Abstract
Uveitis is a sight threatening inflammatory disorder that remains a significant cause of visual loss. We investigated lentiviral gene delivery of IL-1ra or IL-10 to ameliorate murine endotoxin-induced uveitis (EIU). An HIV-1-based vector containing the mIL-1ra or mIL-10 cDNA demonstrated high expression of biologically active cytokine. Following administration of Lenti.GFP into the anterior chamber, transgene expression was observed in corneal endothelial cells, trabecular meshwork and iris cells. To treat EIU, mice were injected with Lenti.IL-1ra, Lenti.IL-10 or a combination of these. EIU was induced 14 days after vector administration and mice were culled 12 h following disease induction. Lenti.IL-1ra or Lenti.IL10 treated eyes showed significantly lower mean inflammatory cell counts in the anterior and posterior chambers compared with controls. The aqueous total protein content was also significantly lower in treated eyes, demonstrating better preservation of the blood-ocular barrier. Furthermore, the treated eyes showed less in vivo fluorescein leakage from inner retinal vessels compared with controls. The combination of both IL-1ra and IL-10 had no additive effect. Thus, lentiviral gene delivery of IL-1ra or IL-10 significantly reduces the severity of experimental uveitis, suggesting that lentiviral mediated expression of immunomodulatory genes in the anterior chamber offers an opportunity to treat uveitis.
Keywords: Gene therapy, uveitis, immunomodulation, lentivirus, IL-10, IL-1ra
Introduction
Uveitis is a sight threatening inflammatory disorder that affects all ages and remains a significant cause of visual loss1, 2, 3. Clinically, chronic progressive or relapsing forms of non-infectious uveitis are treated with topical and/or systemic corticosteroids. However, long-term use of these drugs can result in deleterious ocular and systemic side effects such as glaucoma, cataract, osteoporosis, hypertension and diabetes. Use of alternative steroid-sparing, immunosuppressive agents has also shown clinical benefit, but in themselves carry adverse risks 4. Such alternative approaches include systemic administration of soluble receptors or blocking antibodies as immunosuppressives and potential immunomodulators and experimentally include the use of Th2 cytokines5, 6, 7, 8 However, ocular gene therapy potentially offers advantages over recombinant cytokine or receptor-fusion protein treatment since long-term expression of the therapeutic transgene negates the need for repeated systemic administration or potentially traumatic intraocular injections and administration of expensive recombinant cytokines. In addition, the ocular environment offers an excellent opportunity to study the potential of gene therapy strategies to modulate the immune response. Thus gene therapy, either used alone or as an adjuvant, may provide an effective treatment for blinding ocular inflammatory disease.
Endotoxin-induced uveitis (EIU) in Toll-like receptor-4 (TLR-4) gene-positive mice provides a useful animal model for the more common acute anterior and intermediate uveitis. Lipopolysaccharide (LPS) is a component of gram-negative bacterial cell walls that binds to TLR-4, a pattern recognition receptor of the innate immune system. The local or systemic administration of LPS results in an acute inflammatory response in the anterior and posterior segment of the eye with a breakdown of the blood-aqueous barrier (BAB) and the blood-retinal barrier (BRB) 9, 10. Subsequent inflammation is mediated by activation of the transcription factors, in particular NF-κB, which induces the expression of numerous proinflammatory molecules 11, 12. The pathogenesis of EIU involves elevated expression of pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin 1 (IL-1), interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 2 (MIP-2) 13, 14, 15. MCP-1 and IL-6 were found to be markedly upregulated in the serum and within the eyes of wild-type mice with EIU compared to nearly undetectable levels in healthy controls, while intraocular expression of TNFα, MCP-5, RANTES and interferon-inducible protein 10 (IP10) were also increased in EIU but beneath the limit of detection in healthy controls 16. Other inflammatory mediators such as nitric oxide (NO) and prostaglandin-E2 (PG-E2) are also involved in the pathogenesis of EIU 17, 18.
One of the critical mediators of the inflammatory response in EIU is IL-1. When IL-1α or IL-1β bind to the functionally active IL-1RI receptor, a second chain (IL-1 receptor accessory protein, IL-1R AcP) binds to the IL-1/IL-1RI complex. The cytoplasmic domains of IL-1RI and IL-1R AcP activate signal transduction pathways including NF-κB, JNK/AP-1 and p38 MAPK, initiating the transcription of many proinflammatory genes such as IL-6 and IL-8. The IL-1 inflammatory effects can be inhibited by the IL-1 receptor antagonist (IL-1ra), which is a structural variant of IL-1 that binds the IL-1R with virtually identical avidity as IL-1 but does not activate cells. IL-1ra exists as an intracellular and a secreted form. The secreted form of mouse IL-1ra is a 22-25kDa glycoprotein that is produced by a variety of cell types and competes with IL-1α/β for binding to the IL-1RI. The antagonist activity of IL-1ra comes from its ability to bind IL-1RI but it cannot recruit the IL-1R AcP to IL-1RI, and therefore blocking initiation of signal transduction pathways [16]. The ratio of IL-1 to IL-1ra is about 1 in both health and disease and ≥95% of IL-1RI need to be occupied to abrogate IL-1 activity; therefore, local concentrations of IL-1ra may determine IL-1 activity 19 and high levels of IL-1ra are likely required to be produced in local tissues to inhibit the effects of IL-1. Another important cytokine is interleukin-10 (IL-10) which is produced by several cell types, including macrophages, dendritic cells, monocytes, B- and T-cells (Th2 and Treg cells) and mast cells. It appears to attenuate inflammatory responses by decreasing the expression of MHC and co-stimulatory molecules on antigen presenting cells whilst inhibiting their production of pro-inflammatory cytokines including IL-1, IL-12 and TNFα as well as down-regulating IL-2 production by T-cells 20. Furthermore, IL-10 regulates the proliferation and differentiation of other leukocytes including granulocytes, B- and T-lymphocytes and natural killer cells. IL-10 activates the SOCS-3 gene, which in turn down-regulates the JAK/STAT pathway 21. Many of the functions of IL-10 can be attributed to its inhibitory effect on NF-κB activation. IL-10 inhibits the expression of IL-1β and TNF-α, both of which are implicated in EIU pathogenesis, whilst increasing the expression of anti-inflammatory proteins including IL-1ra and soluble TNF-α receptor 22.
Injection of lentiviral vectors into the anterior chamber of the eye, results in sustained expression of transgene in the anterior segment 23, with reporter gene expression readily detected 6 weeks after vector administration in several different cell types, with expression detected for at least 12 weeks in the corneal endothelium. Anterior chamber administration of a lentiviral vector may therefore enable sufficiently high-level expression of immunomodulatory molecules at the site of inflammation to treat chronic anterior/intermediate uveitis without inducing systemic immunosuppression. In this study, we investigated the effects of anterior chamber lentiviral vector delivery of genes encoding murine IL-1 receptor antagonist (mIL-1ra) and murine IL-10 (mIL-10) on the inflammatory response in a murine EIU model.
