Abstract
There is ample scientific and empirical evidence supporting the use of plant-derived antioxidants for the control of neurodegenerative disorders. Antioxidants may have neuroprotective (preventing apoptosis) and neuroregenerative roles, by reducing or reversing cellular damage and by slowing progression of neuronal cell loss. Although demand for phytotherapeutic agents is growing, there is need for their scientific validation before plant-derived extracts gain wider acceptance and use. We have evaluated antioxidant potential of Peltophorum africanum (weeping wattle), a plant widespread in the tropics and traditionally used, inter alia, for the relief of acute and chronic pain, anxiety and depression. The dried leaves, bark and root of P. africanum were extracted with acetone. Thin layer chromatograms were sprayed with 0.2% 2,2-diphenyl-1-picryl hydrazyl (DPPH) in methanol for screening for antioxidants. Quantification of antioxidant activity was assessed against 6-hydroxy-2, 5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) and L-ascorbic acid (both standard antioxidants), using two free radicals, 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) and DPPH, respectively. Results of our study show that the bark and root extracts had higher antioxidant activity than L-ascorbic acid and Trolox, a synthetic vitamin-E analogue. The respective TEAC (Trolox Equivalent Antioxidant Capacity) values for the bark and root extracts, and Trolox were 1.08, 1.28 and 1.0. EC50 values for L-ascorbic acid (5.04 µg/mL) was more active than the leaf 6.54 (µg/mL), but much less active than the bark (4.37 µg/mL) and root (3.82 µg/mL) extracts. Continued work on P. africanum, and other plants rich in antioxidants, may avail neuroscientists with potent neuroprotective antioxidant therapeutics.
Keywords: Antioxidant, Extracts, Neurodegeneration, Neuroprotection, Oxidative stress, Peltophorum africanum
Introduction
Oxidative stress is the result of an imbalance in the pro-oxidant/antioxidant homeostasis leading to the generation of excess reactive oxygen species (ROS), implicated inter alia in the cause of carcinogenetic, inflammatory, infectious, cardiovascular and neurological diseases in man and animals (Nair et al., 2003). Under normal conditions, the body is equipped with defense mechanisms that scavenge ROS and protect the cells from oxidative damage. However, the detoxifying enzyme processes get overwhelmed, saturated, and faulty under conditions of low dietary antioxidant intake, inflammation, aging or exposure to environmental factors such as irradiation or tobacco smoke, inducing some enzymes like cyclooxygenase-2 (COX-2), lipoxygenase (LOX) and inducible nitric acid synthase (iNOS) that generate intermediaries that damage cellular macromolecules including DNA (Floyd, 1999; Rao and Balachandran, 2000; Nair et al., 2003). The damage is made on proteins, lipids, and nucleic acids signaling cascades leading to disruption of ion homeostasis and modification of the genetic apparatus, with consequence of apoptotic cell death (Sun and Chen, 1998; Sing et al., 2004). The brain is in particular very sensitive to oxidation stress possibly because of its high lipid content, high aerobic metabolic activity and low catalase activity (Halliwell and Gutteridge, 1985; Cao et al., 1988; Floyd and Carney, 1992; Gilgun-Sherki et al., 2001.
