Abstract
Farnesoid X receptor (FXR) is a bile acid–activated transcription factor that is a member of the nuclear hormone receptor superfamily. Fxr-null mice exhibit a phenotype similar to Byler disease, an inherited cholestatic liver disorder. In the liver, activation of FXR induces transcription of transporter genes involved in promoting bile acid clearance and represses genes involved in bile acid biosynthesis. We investigated whether the synthetic FXR agonist GW4064 could protect against cholestatic liver damage in rat models of extrahepatic and intrahepatic cholestasis. In the bile duct–ligation and α-naphthylisothiocyanate models of cholestasis, GW4064 treatment resulted in significant reductions in serum alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase, as well as other markers of liver damage. Rats that received GW4064 treatment also had decreased incidence and extent of necrosis, decreased inflammatory cell infiltration, and decreased bile duct proliferation. Analysis of gene expression in livers from GW4064-treated cholestatic rats revealed decreased expression of bile acid biosynthetic genes and increased expression of genes involved in bile acid transport, including the phospholipid flippase MDR2. The hepatoprotection seen in these animal models by the synthetic FXR agonist suggests FXR agonists may be useful in the treatment of cholestatic liver disease.
Introduction
The enterohepatic circulation of bile acids enables the absorption of fats and fat-soluble vitamins from the intestine and allows the elimination of cholesterol, toxins, and metabolic by-products such as bilirubin from the liver. Cholestasis, an impairment or cessation in the flow of bile, causes hepatotoxicity due to the buildup of bile acids and other toxins in the liver. Cholestasis is a component of many liver diseases, including cholelithiasis, cholestasis of pregnancy, primary biliary cirrhosis (PBC), and primary sclerosing cholangitis. The etiology of cholestasis is varied, and multiple heritable forms have been identified. Progressive familial intrahepatic cholestasis type I (PFIC1), or Byler disease, is seen in individuals with a mutation in FIC1, a gene encoding a putative aminophospholipid transferase (1). As yet, it is unclear how this genetic mutation causes cholestasis. PFIC2 arises from mutations in the BSEP (ABCB11), which encodes the canalicular bile salt export pump (2). To date, more than ten different mutations in this gene have been mapped that give rise to cholestasis. PFIC3 is seen in individuals with a mutation in MDR3 (ABCB4), which encodes a canalicular phospholipid flippase that transports phospholipid into bile (3, 4). The fact that mutations in these genes give rise to severe cholestatic liver disease reveals their importance in maintaining bile flow and healthy liver function. Currently, ursodeoxycholic acid (UDCA) is the only U.S. Food and Drug Administration–approved treatment for PBC. It is increasingly being used to treat all cholestatic conditions because it improves serum liver chemistries (5). UDCA is a polar bile acid that may act by decreasing the hydrophobicity and toxicity of the bile (5). Unfortunately, multiple clinical trials have not demonstrated an increased survival time in patients treated with UDCA (5, 6).
The farnesoid X receptor (FXR; NR1H4) is a member of the nuclear receptor superfamily of ligand-activated transcription factors. FXR is located in the liver, kidney, adrenal glands, and intestine (7). FXR has also been called the bile acid receptor following the discovery that physiological concentrations of bile acids bind and activate FXR (8–10). Activation of FXR induces expression of the canalicular bile transporters BSEP (ABCB11) and multidrug resistance–related protein 2 (MRP2; ABCC2, cMOAT), and represses key genes involved in bile acid biosynthesis, namely sterol 12α-hydroxylase (CYP8B1) and cholesterol 7α-hydroxylase (CYP7A1) (11–16). Fxr-null mice exhibit a phenotype similar to Byler disease with high serum bile acid levels and decreased fecal excretion of bile acids (17–20).
Here we describe the hepatoprotective effects of the potent, selective FXR agonist, GW4064 (21), in rat models of both intrahepatic and extrahepatic cholestasis. Taurine-conjugated UDCA (TUDCA), which does not activate FXR (8–10), was used as the clinical comparator in these studies. Furthermore, we identify an additional canalicular transport gene that is regulated by FXR. MDR2/3 (ABCB4), the gene encoding the canalicular phospholipid flippase, is induced in both human hepatocytes (MDR3) and in rodent (MDR2) liver following treatment with GW4064.
Methods
Materials.
Reagents for measuring serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and total bilirubin were obtained from Instrumentation Laboratory (Lexington, Massachusetts, USA). The bile acid kits, TUDCA, α-naphthylisothiocyanate (ANIT), dexamethasone, olive oil, and corn oil were from Sigma-Aldrich (St. Louis, Missouri, USA). TaqMan primers and probes were from BioSource International (Camarillo, California, USA). The in situ Apoptosis Detection kit was from Intergen Co. (Norcross, Georgia, USA). Cholic-2,2,4,4-d4 acid was from CDN Isotopes Inc. (Pointe-Claire, Quebec, Canada). Primary cultures of human hepatocytes were obtained from Steve Strom (University of Pittsburgh, Pittsburgh, Pennsylvania, USA) or BioWhittaker Inc. (Walkersville, Maryland, USA). TRIzol, Williams’ E medium, penicillin G, streptomycin, dexamethasone, and insulin-transferrin-selenium-G supplement were from Invitrogen Corp. (Carlsbad, California, USA).
