Abstract
A crude acetone extract of the pit of date palm (Phoenix dactylifera L.) was prepared and its antiviral activity evaluated against lytic Pseudomonas phage ATCC 14209-B1, using Pseudomonas aeruginosa ATCC 25668 as the host cell. The antiviral activity of date pits was found to be mediated by binding to the phage, with minimum inhibitory concentration (MIC) of <10 μg ml−1. The decimal reduction time (D-values), the concentration exponent (η) and the phage inactivation kinetics were determined. The date pit extracts show a strong ability to inhibit the infectivity of Pseudomonas phage ATCC 14209-B1 and completely prevented bacterial lysis, which it is hoped will promote research into its potential as a novel antiviral agent against pathogenic human viruses.
Keywords: antiviral, bacteriophage, CAM, MIC, phage, Phoenix dactylifera L, Pseudomonas aeruginosa
Introduction
The pits or seeds of many fruit are used in complementary and alternative medicine (CAM) in an effort to prevent illness, reduces stress, prevent or reduce side effects and symptoms, or control or cure disease. The Mexican people traditionally used crushed apricot pits to treat cancer (https://www.dana-farber.org/can/supportive-care/View.aspx?lang=en&audience=0&doc=CDR0000446369) and also in Russia as early as 1845 (http://www.alternativehealth-uk.com/freeadvice.htm) and recently the patented version called Laetrile a semi-synthetic compound form of amygdalin (found in the pits of many fruits mainly in apricot pits, in raw nuts, and in other plants) is used for cancer treatment in humans worldwide (http://cancerweb.ncl.ac.uk/cancernet/600093.html). On the other hand, the grape seed with antiviral activity (SAA Jassim, unpublished data) has been used as a dietary source of essential fatty acids and tocopherols and as an antioxidant, for liver protective effects, to improve circulation (including conditions such as varicose veins, bruising, swelling, and decreased vision), and to improve skin tone and elasticity (http://www.drugs.com/mtm/grape-seed.html). Whilst, the grapefruit seed extract (GSE) used to treat a major therapeutic breakthrough for patients with chronic parasitic and yeast infections (1). The latter authors also examined the effect of GSE on 770 strains of bacteria and 93 strains of fungus and compared this with 30 effective antibiotics and 18 proven fungicides. GSE was found to perform as well as any and all of the tested agents. Furthermore, the seed oil of both Nigella sativa and Neem has been used traditionally in Asia and Middle East to treat many diseases (2) and several viral diseases (3, http://www.petzlife.co.uk/gse.html). Interestingly a form of pokeweed antiviral protein has been isolated from the seeds of Phytolacca americana (4) while the ribosome-inactivating proteins has been isolated from the seeds of Saponara officinalis L. (soapwort), of Agrostemma githago L. (corn cockle) and of Asparagus officinalis L. (asparagus), and from the latex of Hura crepitans (5). In contrast, the seeds of Momordica charantia, a bitter melon fruit that is widely used as food as well as medicine in Asia, has a particular clinical usefulness similar to MAP30 (Momordica anti-HIV protein; molecular weight: 30 kDa) is believed to have multiple functions that could be beneficial for HIV (3).
Still viral diseases, including emerging and established viruses, are an increasing worldwide health concern. However, the search for antiviral compounds has not been easy and relatively few antiviral drugs are available, while those approved for use often have bad side-effect profiles and are able to cause rapid resistance among targeted viral strains (6). As a consequence, the discovery of new antiviral agents from a variety of sources, including plants, has assumed more urgency than in the past (3). It is essential, therefore, to continue the search for useful and novel natural antiviral agents, which can be expected to prolong the efficacy of drug therapy in subjects infected with HIV. For this reasons the date palm (Phoenix dactylifera L.) is widely used for some traditional medicines (http://en.wikipedia.org/wiki/Phoenix_dactylifera#Traditional_medicinal_uses) in many parts in the Middle East and because it is the main subject in this study, therefore it has been discussed further for their uses in the CAM and antimicrobial activities.
The Phoenix dactylifera L. is a monocotyledoneus woody perennial belonging to the Arecaceae family, which comprises 200 genera and 3000 species (7). The beneficial health and nutrition values of date palm (Phoenix dactylifera L.), for human and animal consumption, have been claimed for centuries (8).