Antioxidants (AOX) are considered a promising therapeutic approach as they may be playing neuroprotective (preventing apoptosis) and neuroregenerative roles (Moosmann and Behl, 2002). Plant-derived antioxidants offer prospects in this regard. In nature, AOX are grouped as endogenous or exogenous. The endogenous group includes enzymes (and trace elements part-of) like superoxidase dismutase (zinc, manganese, and copper), glutathione peroxide (selenium) and catalase, and proteins like albumin, transferrin, ceruloplasmin, metallothionein and haptoglobin. The most important exogenous AOX are dietary phytochemicals (such as polyphenols, quinones, flavonoids, catechins, coumarins, terpenoids) and the smaller molecules like ascorbic acid (Vitamin C), alpha-tocopherol (Vitamin E) and beta-carotene vitamin-E, and supplements. The antioxidant processes occur in cytosol, mitochondria or in plasma (Larson 1988; Namiki et al., 1993; Berger, 2005). Though their mode of action is not yet completely elucidated, and clinical trials involving them are still relatively scarce, AOX offer a promising approach in the control or slowing down progression of neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and ischaemic and haemorrhagic stroke (Maxwell, 1995; Floyd, 1999; Mattson, 2000; Moosmann and Behl, 2002; Nair et al., 2003; Berger, 2005). To write off antioxidants as potentially harmful, is ultimately keeping a potential weapon out of the therapeutic arsenal. Strategies aimed at limiting ROS oxidative stress damage, may slow the progression of neurodegenerative diseases (Halliwell, 2001; Singh et al., 2004). Since endogenous AOX defences are not always completely effective, and since exposure to damaging environmental factors is increasing, exogenous AOX will find more roles in diminishing the cumulative effects of oxidative damage (Gilgun-Sherki et al., 2001). Plant derived AOX are regarded as effective in controlling the effects of oxidative damage, and hence have had influence in what people eat and drink (Viana et al., 1996; Sun et al., 2002; Pinder and Sandler, 2004). As the focus of medicine shifts from treatment of manifest disease to prevention, herbal medicine (with its four pillars of phytochemistry, phytopharmacy, phytopharmacology and phytotherapy) is coming into consideration, being a renaissance of age-old human tradition (Weiss and Fintelmann, 2000). The ‘Green’ movement in Western society has changed attitudes in the general population who now conceive naturally derived substances and extracts as being inherently safer and more desirable than synthetic chemicals products, with the net effect of increase in sales of herbal preparations (Houghton and Raman, 1998; Capasso et al., 2000). About 80% of people in the developing world rely on phytomedicine for primary healthcare for man and livestock (Plotkin, 1992; McCorkle et al., 1996).
However, despite the demand of phytotherapeutic agents growing (Capasso et al., 2000), most medical and veterinary professionals still distrust the use of herbal medicines, due to lack of scientific evidence of efficacy and safety (Sofowora, 1982; Thompson, 1997). Hence the need for their scientific validation before plant-derived extracts gain wider acceptance and use. In this regard, many plants nevertheless have been scientifically proved to be effective in control of acute and chronic nervous disorders (Table 1). As herbal extracts are a complex mixture of compounds, the active molecules, mode of action, bioavailability and pharmacokinetics, and toxicity issues become difficult to evaluate.
Table 1.
Commercialised medicinal plants proven effective in control of nervous/chronic conditions (Van Wyk and Wink, 2004)
| Plant | Common name | Active ingredients | Activity/action |
|
Harpagophytum procumbens |
Devil's claw | Coumarins; phenolic glycosides |
Anti-inflammatory; ant-rheumatic |
|
Hypericum perforatum |
St. John's wort | Phenolic compounds; hyperforin |
Analgesic; psychomotor disturbances; anti-depressant |
|
Withania somnifera |
Winter cherry | Steroids; witherferin | Ant-inflammatory; sedative |
| Ginkgo biloba | Ginkgo | Flavonoids; proanthocyanidins |
Enhances memory & learning; dementia; insomnia |
|
Valeriana officinalis |
Valerian | Valeranone; sesquiterpenoids |
Epilepsy & insomnia; tranquilizer |
|
Rauwolfia serpentium |
Indian snake root | Indole alkaloids | Psychomotor disturbances; tranquilizer |
|
Sutherlandia frutescens |
Cancer bush | Flavonoids; triterpenoids |
Cancer; general tonic |
| Vitis vinifera | Grape vine | Proanthocyanidins; flavonoids |
Circulatory disturbance; antioxidants |
| Humulus lupulus | Hop plant | Phenolics; proanthoyanidins |
Sedative; mood disorders |
|
Papaver somniferum |
Opium poppy | Alkaloids | Narcotic; analgesic |
Peltophorum africanum (weeping wattle), a plant widespread in southern Africa and most tropical areas, is unique in that it is traditionally used to treat more less similar disease conditions in man and domesticated animals. The root and bark decoctions are used to treat wounds, colic (acute pains), joint and back pain (chronic pains), ascites and abdominal disorders, diarrhoea and dysentery, infertility, and depression (Watt and Breyer-Brandwijk, 1962; Venter and Venter, 1996; Van Wyk and Geriche, 2000; Manana, 2003). In southern Africa, women who lose their spouses take the bark/root decoctions for up to a year, possibly for relief of post-traumatic stress. In livestock, the plant is used against diarrhoea, dysentery, colic and as a general tonic. Pastoralists use root as a component in the ‘Kgatla doctors’ mixture to promote well-being, resistance to diseases and fertility (Watt and Breyer-Brandwijk, 1962; Cunningham and Zondi, 1991; Van der Merwe, 2000). From the foregoing, the traditional use point to P. africanum as having antibacterial, anthelmintic, anti-inflammatory and antioxidant activities. Most of the compounds isolated by phytochemists are polyphenols, typical of antioxidants of higher plants (Larson, 1988; Paya et al., 1992; Braca et al., 2002). It is not surprising therefore, that Bizimenyera et al. (2005), reported high concentrations (20–50%) of polyphenols in the extracts of P. africanum.
