventions on clinical outcomes in adults with asthma. Explore (NY) 2007;3:28–36. doi: 10.1016/j.explore.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Chu KA, Wu YC, Lin MH, Wang HC. Acupuncture resulting in immediate bronchodilating response in asthma patients. J Chin Med Assoc. 2005;68:591–4. doi: 10.1016/S1726-4901(09)70099-1. [DOI] [PubMed] [Google Scholar]
- 16.Jeong HJ, Kim BS, Oh JG, Kim KS, Kim HM. Regulatory effect of cytokine production in asthma patients by SOOJI CHIM (Koryo Hand Acupuncture Therapy) Immunopharmacol Immunotoxicol. 2002;24:265–74. doi: 10.1081/iph-120003759. [DOI] [PubMed] [Google Scholar]
- 17.Yim YK, Lee H, Hong KE, Kim YI, Lee BR, Son CG, et al. Electro-acupuncture at acupoint ST36 reduces inflammation and regulates immune activity in Collagen-induced Arthritic Mice. Evid Based Complement Altern Med. 2007;4:51–7. doi: 10.1093/ecam/nel054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JS, Kim YS, Nam SS. The mechanism of immuno modulatory effect by electro-acupuncture in 2-4-dinitrophenylated keyhole limpet protein immunized mice. J Korean Acupuncture and Moxibustion Society. 2005;22:23–35. [Google Scholar]
- 19.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–16. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa T, Kato Y, Nagase H, Atsuta J, Terada A, Iguchi K, et al. Chomokines induce eosinophil degranulation through CCR-3. J Allergy Clin Immunol. 2000;106:507–13. doi: 10.1067/mai.2000.108311. [DOI] [PubMed] [Google Scholar]
- 21.Haas H, Falcone FH, Holland MJ, Schramm G, Haisch K, Gibbs BF, et al. Early interleukin-4: its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]
- 22.Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness-a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ. New directions in allergic diseases: mechanism-based anti-inflammatory therapies. J Allergy Clin Immunol. 2000;106:5–16. doi: 10.1067/mai.2000.107930. [DOI] [PubMed] [Google Scholar]
- 24.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–5. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 25.Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108(Suppl 2):S65–71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- 26.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–90. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–60. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 28.Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278:1815–22. [PubMed] [Google Scholar]
- 29.Okudaira H, Mori A. Simple understanding and optimistic strategy for coping with atopic diseases. IL-5 central hypothesis on eosinophilic inflammation. Int Arch Allergy Immunol. 1998;117:11–9. doi: 10.1159/000023985. [DOI] [PubMed] [Google Scholar]
- 30.Van Wauwe J, Aerts F, Cools M, Deroose F, Freyne E, Goossens J, et al. Identification of R146225 as a novel, orally active inhibitor of interleukin-5 biosynthesis. J Pharmacol Exp Ther. 2000;295:655–61. [PubMed] [Google Scholar]
- 31.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 33.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–75. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]