The date pits, also called pips, stones, kernels or seeds, form part of the integral date fruit. Depending on variety and grade quality, the pits represent about 6–12% of the total weight of the mature date (8). The seed powder is also used in some traditional medicines (http://en.wikipedia.org/wiki/Phoenix_dactylifera#Traditional_medicinal_uses) and has been investigated for human potential health benefits (9), and for addition to animal feed to enhance growth (10–13), the latter an action that has been ascribed to an increase in the plasma level of estrogens (14) or testosterone (15). Date pits have been studied as potential sources of edible oils and pharmaceuticals (16). In addition, date pit extract shows an ability to restore the normal functional status of the poisoned liver, and also to protect against subsequent carbon tetrachloride hepatotoxicity on the liver in rats (17).
In terms of dry weight, the chemical composition of date pits has been reported as containing 5–10% moisture, 5–7% protein, 7–10% oil, 10–20% crude fiber, 55–65% carbohydrates and 1–2% ash (8,16). The carbohydrates, as the largest component of the dry weight, are typically comprised of neutral detergent fiber 75%, acid detergent fiber 57.5%, hemicelluloses 17.5%, lignin 11%, cellulose 42.5% and ash 4% (8,9).
Furthermore, the utilization of date pits in the production of citric acid and protein by Candida lipolytica, Apergillus oryzae and Candida utilis were also investigated (respectively, 18–20). However, the antimicrobial activity of date pits has been poorly investigated (9), and only an ethanolic extract of date pits has been used and this only showed a weak antimicrobial activity on several strains of microorganisms (21). The evidence of the date pits that can be used as CAM to treat human viral diseases are based on a hypothesis is discussed in present study.
Materials and Methods
Media
Luria broth (LB): tryptone 10 g l−1 (HiMedia, Mumbai, India), yeast extract 5 g l−1 (HiMedia, Mumbai, India), and sodium chloride 10 g l−1 (HiMedia, Mumbai, India) at pH 7.2 were used in all the protocols. L-agar (LA), consisted of the above with the addition of 14 g l−1 agar (HiMedia, Mumbai, India) was used for culture maintenance. Bacterial dilutions from 18 h LB cultures grown at 37°C were carried out in phosphate buffered saline (PBS, Oxoid, England). For plaque assay, the ‘soft layer agar’ used was LB prepared in Lambda-buffer [6 mmol l−1 Tris pH 7.2, 10 mmol l−1Mg(SO4)2.7H2O, 50 μg ml−1 gelatin (Oxoid, England)], was supplemented with 4 g l−1 agar bacteriology No. 1 (HiMedia, Mumbai, India).
Bacterial Strain
Pseudomonas aeruginosa ATCC 25668 was used throughout the study. Cultures were stored at −20°C in 15% glycerol (22). Prior to investigation a stock culture of the bacteria was maintained on LA plate. One loopful of the P. aeruginosa was inoculated into a 100 ml Erlenmeyer flask containing 10 ml of LB and incubated for 18 h at 37°C and 90 rev min−1 in an incubator shaker (Innova 4000, New Brunswick Scientific). For experimental tests appropriate serial dilutions were made in LB.
Bacteriophage
A lytic Pseudomonas phage ATCC 14209-B1 was used in this study. The phage stocks were prepared on the host strain, P. aeruginosa ATCC 25668, by a plate lysis procedure essentially equivalent to growing bacteriophage Lambda-derived vectors (23). Briefly, the method for the preparation of a large volume of Pseudomonas phage ATCC 14209-B1 by the soft layer plaque technique was as follows: An aliquot (100 µl) of the phage sample (10-fold serially diluted with Lambda-buffer) was mixed with 100 µl of an overnight LB culture of P. aeruginosa ATCC 25668 in a sterile Eppendorf micro-centrifuge tube (polypropylene; 1.5 ml; Sarstedt) and incubated for 15 min at 37°C to facilitate attachment of the phage to the host cells. The mixture was transferred from the Eppendorf micro-centrifuge tube to a 5 ml Bijou bottle and then 2.3 ml of soft layer agar was added which had been melted and cooled to 40°C in a water bath. The contents of each bottle were then well mixed by swirling, poured over the surface of a plate of LA and allowed to set for 15 min at room temperature. The plates were incubated for 18 h at 37°C, and a plate showing almost confluent plaques was used to prepare a concentrated phage suspension by overlaying with 5 ml of Lambda-buffer [titre 1012 plaque-forming units per ml (pfu ml−1)]. The final purification process used chloroform to separate the bacteriophage from the bacterial cells (23). The phage stocks, at a titer of 1012 pfu ml−1, were maintained in Lambda-buffer at 4°C.