Hence, P. africanum could have potential for neuroprotective antioxidant-based thera-peutics. In the study described hereafter, the comparative antioxidant activity potential of P. africanum extracts was assessed against 6-hydroxy-2, 5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) and L-ascorbic acid (both standard antioxidants), using two free radicals, 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) and 2, 2-diphenyl-1-picryl hydrazyl (DPPH), respectively.
Methodology
Collection, storage and preparation of plant material
Leaves, stem bark and root bark were collected from mature Peltophorum africanum Sond. (Fabaceae) trees, growing naturally at Onderstepoort, South Africa. A voucher specimen (PM 001) is stored in the Medicinal Plant Herbarium, Department of Paraclinical Sciences, University of Pretoria, South Africa. The collected plant material was dried in the shade at ambient temperature, and ground to powder before extraction. A known mass of each of the powdered material was then percolated with ten volumes of acetone at room temperature for 24 hours and filtered. Acetone was used as extractant, as it has been found to extract large quantities of bioactive plant material (Eloff, 1998). The extracts obtained were concentrated under vacuum at 40 °C using a rotary evaporator (Buchi®, Switzerland) to give the crude extracts of each plant material. The dry extracts were stored in sealed vials in the refrigerator prior to further processes.
Chemicals
All chemicals used were of analytical grade. L-ascorbic acid (Merck), potassium persulphate (Sigma), 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) (Sigma), 6-hydroxy-2, 5,7,8-tetramethylchromane-2-carboxylic acid (Trolox®) (Fluka), 2,2-diphenyl-1-picryl hydrazyl (DPPH) (Sigma) and absolute ethanol (Merck).
Evaluation of antioxidant activity
Qualitative screening for antioxidant activity was done using 2, 2-diphenyl-1-picryl hydrazyl (DPPH) according to Takao et al. (1994). Thin layer chromatograms (TLC) of extracts developed in EMW (ethyl acetate/methanol/water (10/1.35 /1) solvent system were sprayed with 0.2% DPPH in methanol. Antioxidant activity is detected on the chromatogram when the initially purple DPPH background turns yellow in bands where an antioxidant is present (Bors et al., 1992).
Quantification of AOX activity was determined spectrophotometrically using two radicals, ABTS and DPPH and the Versa-max® microplate reader (Labotec). In one method, use was made of theTrolox equivalent antioxidant capacity (TEAC) assay (Re et al., 1999) based on the scavenging of the ABTS radical into a colourless product. The absorbance was read at 734 nm. This method was also used in a previous analysis (Bizimenyera et al., 2005). Trolox (6-hydroxy-2, 5,7,8-tetramethylchromane-2-carboxylic acid) is a Vitamin-E analogue. If an extract had antioxidant activity equivalent to Trolox, its TEAC value would be 1 and if the extract were more active its TEAC would be greater than 1.
The second method described by Mensor et al. (2001), employed the DPPH free radical assay. Different concentrations of the extracts were prepared between 20.0 and 1.0 µg/ml. Ten µL of 0.4 mM DPPH in ethanol was added to 25 µL of each concentration of extract tested and allowed to react at room temperature in the dark for 30 minutes. Blank solutions were prepared with each test sample solution (25 µL) and 10 µL ethanol only while the negative control was DPPH solution, 10 µL plus 25 µL ethanol. L-ascorbic acid was the positive control. The decrease in absorbance was measured at 518 nm. Values obtained were converted to percentage antioxidant activity (AOXA%) using the formula: -
AOXA% = 100 − {[(Abssample − Absblank) × 100] / Abs control}
Abssample is the absorbance of the sample, Absblank is the absorbance of the blank and Abscontrol is the absorbance of the control.