Results
Ocular Anterior chamber delivery of Lenti.GFP results in transduction of anterior chamber structures
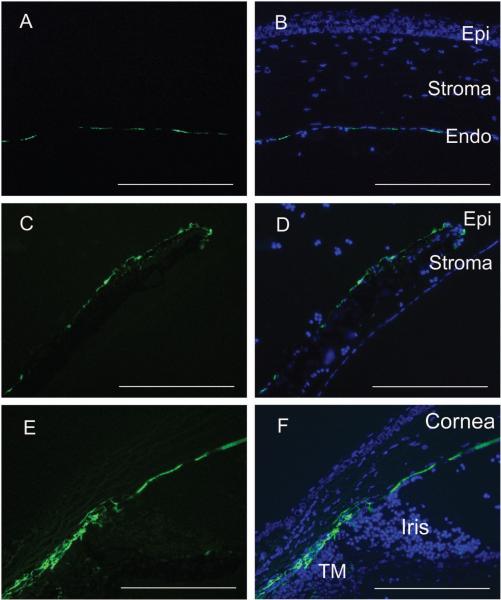
The injection of 4 μl of Lenti.GFP viral suspension into the anterior chamber of the eye produced an efficient and stable transduction of corneal endothelial cells, trabecular meshwork and iris cells (Figure 1). GFP expression could be first detected 4 days after injection and the fluorescence increased over the next 2 weeks. GFP expression was typically observed across the majority of the corneal endothelium cells, and was observed in virtually all of the sections of iris and trabecular meshwork. No infiltrating leukocytes were observed in the anterior or posterior chamber following vector administration, indicating that the Lenti.GFP did not result in a local inflammatory response. The total aqueous protein content in Lenti.GFP injected and normal mice was between 0.2 to 0.6 mg/ml. Furthermore, there was no corneal structure alteration or epithelial corneal oedema in Lenti.GFP injected mice, indicating no endothelial failure (Figure 1).
Figure 1. GFP-expression in the eye 7 days following anterior chamber administration of Lenti-GFP vector.
Representative GFP expression in a mouse that received a single injection of 4 μl of Lenti.GFP. Eyes were enucleated and fixed in 4 % PFA for 1 h before cryosectioning and counterstaining with DAPI. Transgene expression is observed in the corneal endothelium (a-b), iris epithelium (c-d) and trabecular meshwork (e-f), demonstrating that lentiviral vectors are able to transduce several cell and tissue types within the eye. No infiltrating cells were observed in the anterior or posterior chamber in response to vector administration. Scale bars = 200 μm.
Generation of Lenti.mIL-1ra and Lenti.mIL-10 vectors expressing mIL-1ra and mIL-10
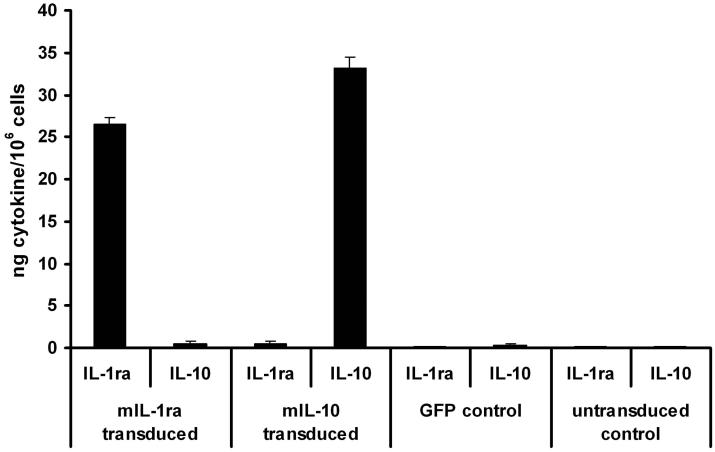
Using a triple transfection method 24, we produced HIV-1-based self-inactivating lentiviral vectors containing the cDNAs for either mIL-1ra or mIL-10 driven by the spleen focus-forming virus (SFFV) promoter and containing a central polypurine tract (cPPT) and Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE). The SFFV promoter was chosen as we have previously shown to drive robust levels of transgene expression within the eye 25, 26 and is not shut off as readily as certain other viral promoters such as the CMV promoter in inflammatory environments 27. These were purified by anion exchange followed by ultracentrifugation and the titre of all vectors was adjusted to 6 × 103 ng p24/ml. To ensure cytokine production by each vector, triplicate wells of 293T cells were transduced in vitro with 1 μl of Lenti.IL-1ra, Lenti.IL-10 or Lenti.GFP. Supernatant was collected 72 h post-infection and cytokines were quantified by ELISA. Lenti.IL-1ra infected cells expressed 27 ± 1 ng/mIL-1ra/106 cells and Lenti.IL-10 infected cells expressed 33 ± 1.4 ng/mIL-10/106 cells (Figure 2a). No mIL-1ra or mIL-10 was detected in supernatants of control Lenti.GFP infected or uninfected control cells.
Figure 2. Schematic representation of lentiviral constructs and determination of cytokine expression and function.
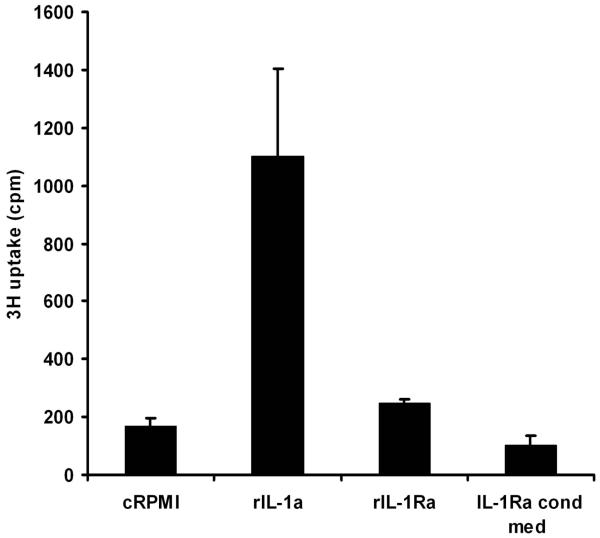
(a) Cytokine production by lentiviral vectors 72 h post-transduction. 293T cells were transduced with Lenti.IL-1ra, Lenti.IL-10 or Lenti.GFP and production of IL-1ra and IL-10 was quantified by ELISA. No IL-1ra or IL-10 expression was observed in control or GFP-transduced cells. (b) Functional assay of vector-produced IL-1ra. The biological function of IL-1ra expressed by the Lenti.IL-1ra vector was tested in vitro for its capability to inhibit the IL-1α-mediated proliferation of mouse thymocytes. Single cell thymocytes suspensions were incubated for 48 h in control media alone, media + 0.5 ng/ml recombinant murine IL-1 α, 0.5 ng/ml recombinant murine IL-1 α + 100 ng/ml recombinant murine IL-1ra, or 0.5 ng/ml recombinant murine IL-1 α + conditioned media from Lenti.IL-1ra-transduced 293T cells. Cells were then pulsed with [3H]-thymidine and proliferation was determined by thymidine uptake. The conditioned media from Lenti.IL-1ra-transduced 293T cells significantly inhibited the IL-1α-dependent proliferation of thymocytes (p = 0.0086) and this inhibition was as effective as recombinant mIL-1ra, indicating the vector produces biologically active IL-1ra.
Biological function of mIL-1ra and mIL-10 produced by lentiviral vectors
In order to verify that the cytokines produced by the transduced cells were functional, biological assays were performed for each cytokine. Murine primary thymocytes proliferate in the presence of IL-1α and this proliferation is inhibited by IL-1ra, which competes with the IL-1 receptor for binding of IL-1α. Single cell suspensions of freshly isolated thymocytes were incubated for 48 h in control RPMI media alone, RPMI + recombinant murine IL-1α, RPMI recombinant murine IL-1α + recombinant murine IL-1ra, or RPMI + recombinant murine IL-1α + conditioned media from Lenti.IL-1ra-transduced 293T cells. Conditioned media from Lenti.IL-1ra-infected 293T cells inhibited the IL-1α-mediated proliferation of murine primary thymocytes as effectively as commercial recombinant mIL-1ra, demonstrating functional efficacy (Figure 2b). Efficacy of the IL-10 cDNA has previously been demonstrated by our group in a functional bioassay 5.