Preparation of Plant Extract
Date fruits were obtained from a local date market in Abu Dhabi, United Arab Emirates. The pits were manually separated from the flesh and were rinsed clear of any flesh by water and dried for 18 h in a drying cabinet (Unitemp) at 40°C, and stored at room temperature. The dried date pits of 100 g were crushed and extracted with acetone (1 : 1 ratio, weight to volume) under agitation at room temperature. After 48 h, the extract was then filtered through Whatman No. 2 filter paper (Whatman International Limited, Kent, England) and the filtrate was evaporated to dryness in a drying cabinet (Unitemp) at 40°C, and stored in a desiccator at room temperature. The bioactive date pit extract was found to be stable at room temperature for many months. The yield of the extract was approximately 30% of the weight of the date pits.
Determination of Minimum Inhibitory Concentration (MIC) Values
The MIC of the test date pit extract was determined for test Pseudomonas phage ATCC 14209-B1 as follows: Sterile Eppendorf micro-centrifuge tubes (polypropylene; 1.5 ml; Sarstedt) were used for this purpose. Each tube contained 1 × 104 colony-forming units (CFU) ml−1of Ps. aeruginosa ATCC 25668 cultures, 1.5 × 107 PFU ml−1 of test Pseudomonas phage ATCC 14209-B1, serially diluted date pit extract in LB (1, 10, 100 and 1000 μg ml−1), and respective growth medium (LB). Triplicate samples were performed for each test concentration. The controls included: (1) bacterial host cell growth medium with serially diluted date pit extract; and (2) inoculated phage and bacterial host cell growth medium without the date pit extract. Sample blanks contained uninoculated medium only. All tubes were incubated at 37°C and bacterial growth was estimated spectrophotometrically (OD 600 nm) after 18 h using a Spectrometer (Beckman DU 520). The MIC for test phage was defined as the minimum concentration of test date pit extract that reduced the host cell turbidity by 0.2 absorbance at 600 nm.
Phage Inactivation Assays
The phage inactivation was determined using a previously described protocol (24). One hundred microlitres of Ps. aeruginosa ATCC 25668 culture of OD600 nm = 0.8 was added to 900 ml of LB (control) and LB supplemented with either 1000 or 100 μg ml−1 of date pit extract to give a final concentration of approximately 1 × 106 CFU ml−1 in sterile Eppendorf micro-centrifuge tubes, and incubated on ice for 15 min. The suspensions were centrifuged for 10 min at 5000×g. The supernatant was discarded, each pellet was resuspended in 1 ml of Lambda-buffer, and the initial cell count was determined by measuring cfu on LA (25). Ten microliters of a solution containing 1 × 1011 PFU ml−1 of Pseudomonas phage ATCC 14209-B1 was immediately added to give a final multiplicity of infection of 1 CFU to 3 PFU. The suspension was mixed thoroughly. After 5 min incubation at 37°C, the number of surviving bacteria was evaluated by 10-fold serial dilution in PBS and plating on LA plates. The data were expressed as the CFU ml−1 Ps. aeruginosa ATCC 25668 enumerated after incubation with Pseudomonas phage ATCC 14209-B1 divided by the CFU ml−1 enumerated immediately prior to the addition of phage, multiplied by 100. Each assay was replicated three times.
Determination of Phage and Bacterial Inhibition Kinetics
Assays to determine the phage and bacterial inhibition kinetics described by Stewart et al. (26) were used, modified as follows (in brief): Pseudomonas phage ATCC 14209-B1 (10 μl at 7 × 109 pfu ml−1) or 10 μl of Ps. aeruginosa ATCC 25668 cultures (1 × 109 cfu ml−1) were placed in a sterile Eppendorf micro-centrifuge tube and 100 μl of the date pit extract solution prepared in sterile distilled water (10, 100 and 1000 μg ml−1), or 100 μl distilled water (as the control), were added. The suspensions were mixed thoroughly. After exposure of phage or bacteria for 1, 5, 15, 30 and 60 min at room temperature, the numbers of phage or bacteria surviving the above protocol were measured by enumerating the pfu or cfu counts, respectively. All determinations were performed in triplicates.
The calculation of the decimal reduction time (D-value) and the value of the concentration exponent (η) were calculated, as described by Bloomfield (27).