L-ascorbic acid (vitamin C) was used as a positive control (antioxidant agent). The antioxidant activity is expressed as effective concentration (EC50) values. The lower the EC50 value, the more effective antioxidant activity. The EC50 value, defined as the concentration of the sample leading to 50% reduction of the initial DPPH concentration, was calculated from the linear regression of plots of concentration of the test extracts (µg/mL) against the mean percentage of the antioxidant activity obtained from three replicate assays. For statistical analysis, the results were expressed as mean ± SEM (standard error of mean) and the EC50 values obtained from the regression plots (SigmaPlotsR 2001, SPSS Science) showed a good coefficient of determination, with most values being r2 ≥ 0.910.
Results
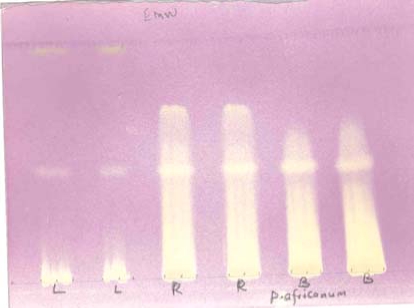
All the extracts (leaf, bark and root), from qualitative screening, contained compounds that exhibited considerable free radical scavenging activity, as shown by the yellow bands (of antioxidant activity) on DPPH chromatograms, Figure 1. The root and bark had more antioxidant activity compared to the leaf. The antioxidant activity of the root and bark extracts was higher than both L-ascorbic acid and Trolox (Table 2). The bark and root extracts had higher TEAC values than Trolox (Vitamin-E analogue), with respective values of 1.08, 1.28 and 1.0. L-ascorbic acid (5.04 µg/mL) was more active than the leaf 6.54 (µg/mL), but much less active than the bark (4.37 µg/mL) and root (3.82 µg/mL) extracts (the higher the µg/mL value, the less AOX activity).
Figure 1.
Chromatogram of 200 µg acetone extracts of leaf (L), bark (B) and root (R) of P. africanum, separated with EMW and sprayed with DPPH. Note the high antioxidant activity, indicated by yellow areas
Table 2.
Trolox (TEAC) and Vitamin-C equivalent# values of acetone extracts of leaf, bark and root of P. africanum
| Plant part | AOX Values* | ||
| TEAC | DPPH(EC50±SEM (µg/ml) |
||
| Leaf | 0.57 | 6.54± 0.49 | |
| Bark | 1.08 | 4.37± 0.41 | |
| Root | 1.28 | 3.82± 0.58 | |
| Standards | Trolox | 1.0 | N/A |
| L-ascorbic acid |
N/A | 5.04± 0.65 | |
{Note: - # If the extract had antioxidant activity equivalent to Trolox, its TEAC value would be 1 and if the extract was more active its TEAC value would be greater than. The reverse is true with DPPH.; the values above 5 mean less antioxidant activity and those lower than 5 mean higher activity}
Discussion and conclusion
Laboratory results showed the extracts had high levels of antioxidant compounds, especially the root and bark extracts. Both Trolox and L-ascorbic acid have been used as standards in quantifications of antioxidant activity (Van den Berg et al., 1999; Fukomoto and Mazza, 2000). The EC50 of the root (3.82 µg/ml) and bark (4.37 µg/ml) extracts indicated higher antioxidant activity than L-ascorbic acid (5.04 µg/ml), and more effective than Ginkgo biloba extract (EGb 761) whose EC50 is 40.72 µg/mL (Mensor et al., 2001; Bridi et al., 2001; Aderogba et al., 2004). The standardised extract of Ginkgo biloba (EGb 761) has been widely employed for its significant benefit in neurodegenerative disorders (Bridi et al., 2001).
The antioxidant activity in plants may largely be due to polyphenols (Thabrew et al., 1998), and in particular polyphenols make up nearly 50% of P. africanum root and bark extracts (Bizimenyera et al., 2005). The presence of antioxidant compounds also agrees with the phytochemical analyses by other investigators (El Sherbeiny et al., 1977; Evans et al., 1985; Bam et al., 1988 and 1990; Khattab and Nasser, 1998; Mebe and Makuhunga, 1992) who isolated flavonoids, coumarins, gallic acid and other polyphenols from P. africanum extracts.