Lenti.IL-1Ra treated eyes were protected from EIU
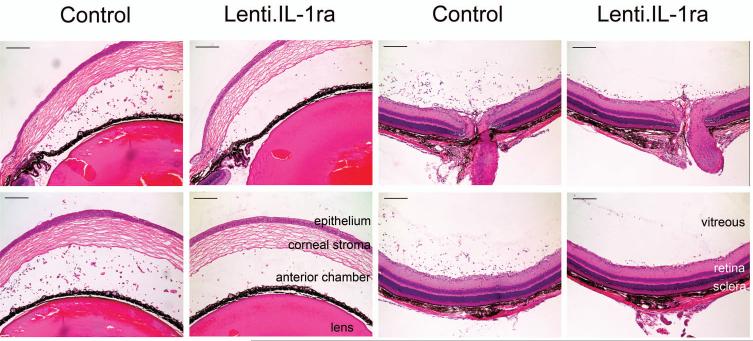
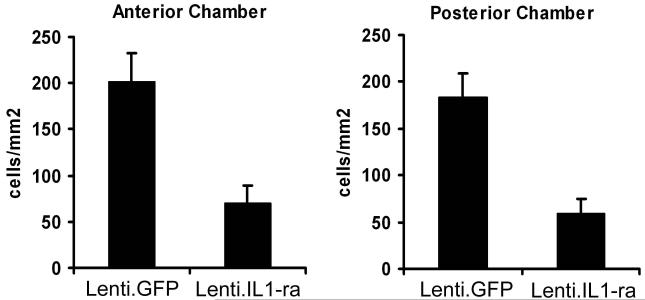
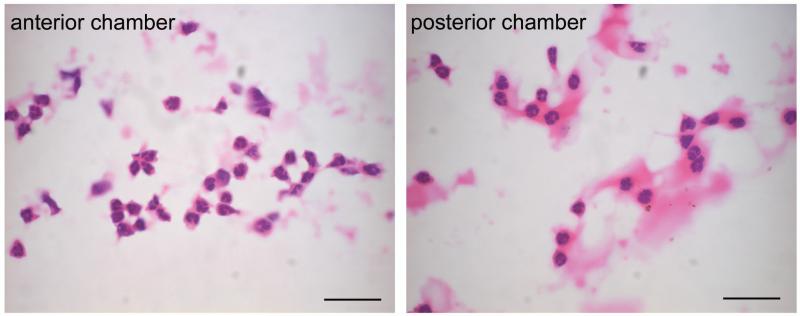
Fourteen days after anterior chamber administration of Lenti.IL-1ra in the right eye, EIU was induced in 8 mice. Twelve hours post EIU induction, when disease was well established, mice were sacrificed, and eyes processed for histological analysis. Sections were analysed by two independent observers and the level of inflammatory cell infiltration in the anterior and posterior eye chamber was quantified. Representative sections are shown in Figure 3a. Paired analysis of Lenti.IL-1ra treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (70.7 ± 18.0 vs. 201.1 ± 31.4 cells/mm2, p = 0.004) and posterior eye segment (59.8 ± 15.7 vs. 183.9 ± 25.3 cells/mm2 p = 0.002) (Figure 3b) and a significantly lower total inflammatory cell count (130.5 ± 32.4 vs. 384.9 ± 46.2, p = 0.0008, data not shown) compared with contralateral Lenti.GFP-injected control eyes, indicating effective local immunosuppression. Eyes injected with Lenti.GFP showed no significant difference in the number of infiltrating cells compared with untreated control EIU eyes (data not shown). In both compartments neutrophils, as determined by cell morphology showing multi-lobed nuclei, were the predominant infiltrating cells with few monocytes/macrophages (Figure 3c). Uninjected normal mouse eyes and LNT-GFP-injected normal mouse eyes (no EIU) showed no evidence of infiltrating cells (data not shown).
Figure 3. Lenti.IL-1ra treated eyes were protected from EIU.
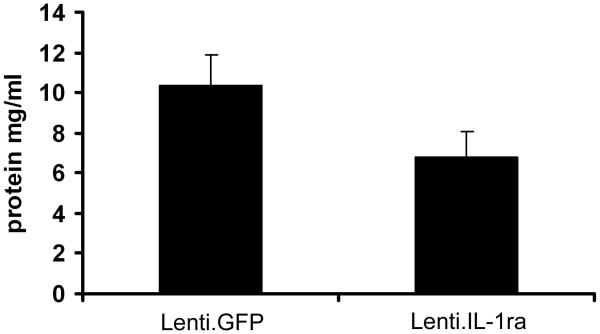
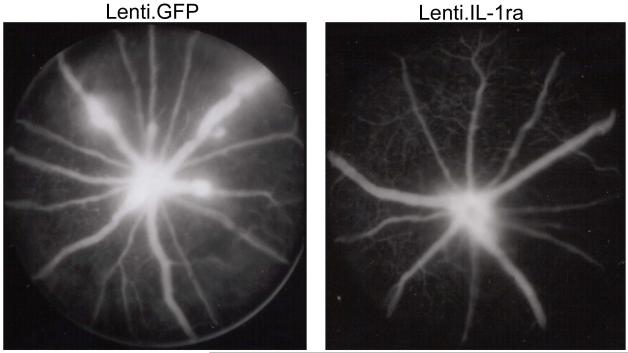
Lenti.IL-1ra was injected into the anterior chamber of the right eye and Lenti.GFP in the left eye of C57Bl/6 mice. Fourteen days after vector administration EIU was induced and mice were culled 12 h following LPS injection (n=8 per group). (a) Eyes were cryosectioned and stained with H&E. Representative sections are shown. Scale bars = 200 μm. (b) Quantification of infiltrating cells in the anterior and posterior chambers. After masking of slides, cells were counted by two independent observers. Lenti.IL-1ra treated eyes contained significantly lower numbers of infiltrating leukocytes in the anterior chamber (70.7 ± 18.0 vs. 201.1 ± 31.4 cells/mm2, p = 0.004) and the posterior eye segment (59.8 ± 15.7 vs. 183.9 ± 25.3 cells/mm2, p = 0.002) compared to Lenti.GFP injected eyes. (c) A representative image of the cells infiltrating the anterior chamber (left panel) and the vitreous (right panel). The main population of infiltrating cells was neutrophils as identified by their multilobed nuclei. Scale bars = 50 μm (d) The total protein content of the aqueous was significantly lower in Lenti.IL-1ra treated eyes compared with Lenti.GFP-injected control eyes (6.77 ± 1.33 vs. 10.37 ± 1.49 mg/ml, p = 0.002), indicating improved preservation of the blood-aqueous barrier. (e) Fluorescence angiography of control eyes and eyes treated with Lenti.IL-1ra 12 h post EIU induction. Images were captured 5 minutes after fluorescein injection. The eyes treated with Lenti.IL-1ra showed less fluorescein leakage, indicating improved preservation of the blood-retinal barrier compared with controls. Representative data is shown.
The total protein content of the aqueous is a function of the integrity of the blood-aqueous barrier (BAB). Acute EIU was induced in 12 mice 14 days after administration of Lenti.IL1ra in the right eye. Twelve hours after LPS injection, paired analysis showed that the total protein concentration of the eyes treated with Lenti.IL-1ra was significantly lower than that of the contralateral Lenti.GFP-injected control eyes (6.77 ± 1.33 vs. 10.37 ± 1.49 mg/ml, p = 0.002), suggesting that the vector mediated expression of IL-1ra prevented the breakdown of the BAB in the treated eyes (Figure 3d). Furthermore, fluorescein angiography showed that there was less leakage of fluorescein from the retinal vessels of treated mice demonstrating substantially less vessel hyperpermeability compared with controls, indicating that there is improved preservation of the blood-retinal barrier (BRB) (Figure 3e).