Statistical Analysis
Experiments were performed in triplicate. Bacteriophage titrations and assays were performed in triplicate. The arithmetic mean ± standard error of the mean (SEM) of control and experimental results were calculated using the Student's t-test. P < 0.05 was considered statistically significant.
Results
MIC
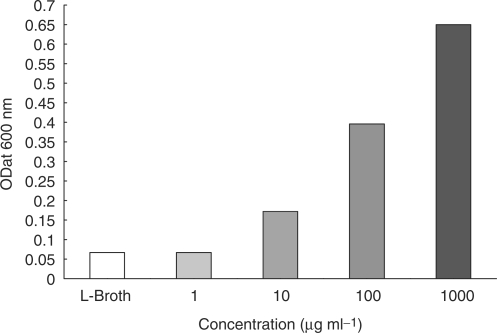
The acetone extract from pits of Phoenix dactylifera L date demonstrated antiviral activity with an MIC value of <10 μg ml−1 for the Pseudomonas phage ATCC 14209-B1 tested (Fig. 1). The MIC activity of date pit extract against the test phage was abolished after heat treatment at 100°C for 5 min (data not shown).
1.
The minimum inhibitory concentrations of date pit extract and LB (‘L-broth’) control against Pseudomonas phage ATCC 14209-B1. A higher number of surviving bacteria reflects the increased inhibitory activity (against the phages) of the higher concentrations of date pit extract.
Inhibitory Activities of Date Pit Extract on Phage Lytic Properties
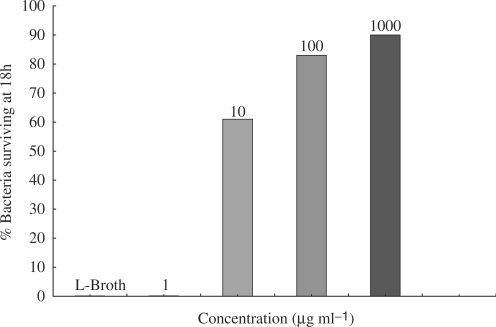
The phage lytic inhibitory properties in LB control and the date pit extract of 10, 100 and 1000 μg m1−1 were evaluated from the date pit MIC assay, a similar pattern was observed: 0, 61, 83 and 90%, respectively (Fig. 2).
2.
Inhibitory effect of date pit extract 1, 10, 100 and 1000 μg ml−1 and L-broth control on the ability of Pseudomonas phage ATCC 14209-B1 to lyse, and thereby eliminate the growth of, Pseudomonas aeruginosa ATCC 25668. Numbers are expressed as the percentage of bacteria remaining after 18 h incubation with Pseudomonas phage ATCC 14209-B1. A higher number of surviving bacteria reflects the increased inhibitory activity (against the phages) of the higher concentrations of date pit extract.
The Kinetics of the Date Pit Extract on the Phage Infectivity
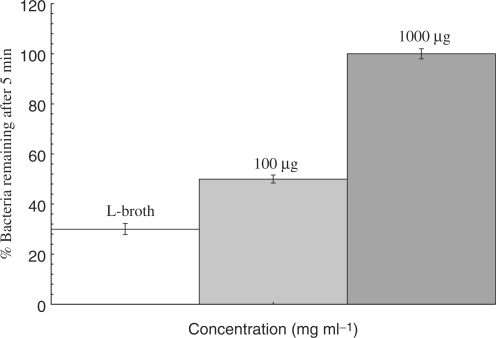
To further understand the nature of this inhibition, a series of experiments was performed to evaluate the kinetics of the date pit extract on the phage infectivity. This assay measured the rate of phage inhibition by determination of the proportion of Ps. aeruginosa which survived exposure to phage ATCC 14209-B1 at a set duration and concentration of date pit extract. Under the conditions used, the mean bacterial survival rate of Ps. aeruginosa which were exposed to LB control, as compared to 100 and 1000 μg ml−1 of date pit extract for 15 min were 30, 50 and 100%, respectively (Fig. 3). The differences in phage-binding activity between samples treated with date pit extract and those treated with control extract were statistically significant (P < 0.001).
3.
Inhibition of Pseudomonas phage ATCC 14209-B1 infectivity to Pseudomonas aeruginosa ATCC 25668 brought about by 100 and 1000 μg ml−1 concentrations of date pit extract, versus L-broth control. Numbers are expressed as the percentage of bacteria remaining after 5 min incubation with Pseudomonas phage ATCC 14209-B1. Error bars indicate SD. A high number of surviving bacteria indicates poor phage infectivity.