The root and bark appear to have the highest concentrations of the AOX. Coincidentally, traditional healers also use the root and bark concoctions (Watt and Breyer-Brandwijk, 1962; Cunningham and Zondi, 1991; Venter and Venter, 1996; Van der Merwe, 2000; Van Wyk and Geriche, 2000; Manana, 2003). In all the traditional treatments, decoctions and infusions are made from the root or bark. That is why the present authors believe the rationale for traditional healers using P. africanum in treating inflammation, pain and depression may be due to high AOX compounds present in the plant.
Various chronic degenerative diseases with different clinical appearances appear to share common biochemical, genetic and cellular alterations that are related to disease pathogenesis (Nair et al., 2003; Barnharm et al., 2004). Empirical evidence suggest P. africanum, due to its with its high AOX activity, could be influential in the control of these conditions. Mixtures of dietary antioxidants or foods rich in antioxidants have been shown to increase the ability of lymphocytes to withstand DNA oxidation (Duthie et al., 1996; Pool-Zobel et al., 1997; Porrini and Riso, 2000). Diets rich in flavonoids appear to be protective against ischemic heart disease (Hertog et al., 1993; Viana et al., 1996; Berger, 2005) and supplementing diets with antioxidants generally appears preventing occurrence and progression of many neurodegenerative diseases (Rice-Evans and Diplock, 1993; Rice-Evans et al., 1995; Singh et al., 2004).
The safety of P. africanum extracts still has to be confirmed. From inquiries from pastoralists (Van der Merwe, 2000) and Pretoria market vendors of traditional herbs, P. africanum extracts were reported safe. Tests in our laboratory involving acetone extracts on monkey kidney cells and brine shrimp (awaiting publication) showed no toxicity effects. Bessong et al. (2005) reported no toxicity of the extracts. This, however, is no cause for complacency, as toxicity of herbal medicines has been reported (Capasso et al., 2000).
This paper reports P. africanum leaf, bark and root extracts as showing considerable antioxidant activity. The root and bark had more antioxidants than the leaf. The antioxidant activity of the root and bark extracts, was higher than L-ascorbic acid (Vitamin-C) and Vitamin-E equivalent (Trolox), both standard antioxidants. The results of these in vitro tests would appear to justify the traditional use of the plant in treatment of acute and chronic nervous disorders. Antioxidants appear to have neuroprotective and neurodegenerative roles, reducing or slowing down neuronal cell death, and have an important role to play in diminishing the cumulative effects of oxidative damage. Further in vivo research, and clinical trials are required for generation of conclusive data for use of the plant in treating neurodegenerative diseases. Continued work on plants like P. africanum, rich in antioxidants, may avail medicine with antioxidant-based neuroprotective therapeutics.
Acknowledgements
Staff Development Programme Makerere University, Uganda, the National Research Foundation, South Africa, and the University of Pretoria funded this work
References
- 1.Aderogba M A, Okoh E K, Adelanwa T A, Obuotor E M. Antioxidant properties of the Nigerian Piliostigma species. J Biol Sci. 2004;4:501–503. [Google Scholar]
- 2.Bam M, Ferreira D, Brandt E. Novel cyanomaclurin analogue from Peltophorum africa-num. Phytochemistry. 1988;27:3704–3705. [Google Scholar]
- 3.Bam M, Malan J C S, Young D A, Brandt E V, Ferreira Profisetinidin-type 4-arylflavin-3-ols and related δ-lactones. Phytochemistry. 1990;29:283–287. [Google Scholar]
- 4.Barnham K J, Masters L, Bush A I. Neurodegenerative diseases and oxidative stress. Nature Rev Drug Disc. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 5.Berger M M. Can oxidative damage be treated nutritionally? Clinical Nutrition. 2005;24:172–183. doi: 10.1016/j.clnu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Bessong P O, Obi C L, Andréola M, Rojas B, Pouységu L, Igumbor E, Meyer J J M, Quideau S, Litvak S. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. J Ethnopharmacol. 2005;99:83–91. doi: 10.1016/j.jep.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 7.Bizimenyera E S, Swan G E, Chikoto H, Eloff JN. Rationale for using Peltophorum africanum (Fabaceae) extracts in veterinary medicine. J S Afri Vet Assoc. 2005;76:54–58. doi: 10.4102/jsava.v76i2.397. [DOI] [PubMed] [Google Scholar]