Lenti.IL-1ra treated eyes were protected from recurrent EIU
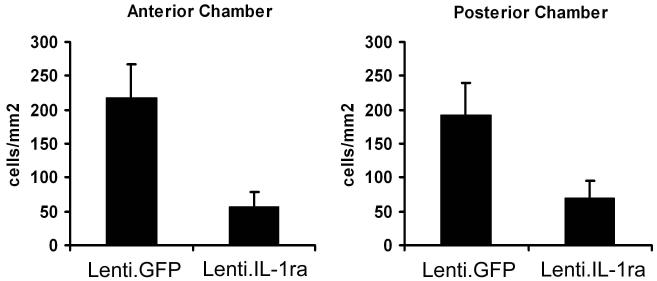
Following administration of Lenti.IL-1ra in the right eye and Lenti.GFP in the left eye of 6 mice, EIU was induced at 14 days post vector administration and then again at 21 days post vector administration. Twelve hours after induction of EIU for the second time, the mice were sacrificed and eyes processed for histology and infiltrating cells counted by two independent observers for the anterior and posterior chamber of each section. Lenti.IL-1ra treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (56.4 ± 22.7 vs. 218.3 ± 48.2 cells/mm2, p = 0.012) and posterior eye segment (68.7 ± 27.0 vs. 192.0 ± 47.3 cells/mm2 p = 0.044) (Figure 4) and a significantly lower total inflammatory cell count (134.9 ± 45.9 vs. 400.5 ± 100.8, p = 0.039, data not shown) when compared with Lenti.GFP-injected contralateral control eyes. This indicates effective local immunosuppression and demonstrates that lentiviral-mediated gene transduction following a single administration of vector can maintain the suppression of inflammation over recurrent episodes of disease. As with the EIU model, the main population of infiltrating cells appeared to be neutrophils. However, in contrast with the results following an initial episode of EIU, Lenti.IL-1ra injected eyes showed no significant difference in the protein concentration of the aqueous compared with Lenti.GFP-injected control eyes (21.02 ± 2.67 vs. 24.56 ± 3.67 mg/ml, p = 0.44, data not shown), suggesting the BAB is not maintained as effectively in the recurrent EIU model.
Figure 4. Lenti.IL-1Ra treated eyes were protected from recurrent EIU.
Following administration of Lenti.IL-1ra in the anterior chamber of the right eye, EIU was induced at 14 days post vector administration and then again at 21 days post vector administration. Twelve hours after the second induction of EIU, the mice were sacrificed and eyes processed for histology (n=6 per group). Eyes were cryosectioned and stained with H&E and after masking of slides, infiltrating cells in the anterior and posterior chambers were counted by two independent observers. Lenti.IL-1ra treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (56.4 ± 22.7 vs. 218.3 ± 48.2 cells/mm2, p = 0.012) and posterior eye segment (68.7 ± 27.0 vs. 192.0 ± 47.3 cells/mm2 p = 0.044) when compared with controls.
Lenti.IL-10 treated eyes were protected from EIU
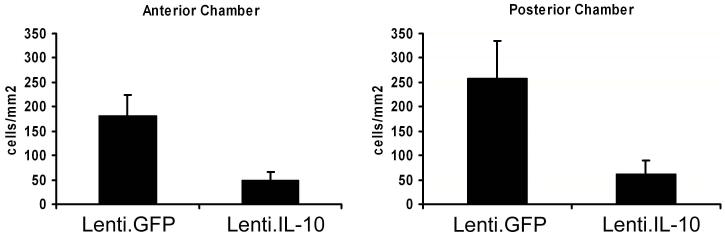
EIU was induced in 8 mice 14 days after administration of Lenti.IL-10 into the anterior chamber of the right eye and the left eye served as a control. Twelve hours post EIU induction mice were sacrificed and eyes processed for histology, stained with H&E and infiltrating cells counted by two independent observers. Paired analysis showed that Lenti.IL-10 treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (48.97 ± 16.70 vs. 181.9 ± 42.42 cells/mm2, p = 0.031) and posterior eye segment (62.44 ± 26.38 vs. 258.5 ± 75.55 cells/mm2 p = 0.047) (Figure 5a) and a significantly lower total inflammatory cell count (111.4 ± 41.3 vs. 440.4 ± 115.5, p = 0.039, data not shown) when compared with contralateral control eyes, indicating effective local immunosuppression. Again, neutrophils were the predominant type of infiltrating cell.
Figure 5. Lenti.IL-10 treated eyes are protected from EIU and partially protected from recurrent EIU.
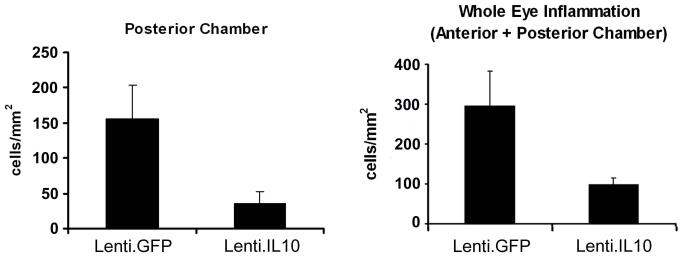
Lenti.IL-10 was injected into the anterior chamber of the right eye of C57Bl/6 mice the left eye served as a control. (a) Fourteen days after vector administration EIU was induced and mice were culled 12 h following LPS injection (n=8 per group). Eyes were cryosectioned and stained with H&E and after masking of slides, infiltrating cells in the anterior and posterior chambers were counted by two independent observers. Lenti.IL-10 treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (48.9 ± 16.7 vs. 181.9 ± 42.4 cells/mm2, p = 0.031) and posterior eye segment (62.4 ± 26.4 vs. 258.5 ± 75.6 cells/mm2 p = 0.047) when compared with control eyes. (b) The effect of IL-10 on recurrent EIU. EIU was induced at 14 days post injection and then again at 21 days post injection. Twelve hours after the second induction of EIU, the mice were sacrificed and eyes processed for histology. Lenti.IL-10 treated eyes showed a lower inflammatory cell count in the posterior eye segment (34.9 ± 17.6 vs. 156.1 ± 48.2 cells/mm2 p = 0.030) and the total inflammatory cell count (98.2 ± 17.2 vs. 295.8 ± 87.2 cells/mm2 p = 0.043) was significantly lower than in control eyes.
The effect of IL-10 on recurrent EIU was assessed in 7 mice. Following administration of Lenti.IL-10 in the right eye, EIU was induced at 14 days post vector administration and then again at 21 days post vector administration. Twelve hours after induction of EIU for the second time, the mice were sacrificed and eyes processed for histology. Paired analysis showed that Lenti.IL-10 treated eyes showed a lower inflammatory cell count in the anterior eye segment, but the difference was not significant (63.29 ± 9.69 vs. 139.70 ± 43.90 cells/mm2, p = 0.1469, data not shown). However, the cell count in the posterior eye segment (34.89 ± 17.58 vs. 156.1 ± 48.13 cells/mm2 p = 0.030) and the total inflammatory cell count (98.18 ± 17.07 vs. 295.8 ± 87.05 cells/mm2 p = 0.043) was significantly lower when compared with contralateral control eyes (Figure 5b).
