Abstract
Earlier we identified adenosine monophosphate (AMP) N1-oxide as a unique compound of royal jelly (RJ) that induces neurite outgrowth (neuritegenesis) from cultured rat pheochromocytoma PC12 cells via the adenosine A2A receptor. Now, we found that AMP N1-oxide stimulated the phosphorylation of not only mitogen-activated protein kinase (MAPK) but also that of cAMP/calcium-response element-binding protein (CREB) in a dose-dependent manner. Inhibition of MAPK activation by a MEK inhibitor, PD98059, did not influence the AMP N1-oxide-induced neuritegenesis, whereas that of protein kinase A (PKA) by a selective inhibitor, KT5720, significantly reduced neurite outgrowth. AMP N1-oxide also had the activity of suppressing the growth of PC12 cells, which correlated well with the neurite outgrowth-promoting activity. KT5720 restored the growth of AMP N1-oxide-treated PC12 cells. It is well known that nerve growth factor suppresses proliferation of PC12 cells before causing stimulation of neuronal differentiation. Thus, AMP N1-oxide elicited neuronal differentiation of PC12 cells, as evidenced by generation of neurites, and inhibited cell growth through adenosine A2A receptor-mediated PKA signaling, which may be responsible for characteristic actions of RJ.
Keywords: adenosine A2A receptor, 5-bromodeoxy uridine, neuronal differentiation, neurotrophic factor
Introduction
Royal jelly (RJ), which is fed to the queen honeybee, has been reported to have a variety of biological activities towards various types of cells (1–4). Although there are few reports so far showing the effects of RJ on the nervous system, we recently found that an extract of RJ induces neurites from cultured PC12 cells, a cell line of rat pheochromocytoma, and identified adenosine monophosphate (AMP) N1-oxide as one of the active components (5). AMP N1-oxide is a unique compound not found in natural products other than RJ; and it suppresses the proliferation of PC12 cells and stimulates the expression of neurofilament M, a protein of mature neurons, thus demonstrating that AMP N1-oxide induces neuronal differentiation of PC12 cells (5), as does nerve growth factor (NGF), a well-known neurotrophic factor that affects PC12 cells. In response to the binding of NGF to receptors p75 and TrkA, PC12 cells stop dividing and extend neurites, differentiating into neurons similar to those found in sympathetic neurons (6). NGF triggers mainly two cascades of cellular signaling that mediate neurite outgrowth, neurofilament M expression and cell survival, i.e. mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1 or 2 (ERK1/2) and phosphatidylinositol 3-kinase/Akt pathways (6,7).
The neurite outgrowth-promoting activity of AMP N1-oxide was found to be mediated by adenylate cyclase-coupled adenosine A2A receptors (5). Adenosine A2A receptor activation leads to an increase in the cAMP level followed by activation of protein kinase A (PKA) and extracellular signal-regulated kinase 1 or 2 (ERK1/2) in PC12 cells (8,9). Activation of ERK1/2 is a checkpoint to assess the activation of the Ras/MAPK cascade. Moreover cAMP elevation induces the development of neurites similarly as treatment with NGF (9). Forskolin, an activator of adenylate cyclase, increases the cAMP level, whose increase is in turn followed by neuronal differentiation (10). Also, the rescuing effect of A2A receptor-mediated cAMP/PKA signals was reported in the case of NGF-induced neurite outgrowth from PC12 cells impaired by suppression of the MAPK cascade (11).
Therefore, in this study, we focused on elucidating the intracellular signaling by AMP N1-oxide responsible for stimulation of neurite outgrowth and/or suppression of cell growth. Also, we speculated about the action of AMP N1-oxide in RJ toward the nervous system.
Materials and Methods
Materials
AMP N1-oxide was chemically synthesized (5). The adenosine A2A receptor antagonist ZM241385 and epidermal growth factor (EGF) were purchased from Sigma (St. Louis, MO). The PKA inhibitor KT5720 was purchased from Biomol GmbH (Hamburg, Germany). The MEK inhibitor PD98059 came from Wako, and the Trk inhibitor K252a, from Santa Cruz Biotechnology (Santa Cruz, CA). NGF was purified from mouse submaxillary glands as described previously (12).