- 8.Bors W, Saran, Eltsner E F. Screening of plant antioxidants. Modern Methods of Plant Analysis. 1992;13:277–295. [Google Scholar]
- 9.Braca A, Sortino C, Politi M, Morelli, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 10.Bridi R, Crossetti F P, Steffen V M, Henriques A T. The antioxidant activity of standardised extracts of Ginkgo biloba (EGb 761) in rats. Phytother Res. 2001;15:449–451. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Carney J M, Duchon A, Floyd R A, Chevion M. Oxygen free radicals involvement in ischemia and repurfusion injury to brain. Neurosci Lett. 1988;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- 12.Capasso R, Izzo A A, Pinto L, Bifulco T, Vitobello C, Mascolo N. Phytotherapy and quality of herbal medicines. Fitoterapia. 2000;71:S58–S65. doi: 10.1016/s0367-326x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham A B, Zondi A S. Cattle owners and traditional medicines used for livestock. Institute of Natural Resourses, University of Natal. Petermaritzburg; 1991. p. 35. Investigational Report No 69. [Google Scholar]
- 14.Duthie S J, Ma A, Ross M A, Collins A R. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996;56:1291–1295. [PubMed] [Google Scholar]
- 15.Eloff J N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 16.El Sherbeiny A E A, El Ansari, Nawwar M A M, El Sayed N H. Flavonol glycosides and flavonol glucoside gallates from Peltophorum africanum. Planta Medica. 1977;32:165–170. doi: 10.1055/s-0028-1097578. [DOI] [PubMed] [Google Scholar]
- 17.Evans S V, Shing T K M, Aplin R T, Fellows L E, Fleet G W J. Sulphate ester of trans-4-hydroxypipecolic acid in seeds of Peltophorum. Phytochemistry. 1985;24:2593–2596. [Google Scholar]
- 18.Floyd R A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proceedings of the Society for Experimental Biology and Medicine. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 19.Floyd R A, Carney J M. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32:S22–S27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto L R, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 21.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacol. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B, Gutteridge J M C. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8:22–26. [Google Scholar]
- 23.Halliwell B. Role of free radicals in neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs & Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 24.Hertog M G L, Feskens E J M, Hollmann P C H. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zuphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 25.Houghton PJ, Raman A. Laboratory handbook for the fractionation of natural extracts. London: Chapman & Hall; 1998. pp. 1–7. [Google Scholar]
- 26.Khattab A M, Nassar M I. Phytochemical and molluscidal studies on Peltophorum africanum and Sesbania sesbani. Bull NatlResCent, Egypt. 1998;23:401–407. [Google Scholar]
- 27.Larson R A. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- 28.Manana J V. Identification of commonly used traditional medicines by planar chromatography for quality control purpose. South Africa: University of Pretoria; 2003. M.Sc. Dissertation. [Google Scholar]
- 29.Mattson M P. Apoptosis in neurodegenerative disorders. Nature Rev Mol Cell Biol. 2000;1:120–130. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell S R J. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 31.McCorkle C M, Mathias-Mundy E, Schillhorn van Veen T W. Ethnoveterinary Research and Development. London: Intermediate technology publications; 1996. [Google Scholar]
- 32.Mebe P P, Makuhunga P. 11-(E)-p-coumaric acid ester of bergenin from Peltophorum africanum. Phytochemistry. 1992;31:3286–3287. [Google Scholar]
- 33.Mensor L L, Menezes F S, Leitao G G, Reis A S, Santos T C, Coube C S, Leitao S G. Screening of Brazilian plants for antioxidant activity by use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 34.Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opinion on Investigational Drugs. 2002;11:1407–1435. doi: 10.1517/13543784.11.10.1407. [DOI] [PubMed] [Google Scholar]
- 35.Nair U, Bartsch H, Nair J. Prevention of degehnerative diseases; clues from studies investigating oxidative stress, Brussels, 13 November 2002. Mutagenesis. 2003;18:477–483. doi: 10.1093/mutage/geg009. [DOI] [PubMed] [Google Scholar]