Virucidal Activity of Date Pit Extract
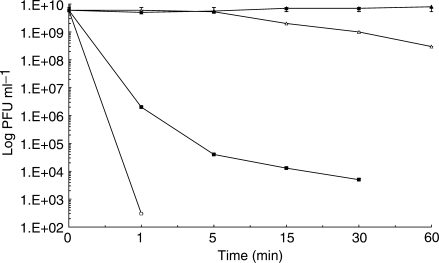
The time course (survivor curves) showing the virucidal action of date pit extract was prepared by enumerating the PFUs, with titers measured at appropriate intervals (Fig. 4). With 1 min of contact, the 1000 μg m1−1 dose of date pit extract brought about an 8-log reduction in phage titers, which effectively eradicated all phage activity by bringing the titers down to the range of the limits of detection. For the next lower dose, that being 100 μg m1−1 of date pit extract, with 1 min of contact an approximate 4 log reduction was achieved, and by 30 min of contact the titers had dropped another 3 logs, for a total 7-log drop in titers.
4.
The effect of contact time and concentration of date pit extract on the viability of Pseudomonas phage ATCC 14209-B1. Date pit extract concentrations (μg ml−1) in L-broth: (filled triangle) 0; (open triangle) 10; (filled squares) 100; (open squares) 1000.
Phage Inhibition Kinetic
The kinetics of inactivation can be described by means of the decimal reduction time (D-value). This is defined as the time taken at a fixed biocide concentration to achieve a 1 log cycle reduction in survivors curve (i.e. its slope) and can be determined from a time vs. log survivors curve such as those in Fig. 4. D-values were determined, for 100 and 1000 μg m1−1 date pit extract, to be 2.36 and 0.25, respectively. From this, the concentration exponent (η) for date pit extract was determined, with an average value of 0.954.
Discussion
This study was primarily designed to evaluate the antiviral efficacy of an acetone extract of the pits of the date palm Phoenix dactylifera L against Pseudomonas phage ATCC 14209-B1 (known to be relatively resistant to disinfection) (28). Bacteriophages have been previously studied to obtain information about mechanisms of virucidal action (28–31).
The study is the first that we know of to reveal that date pit extract has promising antiviral activity, with MIC concentrations found to be >10 μg ml−1 for Pseudomonas phage ATCC 14209-B1 (Fig. 1).
It was also found that the 100 and 1000 μg ml−1 concentrations of date pit extract show a strong ability to inhibit the infectivity of Pseudomonas phage ATCC 14209-B1, as evidenced by the presence of higher numbers of Ps. aeruginosa cells surviving (Fig. 2). The results suggest that the cause of the inhibition could be due to interference with some aspect of the phage's lytic cycle. The lytic cycle of the phage consists of three major phases (26): binding to a suitable host bacterium and injection of its genome; a period of intracellular production of new virions; and then lysis of the cell and release of progeny phage into the environment. To further understand the mechanism by which Ps. aeruginosa cells resisted phage lysis in the presence of date pit extract, the first of these stages, phage binding, was investigated. This effect was shown to be due to a direct effect of the extract on the phage itself rather than an effect on the host cell.
It was found that the ≥100 μg ml−1 of date pit extract strongly inhibited the infectivity of Pseudomonas phage ATCC 14209-B1 to Ps. aeruginosa (Fig. 3), and that this effect was abolished by heating (data not shown). The phage infectivity inhibition may be attributable to date pit extract's heat-labile bioactive component(s) attaching to or modifying the surface of the phages. The bioactive component(s), namely, protein and some derived polyphenolic compounds such as polysaccharides, lignans and bioflavonoids, are present in reasonable amounts in date pit (8,32), which were reported to act principally by binding to the protein coat and thus arrest absorption of the virus (3). However, further studies are needed to investigate the direct effect of date pit protein and polyphenolic compounds on phage binding to host cells, and to determine whether the nucleic acid was damaged inside the phage capsid.
The time course of the survivor curves (Fig. 4) of concentrations of 100 and 1000 μg ml−1 of date pit extract showed respective 4 to 8 log10 reductions in the bacterial CFUs, within 1 min, with D-values of 2.36 and 0.25, respectively. These results were somewhat similar to those reported for extracts of pomegranate (Punica granatum L.) rind, Viburnum plicatum (leaves or flowers), Camellia sinensis (tea leaves) or Acer pseudoplatanus (maple leaves) (26,28). From the above the concentration exponent was derived, with an average value of = 0.954. Similar concentration exponents were reported for formaldehyde, quaternary ammonium and mercury compounds in the range of 1 (27).