Assessments of Neurite Outgrowth and Cell Growth
PC12 cells were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% horse serum and 5% fetal bovine serum (6). For assessment of neurite outgrowth, cells were plated at 104–105 cells/well in six well plates coated with collagen. The cells were exposed to test agents 1 day after plating, and morphological changes were observed under a phase-contrast microscope. Neurite-bearing cells were defined as those with processes longer than the length of the cell body. Four areas, each containing 100–200 cells, were randomly selected in each well, and the neurite-bearing cells were counted. Cell proliferation was evaluated by counting the total cell number, or by incorporation of 5-bromodeoxyuridine (BrdU) (Sigma) as described (13). Cells were cultured on cover glasses coated with poly-l-lysine and collagen in medium containing test samples. The cultures were incubated for another 4 h after the addition of BrdU (10 μM), fixed with 4% paraformaldehyde, and reacted with Alexa Fluor™-conjugated anti-BrdU antibody (Sigma). For estimating total cell number, cell nuclei were stained with propidium iodide (Invitrogen, Carlsbad, CA).
Western Blotting
PC12 cells were lysed with lysis buffer [20 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl, 2 mM EDTA, 1% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 0.1% sodium dodecyl sulfate (SDS) and 1% Na deoxycholate]. The lysates were centrifuged, and the protein concentration of each supernatant was determined with a BCA Protein Assay Kit (Pierce, Rockford, IL). Each sample (5 μg of protein) was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a 10% gel. Proteins were transferred to a polyvinylidene fluoride membrane and blocked with 5% skim milk (Morinaga Milk Products, Tokyo, Japan). Next the membranes were incubated with primary antibody at 4°C overnight, washed and then reacted with alkaline phosphatase-conjugated secondary antibody at room temperature for 1 h (Promega, Madison, WI). Finally, the protein bands were developed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indorylphosphate p-toluidine salt. The primary antibodies used were those against p44/42 MAPK, phospho-p44/42 MAPK, cAMP-response element-binding protein (CREB) and phospho-CREB (Cell Signaling Technology, Danvers, MA).
Immunoprecipitation
AMP N1-oxide or NGF was added to PC12 cell cultures, which were then incubated for 10 min, 1 h or 2 h. The cells were washed with ice-cold PBS, and lysed with the lysis buffer. The cell lysates were incubated overnight at 4°C with anti-Trk antibody (C-14; Santa Cruz Biotechnology), followed by incubation with protein A-Sepharose beads (Amersham Pharmacia Biotech, IL) for 1 h at 4°C. The pellet was then collected, washed with the lysis buffer, and subjected to SDS-PAGE. Phosphorylated TrkA was detected by Western blotting with anti-phosphotyrosine antibody (Santa Cruz Biotechnology).
Results
Correlation Between Antimitotic Activity and Neurite Outgrowth-promoting Activity
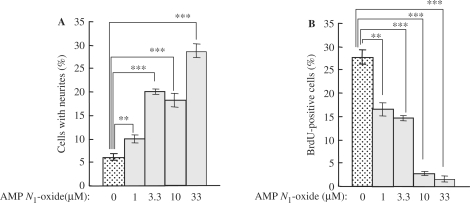
AMP N1-oxide at concentrations between 1 and 33 μM induced neurite outgrowth from PC12 cells in a dose-dependent manner (Fig. 1A) similarly as shown earlier (5). On the other hand, when the antimitotic effect of AMP N1-oxide on PC12 cells was examined by using the BrdU-labeling method, the ratio of BrdU-positive cells to total cells decreased with an increase in the concentration of AMP N1-oxide (Fig. 1B). At the highest concentration tested (33 μM), AMP N1-oxide reduced the ratio of BrdU-positive cells to nearly 2%, but induced the maximal neurite outgrowth. Namely, the rate of cell proliferation was inversely related to the degree of neurite outgrowth. In other words, the antimitotic activity of AMP N1-oxide may be critical for the neurite outgrowth-promoting activity by this molecule.
1.
Reciprocal relationship between neurite outgrowth (A) and cell growth (B) of AMP N1-oxide-treated PC12 cells. PC12 cells were cultured for 1 day in medium containing AMP N1-oxide. A: the ratio of the number of cells with neurite to total cells was calculated. The values are expressed as the mean ± standard error (n = 3). Significant differences from non-treated cells were determined by one-way ANOVA with Tukey's test, ***P < 0.001, **P < 0.01. B: PC12 cells were incubated for 4 h with BrdU (10 μM), fixed, and reacted with Alexa Fluor™-conjugated anti-BrdU antibody. The number of BrdU-positive cells was counted, and the ratio of positive cells to total cells was calculated. The values are expressed as the mean ± standard error (n = 4). Significant differences versus the non-treated control were determined by one-way ANOVA with Tukey's test, ***P < 0.001, **P < 0.01.