Combined Lenti.IL-1ra/Lenti.IL-10 treated eyes were protected from EIU
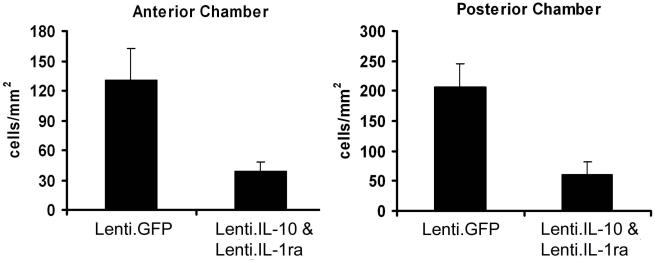
EIU was induced 14 days after vector administration in 9 mice injected with 2μl each of Lenti.IL-1ra and Lenti.IL-10 in the right eye (to give the same total volume as the previous experiments). Mice were sacrificed 12 h post EIU induction. Lenti.IL-1ra and Lenti.IL-10 treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (38.72 ± 9.55 vs. 131.1 ± 31.40 cells/mm2, p = 0.004) and posterior eye segment (61.25 ± 21.21 vs. 206.5 ± 39.77 cells/mm2 p = 0.004) (Figure 6) and a significantly lower total inflammatory cell count (100.5 ± 29.9 vs. 337.6 ± 56.3, p = 0.0004, data not shown) in paired analysis compared with contralateral control eyes, indicating effective local immunosuppression. As with the previous experiments, the infiltrating cells were almost exclusively neutrophils with no detectable monocytes or macrophages. However, there was no additive effect of combined administration of Lenti.IL-1ra/Lenti.IL-10 when compared with the efficacy of either vector administered alone.
Figure 6. Combined Lenti.IL-1ra/Lenti.IL10 protects eyes from EIU but there is no additive effect.
Lenti.IL-1ra + Lenti.IL-10 were injected into the anterior chamber of the right eye of C57Bl/6 mice. Fourteen days after vector administration EIU was induced and mice were culled 12 h following LPS injection (n=9 per group). Eyes were cryosectioned and stained with H&E and after masking of slides, infiltrating cells counted by two independent observers. Lenti.IL-1ra + Lenti.IL-10 treated eyes showed a significantly lower inflammatory cell count in the anterior eye segment (38.7 ± 9.6 vs. 131.1 ± 31.4 cells/mm2, p = 0.004) and posterior eye segment (61.3 ± 21.2 vs. 206.5 ± 39.8 cells/mm2 p = 0.004) when compared with controls. However, there was no additive effect of combined administration of Lenti.IL-1ra/Lenti.IL-10 when compared to the efficacy of either vector administered alone.
Intraocular administration of Lenti.IL-1ra or Lenti.IL-10 does not cause systemic immune suppression
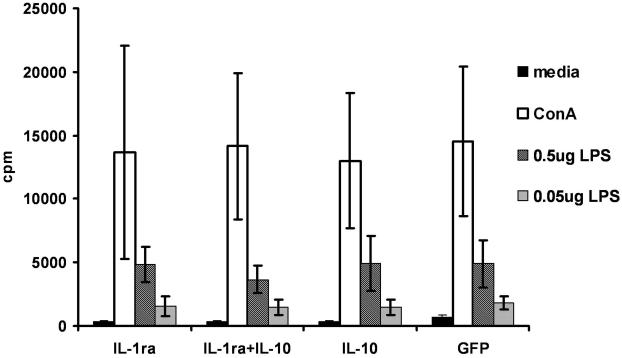
The main purpose of administering local immunosuppressive therapy is to avoid systemic immunosuppression. To test that no systemic immunosuppression was induced following administration of Lenti.IL-1ra or Lenti.IL-10, 4 μl of either vector or Lenti.IL-1ra+Lenti.IL-10 (2 μl of each) were injected into the anterior chamber. Control mice received anterior injection of Lenti.GFP. Mice were sacrificed 14 days later and splenocytes were co-cultured for 48 h with either LPS, (0.5 μg/ml or 0.05 μg/ml) Concanavilin A (positive control) or media alone (negative control). A proliferation assay was then performed. The splenocytes from mice that had received anterior chamber injections of lentiviral vectors expressing IL-1ra or IL-10 showed no difference in their proliferative capacity compared with Lenti.GFP injected mice or unprocedured controls (Figure 7), demonstrating no attenuation of systemic immune responses.
Figure 7. Administration of Lenti.IL-1ra and/or Lenti.IL-10 in the anterior chamber does not induce systemic immunosuppression.
Splenocytes were harvested from mice 14 days after anterior chamber injection of Lenti.IL-1ra, Lenti.IL-10, Lenti.IL-1ra + Lenti.IL-10 or Lenti.GFP and cultured with LPS, Concanavilin A (positive control) or media alone (negative control) for 48 h. A proliferation assay was then performed; cells were then pulsed with [3H]-thymidine for 18 h and harvested onto glass filter mats. Proliferation was determined by thymidine uptake. The splenocytes from mice that had received the cytokine vectors showed equivalent proliferation to the control group that received the GFP vector, demonstrating no reduction in induction of systemic immunity.
Discussion
Murine endotoxin-induced uveitis is employed to assess the efficacy of immunotherapies as a surrogate for anterior and intermediate uveitis in man. We used this model to investigate the therapeutic effects of IL-1ra and IL-10 delivered using a lentiviral vector. We show not only that anterior chamber lentiviral delivery of mIL-1ra or mIL-10 significantly suppress EIU but this inhibition of leukocyte infiltration into the posterior and anterior segments is also maintained in recurrent disease.
Secreted IL-1ra is produced by a variety of cell types and competitively inhibits the binding of IL-1α/β to IL-1RI. IL-1ra bound to IL-1RI cannot induce downstream signalling, thereby inhibiting NF-κB-mediated inflammation. IL-1ra levels are raised in the circulation of patients with a variety of inflammatory conditions and it is likely that IL-1ra diffuses into tissues from the circulation, thereby influencing the local IL-1 to IL-1ra ratio. However, as around 100-fold greater levels of IL-1ra over IL-1 are required to prevent the biological effects of IL-1 on target cells, the local concentration of IL-1ra is critical for its effects. Studies in many inflammatory-mediated conditions have shown the efficacy of IL-1ra treatment. Exogenous administration of IL-1ra blocked colonic inflammation in immune-complex colitis in rabbits and acetic-acid-induced colitis in rats28, 29, 30. Furthermore, in a rat model of granulomatous colitis, treatment with IL-1ra attenuated the acute and chronic enterocolitis 31. Recombinant IL-1ra has also shown efficacy in EIU 32 and has been used clinically to treat uveitis 33.
Previous studies showed the ability of recombinant IL-10 to attenuate inflammatory conditions 34, 35. IL-10 has also been shown to down-regulate ocular inflammation or to contribute to a higher threshold of resistance to uveitis using both recombinant protein 22 or gene transfer 5, 36, 37 and IL-1ra has also demonstrated therapeutic efficacy in inflammatory conditions 19, 38, 39.
While administration of recombinant cytokines can show therapeutic efficacy, many therapeutic regimens require daily administration due to the short half-life of the cytokine molecules and swift clearance from the circulation. Indeed, the serum half-life of recombinant IL-10 is between 2.3-3.7 hours 40, and therefore the cytokine may be cleared before reaching the target organ. Furthermore, extremely high doses may be necessary to reach a therapeutically active concentration of cytokine in the serum, and achieving such high systemic levels can be associated with deleterious side effects. Gene transfer techniques therefore offer several advantages over recombinant cytokine administration, including continuous production of therapeutic concentrations of cytokine and local expression of cytokine in target tissues. In vitro studies have shown that gene transfer of IL-1ra is more effective that recombinant IL-1ra at inhibiting the biological actions of IL-1β in human synovial fibroblast cultures 41. This is also demonstrated with in vivo models; bacterial cell wall induced arthritis in rats showed that locally expressed IL-1ra from ex vivo gene therapy was four-fold more therapeutically efficient than systemically administered recombinant IL-1ra protein 38. An adenoviral vector containing IL-1ra was protective against LPS-induced arthritis in rats 39, while Ad-IL-10 treatment significantly inhibited tissue damage in a rat experimental colitis 42. The protective role of IL-1ra in inflammatory disease is further highlighted by the observation that neutralisation of IL-1ra exacerbates disease in LPS-induced arthritis in rabbits 43.