- 36.Namiki M, Yamashita K, Osawa . Active oxygen, lipid peroxidases and antioxidants. Tokyo: Japan Science Society Press; 1993. Food-related antioxidants and their activities in vivo; pp. 319–332. [Google Scholar]
- 37.Paya M, Halliwell B, Hoult J R S. Interaction of a series of coumarins with reactive oxygen species. Biochem Pharmacol. 1992;44:205–214. doi: 10.1016/0006-2952(92)90002-z. [DOI] [PubMed] [Google Scholar]
- 38.Plotkin M J. Natural resources and human health: plants of medicinal and nutritional value. Amsterdam: Elsevier; 1992. Ethnomedicine: past, present and future; pp. 79–86. Proceedings. [Google Scholar]
- 39.Pinder R M, Sandler M. Alcohol, wine and mental health: focus on dementia and stroke. J Psychopharm. 2004;18:449–456. doi: 10.1177/0269881104047272. [DOI] [PubMed] [Google Scholar]
- 40.Pool-Zobel B L, Bub A, Muller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–1850. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 41.Porrini M, Riso P. Lymphocyte lycopene concentration and DNA protection from oxidative damage is is increased in women after a short period of tomato consumption. J Nutri. 2000;130:189–192. doi: 10.1093/jn/130.2.189. [DOI] [PubMed] [Google Scholar]
- 42.Prasad K N, Cole W C, Hovland A R, Prasad K C, Nahreini P, Kumar B, Edwards-Prasad J, Andreatta C P. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biological rationale. Curr Opin Neurol. 1999;12:761–770. doi: 10.1097/00019052-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Rao A V, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutri Neurosci. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- 44.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans CA. Antioxidant activity applying an improved ABTS radical cation decolourising assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 45.Rice-Evans C A, Diplock A T. Current status of antioxidant therapy. Free Rad Biol Med. 1993;15:77–96. doi: 10.1016/0891-5849(93)90127-g. [DOI] [PubMed] [Google Scholar]
- 46.Rice-Evans C A, Miller N J, Bolwell G P, Bramley P M, Pridham J B. The relative antioxidant activities of plat-derived polyphenolic favonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 48.Singh R P, Sharad S, Kapur S. Free radicals and oxidative stress in neurodegenerative diseases: relevance of dietary antioxidants. J Ind Acad Clin Med. 2004;5:218–225. [Google Scholar]
- 49.Sofowora A. Medicinal Plants and traditional medicine in Africa. Chichester, UK: John Wiley and Sons; 1982. [Google Scholar]
- 50.Sun A Y, Chen Y. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- 51.Sun A Y, Simonyi A, Sun G Y. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002;32:314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 52.Takao T, Kitatani F, Watanabe N, Yagi A, Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotech Biochem. 1994;58:1780–1783. [Google Scholar]
- 53.Thabrew M I, Hughes R D, McFarlane I G. Antioxidant activity of Osbeckia aspera. Phytother Res. 1998;12:288–290. [Google Scholar]
- 54.Thompson A. As patients embrace herbal remedies, dearth of scientific evidence frustrates clinicians. Am J Health Syst Pharm. 1997;54:2656–2664. doi: 10.1093/ajhp/54.23.2664. [DOI] [PubMed] [Google Scholar]
- 55.Van der Merwe D. Use of ethnoveterinary medicine plants in cattle by Setwana-speaking people in the Madikwe area of the North West Province. South Africa: University of Pretoria; 2000. M.Sc Dissertation. [DOI] [PubMed] [Google Scholar]
- 56.Van den Berg R, Haenen G R M M, Van der Berg H, Bast A. Application of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity of measurement of mixtures. Food Chem. 1999;66:511–517. [Google Scholar]
- 57.Van Wyk B E, Gericke N. People's plants. Pretoria: Briza Publications; 2000. p. 130. [Google Scholar]
- 58.Van Wyk B E, Wink M. Medicinal plants of the world. 1st Ed. Pretoria: Briza; 2004. 2nd Revised impression. [Google Scholar]
- 59.Venter F, Venter J A. Making the most of Indigenous Trees. Pretoria: Briza publications; 1996. pp. 20–21. [Google Scholar]
- 60.Viana M, Barbas C, Banet B, Bonet M V, Castro M, Fraile M U, Herrela In vitro effect of a flavonoid-rich extract on LDL oxidation. Athelosclerosis. 1996;123:83–91. doi: 10.1016/0021-9150(95)05763-3. [DOI] [PubMed] [Google Scholar]
- 61.Watt J M, Breyer-Brandwijk M G. The medicinal and poisonous plants of Southern and Eastern Africa. 2nd Ed. London: E& S Livingstone; 1962. p. 638. [Google Scholar]
- 62.Weiss R F, Fintelmann V. Herbal Medicine. 2nd edition. Stuttgart: Thieme; 2000. pp. 3–20. [Google Scholar]