Phosphorylation of CREB and MAPK by AMP N1-oxide
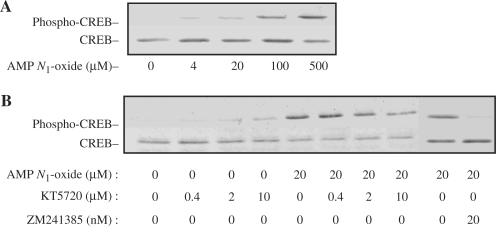
We first examined the phosphorylation of CREB in response to AMP N1-oxide, since we had earlier shown that the actions of AMP N1-oxide were mediated by the adenylcyclase-coupled adenosine receptor A2A (5). As Fig. 2A shows, phosphorylation of CREB was increased dose dependently by AMP N1-oxide up to 500 μM, and diminished by the selective A2A receptor antagonist ZM241385 (Fig. 2B), suggesting that the phosphorylation of CREB was evoked by AMP N1-oxide through A2A receptor-mediated PKA activation.
2.
Phosphorylation of CREB in PC12 cells after treatment with various agents. PC12 cells were cultured for 30 min in medium supplemented with various concentrations of AMP N1-oxide (A) or in medium supplemented or not with 20 μM AMP N1-oxide in the presence or absence of various concentrations of KT5720 or ZM 241385 for 30 min (B). The cell lysates were subjected to PAGE (5 μg protein/lane) and blotted onto membranes for Western blotting with anti-phosphorylated-CREB and anti-CREB antibodies.
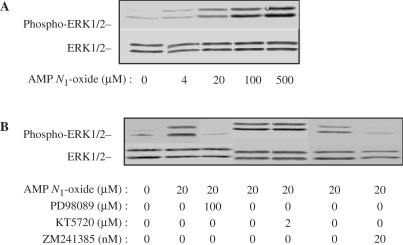
MAPK was also phosphorylated in a dose-dependent manner by AMP N1-oxide from 4 to 500 μM (Fig. 3A). The MEK inhibitor PD98059 or A2A receptor antagonist ZM241385 clearly inhibited the phosphorylation of MAPK, whereas the PKA inhibitor KT5720 did not affect it even when administered at concentrations higher than 2 μM (Fig. 3B), demonstrating that MAPK phosphorylation was evoked through A2A receptor-mediated MEK activation independent of PKA activity.
3.
Phosphorylation of ERK1/2 in PC12 cells after treatment with various agents. The cells were cultured in medium supplemented with various concentrations of AMP N1-oxide for 10 min (A) or in medium supplemented or not with 20 μM AMP N1-oxide in the presence or absence of PD98059, KT5720 or ZM 241385 for 30 min (B). The cell lysates were subjected to PAGE (5 μg protein/lane) and blotted onto membranes for Western blotting with anti-phosphorylated- ERK1/2 and anti-ERK1/2 antibodies.
Involvement of MAPK or PKA Signaling in the Action Mechanism of AMP N1-oxide
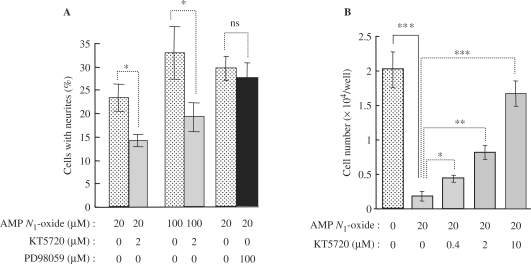
Involvement of MAPK activation in neurite outgrowth was next examined. The MEK inhibitor PD98059 did not inhibit AMP N1-oxide-induced neurite outgrowth (Fig. 4A), suggesting the induced outgrowth to be independent of the MAPK signaling pathway. The suppression of cell growth was not dependent on MAPK, either, since the growth of PC12 cells was also slow when cells were treated with AMP N1-oxide and PD98059 at the same time. However, the PKA inhibitor KT5720 significantly reduced a percentage of the cells with the AMP N1-oxide-induced neurites (Fig. 4A), and simultaneously attenuated the AMP N1-oxide-induced suppression of cell proliferation (Fig. 4B). These observations suggest the participation of PKA signaling not only in the neuritegenic activity of AMP N1-oxide but also in its cell growth-suppressing activity.