The pits of date palm (Phoenix dactylifera L.) are an inexpensive component found in abundance in the Middle East. In this study, date palm pit extract has demonstrated positive antiviral results (this effect was rapid, dose dependent, and appeared to be related to the adsorption stage of the phage replication cycle) and it seems reasonable to conclude that a further phytochemical characterization of the active ingredients may reveal useful compounds, and may also provide the basis for further refinement of antiviral drug design and development as potential biotherapeutic agents against medically-important viruses, such as the human immunodeficiency virus (HIV). Since HIV is an RNA virus it is not subjected to the ‘proofreading’ that occurs during DNA transcription. As a consequence this kind of virus mutates very often. This enables HIV to grow resistant to antiretroviral pharmaceuticals quickly, and is one of the main reasons why an effective vaccine for HIV has not been developed yet (6).
In this regard, date palm pit extract could play an important role in a control replication of HIV-1 this hypothesis has been based from the results of date pit extract which has strongly inhibited the infectivity of the lytic Pseudomonas phage ATCC 14209-B1 to Ps. aeruginosa as a result of novel mechanism of interaction with binding of the phage to the host bacterium and injection of its genome. Therefore, it could conceivably play a role in combination therapy or even as a novel new class of anti-HIV phyto chemo-type therapy for drugs development ‘fusion and attachment inhibitors’ similar to the Enfuvirtide (T-20) in which inhibit or blocks HIV from entering the CD4 cell are important drugs for the treatment of AIDS (6). Thus, further studies are needed to elucidate the mechanism(s) of our findings and, of equal importance, proceed to address further testing with the HIV.
References
- 1.Ionescu G, Kiehl R, Wichmann-Kunz F, Williams CH, Bauml L, Levine S. Oral citrus seed extract in atopic eczema: In vitro and in vivo studies on intestinal microflora. J Orthomolecular Med. 1990;5:155–57. [Google Scholar]
- 2.Puri HS. The Divine Tree. Azadirachta indica. Amsterdam: Harwood Academic Publications; 1999. Neem; pp. 25–91. [Google Scholar]
- 3.Jassim SAA, Naji MA. Review/novel antiviral agents: A medicinal plant perspective. J Appl Microbiol. 2003;95:412–27. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri L, Aron GM, Irvin JD, Stirpe F. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolacca americana L. (pokeweed) Biochem J. 1982;203:55–59. doi: 10.1042/bj2030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stirpe F, Gasperi-Campani A, Barbieri L, Falasca A, Abbondanza A, Stevens WA. Ribosome-inactivating proteins from the seeds of Saponara officinalis L. (soapwort), of Agrostemma githago L. (corn cockle) and of Asparagus officinalis L. (asparagus), and from the latex of Hura crepitans L. (sandbox tree) Biochem J. 1983;216:617–25. doi: 10.1042/bj2160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jassim SAA. Review/novel phyto-anti-HIV drugs: A cause for optimism. Biologist. 2005;52:268–72. [Google Scholar]
- 7.McClintock E. Arecaceae palm family. The Jepson Manual: http://ucjeps.berkeley.edu/cgi-bin/get_JM_treatment.pl?Phoenix+dactylifera (Accessed on October 18, 2007)
- 8.Barreveld WH. Date Palm Products. FAO Agricultural Services Bulletin No. 101. ( http://www.fao.org/docrep/t0681E/t0681e00.htm#con). (Accessed on October 18, 2007)
- 9.Hamada JS, Hashim IB, Sharif FA. Preliminary analysis and potential uses of date pits in foods. Food Chem. 2002;76:135–7. [Google Scholar]
- 10.Afifi M, Abdou F, El-Sayed M. Date stone meal as a substitute for barley in chick rations. Trop Agric. 1966;43:12–7. [Google Scholar]
- 11.Jumah HF, Al-Azzawi II, Al-Hashimi SA. Some nutritional aspects of feeding ground date pits for broilers. Mesopotamia J Agric. 1973;8:139–45. [Google Scholar]