Our data shows that significantly fewer infiltrating leukocytes were present in the Lenti.IL-1ra treated eyes compared with controls. Intercellular adhesion molecule-1 (ICAM-1) is expressed endogenously on endothelial cells but can be upregulated by many proinflammatory stimuli. It is very important for the adherence to blood vessels walls of leukocytes, particularly neutrophils, prior to their transmigration through the vessel into the surrounding tissues 44. As discussed above, IL-1 activates the NK-κB signalling pathway, and the ICAM-1 promoter has multiple NK-κB binding sites. Thus, IL-1 induces increased ICAM-1 expression 45, 46, which has been shown to be necessary for the development of EIU. During EIU development, ICAM-1 is upregulated on vascular endothelial cells in the iris and ciliary body following LPS injection 47, and EIU pathogenesis can be inhibited by antibodies against ICAM-1 or its receptor (the CD18 integrins) 48. Leukocyte adhesion can lead to leukocyte-mediated endothelial injury and apoptotic cell death. This reduction in vasculature integrity was correlated with an increase of the breakdown of the blood-ocular barrier 49. We also observed reduced levels of total protein in the aqueous humour of eyes treated with Lenti.IL-1ra compared with controls, indicating better preservation of the blood-ocular barrier. Previous work in a murine ischemic brain injury model showed that IL-1ra administration decreased ICAM-1 expression 50 and therefore IL-1ra-mediated down-regulation of ICAM-1 provides a potential functional mechanism for the reduced levels of leukocyte infiltration and improved preservation of the blood-ocular barrier we observe in Lenti.IL-1ra-treated eyes.
In contrast, the Lenti.IL-1ra treated eyes with recurrent EIU showed no preservation of the BAB. An explanation may be a permanent alteration of the integrity of iris blood vessels after the second uveitis episode causing chronic breakdown of the BAB as we can see in patients with recurrent forms of anterior uveitis resulting in a chronic flare (protein exudation into the anterior eye chamber seen with the biomicroscope during clinical examination) that is unresponsive to treatment.
We and others have previously shown the efficacy of IL-10 gene transfer using an adeno-associated virus (AAV) vector in another model of murine uveitis 5, 8, with one study using a tetracycline-inducible system8. Such systems have the advantage of the ability to regulate gene expression through the presence/absence of antibiotics, but we do not believe an inducible system is necessary in this experimental setting, and would not confer any advantage over sustained intraocular expression of IL-1ra and IL-10. A lentiviral vector was used in this study as the onset of transgene expression is faster (within 3-4 days) than the onset of expression with the most frequently used AAV serotypes (about 3 weeks for AAV 51). Furthermore, we have previously shown efficient transduction of several structures within the anterior chamber with a lentiviral vector 23 and this vector transduces a broader range of cell types within the anterior chamber than AAV 52. Furthermore, HIV-1-based lentiviral vectors have been shown to have low immunogenicity and do not induce anti-vector responses in immune privileged tissue 53. The demonstration here that IL-10 gene delivery is effective in preventing EIU and posterior segment disease in recurrent EIU shows that IL-10 gene delivery is effective against pathologies caused by both innate and adaptive immune responses. We did not observe a synergistic or additive effect when the combination of the two cytokines was administered together. Several explanations can be given: firstly, both cytokines reduce the NF-κB activation so synergism is not observed; secondly, there remains, despite IL-1 neutralisation and in the presence of IL-10 a maintained immune drive from other pro-inflammatory cytokines that mediate EIU such as TNF-α 48. Therefore inhibition of TNF-α in combination with IL-1ra and IL-10 administration may further improve outcome in EIU. Thirdly, IL-10 in the anterior chamber when chronically expressed may modulate anterior chamber inflammation, without inducing remission of cellular infiltration as seen in persistent inflammation in subtypes of human anterior uveitis54.
Many types of ocular inflammation including acute and chronic uveitis are treated with topical and/or systemic corticosteroids. However, the detrimental ocular side effects such as glaucoma and systemic side effects such as osteoporosis and hypertension associated with long-term use of these drugs are well documented. The development of alternative anti-inflammatory treatments may therefore allow the use of steroids to be reduced. This study shows that anterior chamber delivery of viral vectors expressing IL-1ra and IL-10 can effectively treat murine experimental uveitis without resulting in systemic immune suppression suggesting that intra-ocular delivery of immunomodulatory genes might offer an opportunity to develop more effective treatments for uveitis.
Material and methods
Animals
Female 4-6 weeks old C57BL/6 mice (Harlan, UK) were used and all experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. At the end of the experiments, mice were euthanased by an overdose of pentobarbital.
Viral vectors
Second generation HIV-1-based self-inactivating lentiviral vectors pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G) envelope contained either the murine IL-1ra cDNA (Lenti.IL-1ra), the murine IL-10 cDNA (Lenti.IL-10) or the GFP reporter gene (Lenti.GFP). All vectors contain deletions in the 3′LTR, making them self-inactivating and include the central polypurine tract (cPPT), which is necessary for second strand DNA synthesis, and may enhance the nuclear transport of the provirus. All cDNAs are driven by the spleen focus forming virus (SFFV) promoter each vector contains the Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) to stabilise mRNA and increase expression. Vectors were produced using transient triple transfection of 293T cells as described previously 24. 28-72 h post-transfection supernatant was harvested and filtered through a 0.45 μm filter. The virus particles concentrated using a Resource Q anionic exchange column (Amersham, UK) and eluted with a NaCl gradient. The eluted fractions were spun in an ultra centrifuge at 90,000 x g for 2 h and the pelleted virus was resuspended in OptiMEM and stored at −80°C. Virus was titred using a p24 ELISA kit (Beckman Coulter) according to the manufacturer’s instructions.
Production and Biological Function of Transgenic mIL-1ra and mIL-10
Expression and function of the IL-1ra and IL-10 transgenes were tested by infecting 293T cells with Lenti.mIL-1ra and Lenti.mIL-10. 1 μl of virus with a titre of 6 × 106 pg p24/ml was added to 5 × 104 293T cells. Supernatants were collected after 72 h and the concentration of mIL-1ra and mIL-10 was determined by ELISA (mIL-10 Duoset and mIL-1ra Quantikine, both R&D Systems, UK), as per manufacturer’s instructions.
The biological function of IL-1ra was also determined by an in vitro assay 55. Thymocytes were isolated and brought to single cell suspension by mechanical disruption from a 4 week old mouse thymus. Thymocytes were then resuspended in complete medium and 5 × 105 cells per well added to 96-well round bottomed plate. Triplicate test samples of pure media, media containing recombinant murine IL-1α (0.5 ng/ml R&D Systems) with or without recombinant murine IL-1ra (100 ng/ml, R&D Systems) were added to wells. Conditioned media from Lenti.IL-1ra infected 293T cells was added to further wells in triplicate. Media containing recombinant mIL-1α and recombinant mIL-1ra was preincubated for 1 h prior to addition to thymocytes. 0.5 μCi of [3H]-thymidine was added to each well after 48 h and cells were harvested onto glass filter paper after a further 18 h. Viability was determined by Trypan blue exclusion. Thymocyte proliferation was determined by [3H]-thymidine uptake by reading on a Perkin Elmer β-scintillation counter. The biological function of the mIL-10 cDNA has been previously demonstrated 5.
In vivo experiments
All experiments were repeated at least twice
Induction of EIU
Uveitis was induced by intravitreal injection of 2 μl PBS containing 1 ng of LPS from Salmonella typhimurium (Sigma, UK). The mice were culled 12 h after the LPS challenge.