4.
Effects of inhibitors of PKA and MAPK on the neurite outgrowth of AMP N1-oxide-treated PC12 cells (A) and on suppression of growth of PC12 cells elicited by AMP N1-oxide (B). The cells were precultured for 30 min in medium containing KT5720 (2 μM) or PD98059 (100 μM). Then, AMP N1-oxide (20 or 100 μM) was added, and the cells were cultured for another 1 day. A: the number of process-bearing cells was counted, and the ratio of them to total cells was calculated. The values are expressed as the mean ± standard error (n = 6). Significant difference from the non-treated cells was determined by one-way ANOVA with Tukey's test, *P < 0.05. ‘ns’ means non-significant relationship. B: the cells were cultured for 4 days in medium containing the indicated concentrations of AMP N1-oxide and KT5720. The number of cells was then counted, and the values expressed as the mean ± standard error (n = 3). Significant differences from the values of the corresponding cells untreated with the inhibitor were determined by one-way ANOVA with Tukey's test, ***P < 0.001, **P < 0.01, *P < 0.05. Significance of differences in values between non-treated control cells and the cells treated with 20 μM AMP N1-oxide was determined by Student's t-test, ***P < 0.001.
Interaction of the Action Mechanism of AMP N1-oxide with TrkA Signal Pathway
Transactivation of mitogenic kinase receptors through G protein-coupled receptors was described earlier (14,15). Namely, adenosine and adenosine receptor agonists were reported to activate Trk receptors through a mechanism that requires the adenosine A2A receptor (16). Therefore, we examined whether TrkA was activated during neurite outgrowth by AMP N1-oxide. However, we could not obtain any positive results indicating activation (data not shown). Furthermore, treatment of cells with a selective TrkA inhibitor, K252a, greatly reduced the phosphorylation level of MAPK of cells incubated with NGF, but the level obtained with AMP N1-oxide was unaffected (data not shown). The neurite outgrowth by AMP N1-oxide was also unaffected by K252a. Thus, TrkA activation seems not to be involved in the response of cells to AMP N1-oxide.
Discussion
Adenosine plays an essential role in modulating neuronal function via adenosine receptors (17). In the central nervous system (CNS), the A2A receptor gene is strongly expressed and is suggested to be involved in the regulation of synaptic plasticity and to play a critical role in early neuronal development (18). In such a context, adenosine derivatives may have various effects on the CNS, and be expected to modulate brain functions.
Here, we demonstrated that MAPK phosphorylation was evoked through A2A receptor-mediated MEK activation in a manner independent of PKA signaling (Fig. 3). Recently, Kim et al. (19) reported that secretin-induced neurite outgrowth of PC12 cells and that the activity was dependent on a cAMP-MAPK pathway. According to them, a PKA inhibitor suppressed the phosphorylation of MAPK, which is inconsistent with our results. MAPK can be phosphorylated in response to cAMP in a manner independent of PKA, whose phosphorylation is mediated by the Rap1-B-Raf pathway (20) or an Src family kinase (21). This pathway might be involved in the mechanism of AMPN1-oxide-evoked MAPK activation.
Integrin signals activate the MAPK signaling pathway, which is required for Mn2+-induced neurite outgrowth of PC12 cells (22). TrkA signals evoked by NGF also cause MEK activation followed by MAPK phosphorylation necessary for neurite outgrowth (23). Thus, it is obvious that both Mn2+- and NGF-induced neurite outgrowth require activation of MAPK. However, AMP N1-oxide-induced neurite outgrowth was independent of the activation of the MAPK signaling pathway (Fig. 4). This mechanism may be supported by A2A signal-mediated activation of CREB, which is known to induce neurite outgrowth without MAPK signaling (24). In this study, we found that the AMP N1-oxide-induced CREB phosphorylation dependently on A2A receptor-mediated PKA signaling (Fig. 2). However, we have not yet had direct evidence whether the CREB phosphorylation is correlated with neurite outgrowth or cell growth suppression elicited by AMP N1-oxide.