- 12.Kamel BS, Diab MF, Ilian MA, Salman AJ. Nutritional value of whole dates and date pits in broiler rations. Poult Sci. 1981;60:1005–11. [Google Scholar]
- 13.Hussein AS, Alhadrami GA. Effect of enzyme supplementation and diets containing date pits on growth and feed utilization of broiler chicks. Agric Mar Sci. 2003;8:67–71. [Google Scholar]
- 14.Elgasim EA, Alyousif YA, Homeida AM. Possible hormonal activity of date pits and flesh fed to meat animals. Food Chem. 1995;52:149–50. [Google Scholar]
- 15.Ali BH, Bashir AK, Al Hadrami G. Reproductive hormonal status of rats treated with date pits. Food Chem. 1999;66:437–41. [Google Scholar]
- 16.Al-Shahib W, Marshall RJ. Fatty acid content of the seeds from 14 varieties of date palm Phoenix dactylifera L. Int J Food Sci Tech. 2003;38:709–12. [Google Scholar]
- 17.Al-Qarawi AA, Mousa HM, Ali BEH, Abdel-Rahman H, El-Mougy AA. Protective effect of extracts from dates (Phoenix dactylifera L.) on carbon tetrachloride–induced hepatotoxicity in rats. Int J Appl Res Vet Med. 2004;2:176–80. [Google Scholar]
- 18.Abou-Zeid A-ZA, Baghlaf AO, Khan JA, Makhashin SS. Utilization of date seeds and cheese whey in the production of citric acid by Candida Lipolytica. Agric Wastes. 1983;8:131–42. [Google Scholar]
- 19.Ogaidi AL-HK, Khalifa S, Hadi H, Al-Nakash S. Production of protein from date stones by Aspergillus oryzae (Arabic) J Agric Water Resour Res. 1985;4:317–22. [Google Scholar]
- 20.Ogaidi AL-HK, Khalifa S, Hadi H, Al-Nakash S. Utilization of date stone in single cell protein production. Date Palm J. 1988;6:151–9. [Google Scholar]
- 21.Mossa JS, Hifnawy MS, Mekkawi AG. Phytochemical and biological investigations on date seeds (Phoenix dactylifera L.) produced in Saudi Arabia. Arab Gulf J Sci Res. 1986;4:495–507. [Google Scholar]
- 22.Favrin SJ, Jassim SAA, Griffiths MW. Application of a novel immunomagnetic separation-bacteriophage assay for the detection of Salmonella enteritidis and Escherichia coli O157: H7 in food. Int J Food Microbiol. 2003;85:63–71. doi: 10.1016/s0168-1605(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 23.Ausubel FM, Brent R, Kingstone RE, Moore DD, Seidman JG, Smith JA, et al. Current Protocols in Molecular Biology. New York: Wiley Interscience; 1991. Growing lambda-derived vectors; pp. 1.12.1–1.12.3. [Google Scholar]
- 24.Adams MH. Bacteriophages. New York: Inter Science Publisher; 1959. [Google Scholar]
- 25.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg Cambridge. 1938;38:32–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart GSAB, Jassim SAA, Denyer SP, Newby P, Linley K, Dhir VK. The specific and sensitive detection of bacterial pathogens within 4 h using bacteriophage amplification. J Appl Bacteriol. 1998;84:77–783. doi: 10.1046/j.1365-2672.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 27.Bloomfield SF. Methods for antimicrobial activity. In: Denyer SP, Hugo WB, editors. Mechanisms of Action of Chemical Biocides: Their Study and Exploitation, SAB Technical Series 27. Oxford: Blackwell Scientific Publications; 1991. pp. 1–22. [Google Scholar]
- 28.Jassim SAA, Stewart GSAB, Denyer SP. Antiviral and antifungal composition and method. PCT patent WO 95/22254 1995. [Google Scholar]
- 29.McDonnell G, Russell DA. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindemann BF, Klug C, Schwienhorst A. Evolution of bacteriophage in continuous culture: a model system to test antiviral gene therapies for the emergence of phage escape mutants. J Virol. 2002;76:5784–92. doi: 10.1128/JVI.76.11.5784-5792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipson SM, Sethi L, Cohen P, Gordon RE, Tan IP, Burdowski A, et al. Antiviral effects on bacteriophages and rota virus by cranberry juice. Phytomedicine. 2007;14:23–30. doi: 10.1016/j.phymed.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutlak HH, Alywi FF, Maysara MS. Some flavonoid compounds in date seeds. Date Palm J. 1987;5:257–81. [Google Scholar]