Recurrent EIU
To simulate recurrent relapsing uveitis, a common feature of chronic uveitis in humans, mice received a second intravitreal LPS injection (1 ng LPS) 7 days after the first LPS challenge. Preliminary experiments showed a peak inflammatory response after the first LPS injection at 9 to 12 h, which resolved by day 7. A second intravitreal LPS injection at day 7 induced a new inflammatory response with the same kinetics as the first injection. In the recurrent EIU experiments, inflammation was confirmed by 24 h after the first LPS injection. The mice were culled 12 h after the second injection 10.
Histological Assessment of Disease
Enucleated eyes were fixed in 4% paraformaldehyde for 20 mins at room temperature. Samples were embedded in Tissue-Tek (Sakura Finetek Europe, The Netherlands) optimal cutting temperature (OCT) compound and cryosectioned into 8-10 μm vertical serial sections through the papillary-optic nerve axis and then stained with haematoxylin and eosin. Sections were coded and the number of inflammatory cells in the anterior and posterior segment were counted by two independent observers. Four histological sections per eye were evaluated. The inflammatory cells were counted under 400x magnification (40x objective and 10x ocular) in five fields, each of the anterior and posterior chambers (each field corresponds to 0.2 mm2, verified with the grid of a Neubauer heamocytometer). The number of inflammatory cells was expressed as the mean cell number / mm2 per eye segment.
Protein Concentration in Aqueous
Aqueous was collected by anterior chamber puncture with a 30-gauge needle and a 10 μl microcapillary pipette (Microcaps, Sigma, UK) 12 h after LPS injection. Total protein concentration was determined with a Coomassie blue protein assay kit (Bradford Protein assay, Biorad, UK) and the absorbance at 595 nm was measured with a microplate reader.
Intraocular Injection of Viral Vectors into the Anterior Chamber
For all experiments mice were anesthetized with an intraperitoneal injection of 0.15 ml of a mixture of Domitor (medetomidine hydrochloride 1 mg/ml, Pfizer Pharmaceuticals, Kent, UK) and ketamine (100 mg/ml, Fort Dodge Animal Health, Southampton, UK) mixed with sterile water for injections in the ratio 5:3:42. The pupils of all animals were dilated using topical 1% tropicamide and 2.5% phenylephrine (Chauvin Pharmaceuticals, Essex, UK). Viral vector injections into the anterior eye chamber were performed through the peripheral cornea under the direct control of a surgical microscope with the tip of a 10 mm 34-gauge hypodermic needle mounted on a 5 μl syringe (Hamilton AG, Bonaduz, Switzerland). During the paracentesis a certain amount of aqueous humour outflow could be observed. 4 μl of vector suspension (Lenti.IL-1ra, Lenti.IL-10 or Lenti.IL-1ra and Lenti.IL-10 combined) was then injected into the anterior eye chamber of the right eye and the needle was left in place for 20 seconds to reduce a possible backflow of the viral suspension. The contralateral left eyes were injected with 4 μl of Lenti.GFP or sterile PBS as internal controls. After vector administration patent retinal vasculature was observed in all animals. Anaesthesia was reversed using 0.15 ml of Antisedan (atipamezole hydrochloride 0.10 mg/ml, Pfizer, Kent, UK) and all animals received chloramphenicol 1% eye ointment to the cornea.
Fluorescein angiography
In vivo fundus fluorescein angiography was performed 12 h after EIU induction. Mice were anaesthetised and pupils dilated as described above. Fluorescein (0.2 ml of 2% fluorescein sodium in water) was administered by intraperitoneal injection 5 minutes after administration of the anaesthetic. A Kowa Genesis small animal fundus camera equipped with appropriate excitation and barrier filters was used to obtain fluorescein angiograms. Images were captured 5 minutes after fluorescein injection.
Assessment of systemic immunosuppression
Mice were received anterior chamber injections of 4 μl Lenti.IL-1ra, Lenti.IL-10, Lenti.IL-1ra+Lenti.IL-10 or Lenti.GFP as described above. Fourteen days post-injection mice were euthanased and single cell suspensions of splenocytes were prepared. Cells were cultured for 48 hours in 96-well round bottomed plates (2.5 × 105 cells per well) in the presence of 2.5 μg/ml Concanavilin A (Sigma, Poole, UK), 0.5 μg LPS, 0.05 μg LPS or media alone. Cells were then pulsed with 0.5 μCi of [3H]-thymidine and harvested onto glass fibre mats 18 hours later. Cell proliferation was measured using a Perkin Elmer β-scintillation counter.
Statistical Analysis
All data is shown as mean ± standard error of the means (SEM). To analyse the distribution of the data sets the Shapiro-Wilk normality test and D’Agostino and Pearson omnibus normality test were used. Comparisons between normal distributed paired samples (Lenti.IL-1ra, Lenti.IL-10, Lenti.IL-1ra/Lenti.IL-10 and controls in EIU and recurrent EIU) were performed using a two-tailed paired t-test. To test for a possible additive effect of the combined Lenti.IL-1ra/Lenti.IL-10 group over the Lenti.IL-1ra or the Lenti.IL-10 groups, a parametric one-way analysis for variance with the Tukey’s multiple comparison test was used. P<0.05 was considered to be statistically significant. All statistical evaluations were conducted using the GraphPad Prism version 4.03 software (GraphPad Software, San Diego, USA).
Acknowledgments
This work was supported by The Sir Jules Thorn Charitable Trust, Moorfields Eye Hospital Special Trustees, the UK Department of Health and the Department of Health’s National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital and the UCL Institute for Ophthalmology. Personal support was provided to PT by the Swiss National Science Foundation, the Alfred A. Vogt Foundation and the Swiss Fund for Preventing and Fight of the Blindness.