Neuronal differentiation is accompanied by suppressed cell proliferation as reported earlier (25). Although the mechanism underlying cell growth suppression by AMP N1-oxide is not known, we showed earlier that activities of both cell growth suppression and neurite outgrowth are mediated by the adenosine A2A receptor (5). Other investigators recently identified translin-associated protein X (TRAX) as a novel protein that interacts with the C-terminal part of the adenosine A2A receptor, and considered TRAX to be an essential molecule for mediating A2A receptor-induced inhibition of cell proliferation (26). It is likely that AMP N1-oxide interacts with TRAX at higher affinity than does AMP. This hypothesis may explain the preferential neurite outgrowth by AMP N1-oxide. Thus, AMP N1-oxide may modulate the activities of various neurotrophic factors through TRAX and A2A receptors to a greater extent than AMP. Therefore, AMP N1-oxide would be expected to have beneficial effects on the CNS when used in combination with other agents such as favorable neurotrophic factors.
AMP N1-oxide is a unique compound not found in natural resources so far other than RJ. Therefore, it may be the molecule responsible for RJ-specific physiological actions on the CNS. Our study provides molecular-based evidence that RJ regulates neuronal functions through A2A receptor signaling enhanced by AMP N1-oxide. We expect neuroprotective effects of AMP N1-oxide on the brain as a potent agonist of A2A receptor, because particular ligands of A2A receptors including selective agonists are reported to protect neurons against kainate-induced excitotoxicity in vivo (27) or to attenuate the A1 receptor-mediated synaptic depression in the CA1 area of the hippocampus in vitro (28). In addition, A2A receptor antagonists can also reduce damage produced by combinations of sub-threshold doses of the endogenous excitotoxin quinolinic acid and free radicals (29). These observations suggest that up or down regulation of A2A receptor-linked signaling pathway appear to be promising for the prevention of neuronal damage (30). As A2A receptors are predominantly expressed in dendrites of medium spiny neurons of the striatum in rats, Cabeza et al. (31) demonstrated that A2A receptor agonist, CGS-21680, activated both ERK1/2 and CREB in the caudate-putamen, suggesting that AMP N1-oxide modulates neuronal signaling in various brain regions including caudate-putamen.
Elucidation of the physiological roles of AMP N1-oxide in brain function is important to develop RJ as an evidence-based complementary and alternative medicine. Taking RJ as a source of AMP N1-oxide may be promising to maintain healthy brain functions.
References
- 1.Oka H, Emori Y, Kobayashi N, Hayashi Y, Nomoto K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int Immunopharmacol. 2001;1:521–32. doi: 10.1016/s1567-5769(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi Y, Kohno K, Inoue S, Koya-Miyata S, Okamoto I, Arai N, et al. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2003;3:1313–24. doi: 10.1016/s1567-5769(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 3.Hidaka S, Okamoto Y, Uchiyama S, Nakatsuma A, Hashimoto K, Ohnishi T, et al. Royal Jelly Prevents Osteoporosis in Rats: beneficial effects in ovariectomy model and in bone tissue culture model. Evid Based Conmplement Alternat Med. 2006;3:339–48. doi: 10.1093/ecam/nel019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K, Isohama Y, Maruyama H, Yamada Y, Narita Y, Ohta S, et al. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid Based Complement Alternat Med. 2007 doi: 10.1093/ecam/nem036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattori N, Nomoto H, Mishima S, Inagaki S, Goto M, Sako M, et al. Identification of AMP N1-oxide in royal jelly as a neurotrophic component for cultured rat pheochromocytoma PC12 cells. Biosci Biotechnol Biochem. 2006;70:897–906. doi: 10.1271/bbb.70.897. [DOI] [PubMed] [Google Scholar]
- 6.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene LA, Kaplan DR. Early events in neurotrophin signaling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–87. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 8.Charles MP, Adamski D, Kholler B, Pelletier L, Berger F, Wion D. Induction of neurite outgrowth in PC12 cells by the bacterial nucleoside N6-methyldeoxyadenosine is mediated through adenosine A2A receptors and via cAMP and MAPK signaling pathways. Biochem Biophys Res Commun. 2003;304:795–800. doi: 10.1016/s0006-291x(03)00666-1. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, Wandosell F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem Int. 2004;44:231–42. doi: 10.1016/s0197-0186(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 10.Piiper A, Dikic MP, Lutz J, Leser B, Kronenberger R, Elez H, et al. Cyclic AMP induces transactivation of the receptors for epidermal growth factor and nerve growth factor, thereby modulating activation of MAP kinase, Akt, and neurite outgrowth in PC12 cells. J Biol Chem. 2002;277:43623–30. doi: 10.1074/jbc.M203926200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng HC, Shih HM, Chern Y. Essential role of cAMP-response element-binding protein activation by A2A adenosine receptors in rescuing the nerve growth factor-induced neurite outgrowth impaired by blockage of the MAPK cascade. J Biol Chem. 2002;277:33930–42. doi: 10.1074/jbc.M201206200. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Kamo I, Furukawa Y, Akazawa S, Sotoyoshi E, Itoh K, et al. A highly sensitive enzyme immunoassay for mouse beta nerve growth factor. J Neurochem. 1983;40:734–44. doi: 10.1111/j.1471-4159.1983.tb08040.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Zhizhin I, Perlman RL, Mangoura D. Prolactin-induced cell proliferation in PC12 cells depends on JNK but not ERK activation. J Biol Chem. 2000;275:23326–32. doi: 10.1074/jbc.M001837200. [DOI] [PubMed] [Google Scholar]
- 14.Richter-Landsberg C, Jastorff B. The role of cAMP in nerve growth factor-promoted neurite outgrowth in PC12 cells. J Cell Biol. 1986;102:821–9. doi: 10.1083/jcb.102.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao YP, Buckley DJ, Buckley AR. Rapid activation of mitogen-activated protein kinase and p21 rats by prolactin and interleukin 2 in rat Nb2 node lymphoma cells. Cell Growth Differ. 1995;6:1235–44. [PubMed] [Google Scholar]
- 16.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–60. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daval JL, Nehlig A, Nicolas F. Physiological and pharmacological properties of adenosine: therapeutic implications. Life Sci. 1991;49:1435–53. doi: 10.1016/0024-3205(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 18.Weaver DR. A2A adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–27. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Yumkham S, Kim SH, Yea K, Shin YC, Ryu SH, et al. Secretin induces neurite outgrowth of PC12 through cAMP-mitogen-activated protein kinase pathway. Exp Mol Med. 2006;38:85–93. doi: 10.1038/emm.2006.10. [DOI] [PubMed] [Google Scholar]
- 20.Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci. 2004;117:6085–94. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- 21.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic billary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–37. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Walowitz JL, Roth JA. Activation of ERK1 and ERK2 is required for manganese-induced neurite outgrowth in rat pheochromocytoma (PC12) cells. J Neurosci Res. 1999;57:847–54. [PubMed] [Google Scholar]
- 23.Piiper A, Dikic I, Lutz MP, Leser J, Kronenberger B, Elez R, et al. Cyclic AMP induces transactivation of the receptors for epidermal growth factor and nerve growth factor, thereby modulating activation of MAP kinase, Akt, and neurite outgrowth in PC12 cells. J Biol Chem. 2002;277:43623–30. doi: 10.1074/jbc.M203926200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Tarone G, Turner DC. Expression of integrin alpha 1 beta 1 is regulated by nerve growth factor and dexamethasone in PC12 cells. Functional consequences for adhesion and neurite outgrowth. J Biol Chem. 1993;268:5557–65. [PubMed] [Google Scholar]
- 25.Erhardt JA, Pittman RN. p21WAF1 induces permanent growth arrest and enhances differentiation, but does not alter apoptosis in PC12 cells. Oncogene. 1998;16:443–51. doi: 10.1038/sj.onc.1201577. [DOI] [PubMed] [Google Scholar]
- 26.Sun CN, Cheng HC, Chou JL, Lee SY, Lin YW, Lai HL, et al. Rescue of p53 blockade by A(2A) adenosine receptor via a novel interacting protein, translin-associated protein X. Mol Pharmacol. 2006;70:454–66. doi: 10.1124/mol.105.021261. [DOI] [PubMed] [Google Scholar]
- 27.Jones PA, Smith RA, Stone TW. Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience. 1998;85:229–37. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- 28.Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;128:1035–44. doi: 10.1038/sj.bjp.0702888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pèzzola A, et al. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–75. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone TW. Purines and neuroprotection. Adv Exp Med Biol. 2002;513:249–80. doi: 10.1007/978-1-4615-0123-7_9. [DOI] [PubMed] [Google Scholar]
- 31.Cabeza de Vaca S, Kannan P, Pan Y, Liang N, Sun Y, Carr KD. The adenosine A2A receptor agonist, CGS-21680, blocks excessive rearing, acquisition of wheel running, and increases nucleus accumbens CREB phosphorylation in chronically food-restricted rats. Brain Res. 2007;1142:100–9. doi: 10.1016/j.brainres.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]