References
- 1.Reeves SW, Sloan FA, Lee PP, Jaffe GJ. Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006;113:307. doi: 10.1016/j.ophtha.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003;135:676–680. doi: 10.1016/s0002-9394(02)02148-7. [DOI] [PubMed] [Google Scholar]
- 3.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Imrie FR, Dick AD. Nonsteroidal drugs for the treatment of noninfectious posterior and intermediate uveitis. Curr Opin Ophthalmol. 2007;18:212–219. doi: 10.1097/ICU.0b013e3281107fef. [DOI] [PubMed] [Google Scholar]
- 5.Broderick CA, Smith AJ, Balaggan KS, Georgiadis A, Buch PK, Trittibach PC, et al. Local administration of an adeno-associated viral vector expressing IL-10 reduces monocyte infiltration and subsequent photoreceptor damage during experimental autoimmune uveitis. Mol Ther. 2005;12:369–373. doi: 10.1016/j.ymthe.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Robertson M, Liversidge J, Forrester JV, Dick AD. Neutralizing tumor necrosis factor-alpha activity suppresses activation of infiltrating macrophages in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44:3034–3041. doi: 10.1167/iovs.02-1156. [DOI] [PubMed] [Google Scholar]
- 7.Tynjala P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:548–550. doi: 10.1136/ard.2006.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JR, Verwaerde C, Rolling F, Naud MC, Delanoye A, Thillaye-Goldenberg B, et al. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum Gene Ther. 2005;16:1037–1046. doi: 10.1089/hum.2005.16.1037. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 10.Forrester JV, Worgul BV, Merriam GR., Jr. Endotoxin-induced uveitis in the rat. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213:221–233. doi: 10.1007/BF00417543. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 12.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 13.de Vos AF, Klaren VN, Kijlstra A. Expression of multiple cytokines and IL-1RA in the uvea and retina during endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994;35:3873–3883. [PubMed] [Google Scholar]
- 14.Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994;35:924–930. [PubMed] [Google Scholar]
- 15.Shen DF, Buggage RR, Eng HC, Chan CC. Cytokine gene expression in different strains of mice with endotoxin-induced uveitis (EIU) Ocul Immunol Inflamm. 2000;8:221–225. doi: 10.1076/ocii.8.4.221.6461. [DOI] [PubMed] [Google Scholar]
- 16.Tuaillon N, Shen DF, Berger RB, Lu B, Rollins BJ, Chan CC. MCP-1 expression in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2002;43:1493–1498. [PubMed] [Google Scholar]
- 17.Jacquemin E, De, Kozak Y, Thillaye B, Courtois Y, Goureau O. Expression of inducible nitric oxide synthase in the eye from endotoxin-induced uveitis rats. Invest Ophthalmol Vis Sci. 1996;37:1187–1196. [PubMed] [Google Scholar]
- 18.Bellot JL, Palmero M, Garcia-Cabanes C, Espi R, Hariton C, Orts A. Additive effect of nitric oxide and prostaglandin-E2 synthesis inhibitors in endotoxin-induced uveitis in the rabbit. Inflamm Res. 1996;45:203–208. doi: 10.1007/BF02285162. [DOI] [PubMed] [Google Scholar]
- 19.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 20.Hill N, Sarvetnick N. Cytokines: promoters and dampeners of autoimmunity. Curr Opin Immunol. 2002;14:791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- 21.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–16521. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi S, Guex-Crosier Y, Delvaux A, Velu T, Roberge FG. Interleukin 10 inhibits inflammatory cells infiltration in endotoxin-induced uveitis. Graefes Arch Clin Exp Ophthalmol. 1996;234:633–636. doi: 10.1007/BF00185297. [DOI] [PubMed] [Google Scholar]
- 23.Bainbridge JW, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher AJ, et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–1668. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- 24.Demaison C, Brouns G, Blundell MP, Goldman JP, Levinsky RJ, Grez M, et al. A defined window for efficient gene marking of severe combined immunodeficient-repopulating cells using a gibbon ape leukemia virus-pseudotyped retroviral vector. Hum Gene Ther. 2000;11:91–100. doi: 10.1089/10430340050016184. [DOI] [PubMed] [Google Scholar]
- 25.Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JW, et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- 26.Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 27.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 28.Cominelli F, Nast CC, Clark BD, Schindler R, Lierena R, Eysselein VE, et al. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86:972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cominelli F, Nast CC, Duchini A, Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992;103:65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- 30.Thomas TK, Will PC, Srivastava A, Wilson CL, Harbison M, Little J, et al. Evaluation of an interleukin-1 receptor antagonist in the rat acetic acid-induced colitis model. Agents Actions. 1991;34:187–190. doi: 10.1007/BF01993274. [DOI] [PubMed] [Google Scholar]
- 31.McCall RD, Haskill S, Zimmermann EM, Lund PK, Thompson RC, Sartor RB. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994;106:960–972. doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 32.Lim WK, Fujimoto C, Ursea R, Mahesh SP, Silver P, Chan CC, et al. Suppression of immune-mediated ocular inflammation in mice by interleukin 1 receptor antagonist administration. Arch Ophthalmol. 2005;123:957–963. doi: 10.1001/archopht.123.7.957. [DOI] [PubMed] [Google Scholar]
- 33.Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91:263–264. doi: 10.1136/bjo.2006.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grool TA, van, Dullemen H, Meenan J, Koster F, ten Kate FJ, Lebeaut A, et al. Anti-inflammatory effect of interleukin-10 in rabbit immune complex-induced colitis. Scand J Gastroenterol. 1998;33:754–758. doi: 10.1080/00365529850171710. [DOI] [PubMed] [Google Scholar]
- 35.Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160:5213–5220. [PubMed] [Google Scholar]
- 36.Fang IM, Lin CP, Yang CH, Chiang BL, Yang CM, Chau LY, et al. Inhibition of experimental autoimmune anterior uveitis by adenovirus-mediated transfer of the interleukin-10 gene. J Ocul Pharmacol Ther. 2005;21:420–428. doi: 10.1089/jop.2005.21.420. [DOI] [PubMed] [Google Scholar]
- 37.De, Kozak Y, Thillaye-Goldenberg B, Naud MC, Da Costa AV, Auriault C, Verwaerde C. Inhibition of experimental autoimmune uveoretinitis by systemic and subconjunctival adenovirus-mediated transfer of the viral IL-10 gene. Clin Exp Immunol. 2002;130:212–223. doi: 10.1046/j.1365-2249.2002.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makarov SS, Olsen JC, Johnston WN, Anderle SK, Brown RR, Baldwin AS, Jr., et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc Natl Acad Sci U S A. 1996;93:402–406. doi: 10.1073/pnas.93.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43:289–297. doi: 10.1002/1529-0131(200002)43:2<289::AID-ANR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Huhn RD, Radwanski E, O’Connell SM, Sturgill MG, Clarke L, Cody RP, et al. Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood. 1996;87:699–705. [PubMed] [Google Scholar]
- 41.Gouze JN, Gouze E, Palmer GD, Liew VS, Pascher A, Betz OB, et al. A comparative study of the inhibitory effects of interleukin-1 receptor antagonist following administration as a recombinant protein or by gene transfer. Arthritis Res Ther. 2003;5:R301–R309. doi: 10.1186/ar795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbara G, Xing Z, Hogaboam CM, Gauldie J, Collins SM. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344–349. doi: 10.1136/gut.46.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukumoto T, Matsukawa A, Ohkawara S, Takagi K, Yoshinaga M. Administration of neutralizing antibody against rabbit IL-1 receptor antagonist exacerbates lipopolysaccharide-induced arthritis in rabbits. Inflamm Res. 1996;45:479–485. doi: 10.1007/BF02252320. [DOI] [PubMed] [Google Scholar]
- 44.Seely AJ, Pascual JL, Christou NV. Science review: Cell membrane expression (connectivity) regulates neutrophil delivery, function and clearance. Crit Care. 2003;7:291–307. doi: 10.1186/cc1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda M, Ikeda U, Shimada K, Minota S, Kano S. Regulation of ICAM-1 expression by inflammatory cytokines in rat mesangial cells. Cytokine. 1996;8:109–114. doi: 10.1006/cyto.1996.0015. [DOI] [PubMed] [Google Scholar]
- 46.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- 47.Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- 48.Whitcup SM, DeBarge LR, Rosen H, Nussenblatt RB, Chan CC. Monoclonal antibody against CD11b/CD18 inhibits endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1993;34:673–681. [PubMed] [Google Scholar]
- 49.Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, et al. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- 50.Yang GY, Mao Y, Zhou LF, Gong C, Ge HL, Betz AL. Expression of intercellular adhesion molecule 1 (ICAM-1) is reduced in permanent focal cerebral ischemic mouse brain using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist. Brain Res Mol Brain Res. 1999;65:143–150. doi: 10.1016/s0169-328x(98)00335-0. [DOI] [PubMed] [Google Scholar]
- 51.Sarra GM, Stephens C, Schlichtenbrede FC, Bainbridge JW, Thrasher AJ, Luthert PJ, et al. Kinetics of transgene expression in mouse retina following sub-retinal injection of recombinant adeno-associated virus. Vision Res. 2002;42:541–549. doi: 10.1016/s0042-6989(01)00230-9. [DOI] [PubMed] [Google Scholar]
- 52.Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE. Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9:804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- 53.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muhaya M, Calder VL, Towler HM, Jolly G, McLauchlan M, Lightman S. Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol. 1999;116:410–414. doi: 10.1046/j.1365-2249.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Symons JA, Dickens EM, Di Giovine F, Duff GW. Measurement of Interleukin-1 activity. 1987:270–272. [Google Scholar]