Animals.
Adult male CRL:CD(SD)IGS rats weighing 300–350 g, purchased from Charles River Laboratories (Wilmington, Massachusetts, USA), were housed in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited GlaxoSmithKline, Research Triangle Park, facility. Rats were housed three per cage under a 12-hour light/12-hour dark cycle at 72 ± 2°F, 50% humidity, and allowed free access to food and water. Experimental protocols were approved by the GlaxoSmithKline Institutional Animal Care and Use Committee.
Bile duct ligation model of cholestasis.
Under isoflurane anesthesia and sterile surgical conditions, the common bile duct was ligated in three locations and transected between the two distal ligatures. Sham controls underwent laparotomy, without ligation of the bile duct. The rats received a single analgesic dose of oxymorphone following surgery. Twenty-four hours after laparotomy, groups of rats (n = 6) received intraperitoneal injections once daily for 4 days. Bile duct–ligated (BDL) rats were treated with 5 ml/kg corn oil as vehicle, 30 mg/kg GW4064 in corn oil, or 15 mg/kg TUDCA in corn oil. Sham-operated animals received 5 ml/kg corn oil vehicle. Four hours after the final dose, serum and livers were collected for analysis.
ANIT-induced cholestasis.
Once daily for 4 days, rats (n = 6–8) received intraperitoneal injections of vehicle, GW4064, or TUDCA, as described above. On day 2 of treatment, 4 hours after the intraperitoneal injection, the vehicle-, GW4064-, and TUDCA-treated groups received a single, orally administered, 50 mg/kg dose of ANIT in olive oil. A second set of vehicle-treated rats was given an oral dose of olive oil (5 ml/kg) in place of ANIT to serve as the normal control. Serum and liver samples were collected as outlined above, 4 hours after the final dose.
Serum biochemistry analysis.
Serum ALT, AST, LDH, ALP, total bilirubin, and bile acids were measured using the Instrumentation Laboratory ILab600 clinical chemistry analyzer according to the manufacturer’s directions.
Histopathology.
Liver samples from each rat were fixed in 10% buffered formalin and processed by standard histological techniques. Slides were stained with H&E using standard protocols and examined by light microscopy for necrosis and other structural changes. Bile duct proliferation was assessed by quantitation of the area occupied by cholangiocytes in 40–50 randomly selected fields under ×400 magnification, aided by a grid of 100 squares. Quantitation of mitotic nuclei was accomplished by dividing the number of mitotic cells by the total number of hepatocytes.
Reverse transcription quantitative PCR.
Total RNA was extracted from rat tissues or human hepatocytes using TRIzol reagent (Invitrogen Corp.) according to the manufacturer’s directions. The RNA was treated with DNase I (Ambion Inc., Austin, Texas, USA) at 37°C for 30 minutes, followed by inactivation at 75°C for 5 minutes. RNA was then quantitated using the RiboGreen RNA quantitation kit (Molecular Probes Inc., Eugene, Oregon, USA). RNA expression was measured by reverse transcription quantitative PCR (RTQ-PCR) using an ABI Prism 7700 or 7900 Sequence Detection System (PE Applied Biosystems, Foster City, California, USA), as described previously (22). Sequences of the gene-specific primers and probes used for RTQ-PCR are listed in Table 1.
Table 1.
Primer-probe sets and gene abbreviations
Analysis of liver bile acid concentration.
Bile acid concentrations were determined by atmospheric pressure ionization-liquid chromatography mass spectrometry (API-LCMS). Briefly, 1-ml aliquots of liver samples homogenized in methanol (0.5 g/ml) were spiked with 50 μl of 20 μg/ml of 2,2,4,4-d4-cholic acid (D4-cholic acid) in methanol. Samples were sonicated, centrifuged (3,000 g for 10 minutes), and filtered through a 0.45-μm filter unit before injection onto the analytical column of an API-LCMS instrument (Hewlett Packard Series 1100 Liquid Chromatograph Mass Selective Detector; Hewlett-Packard, Palo Alto, California, USA). Bile acids and D4-cholic acid were detected as molecular ions ([M-H]–) in the negative-selected ion-monitoring mode of the instrumentation. Bile acid concentrations in the study samples were calculated by comparison with standard solutions of bile acids containing D4-cholic acid as the internal standard.
Primary culture of human hepatocytes.
Primary human hepatocytes were cultured on Matrigel-coated six-well plates at a density of 1.5 × 106 cells per well. The culture media consisted of serum-free Williams’ E medium supplemented with 100 nM dexamethasone, 100 U/ml penicillin G, 100 μg/ml streptomycin, and ITS-G. Forty-eight hours after isolation, cells were treated for 12 or 48 hours with GW4064 or chenodeoxycholic acid (CDCA), which was added to the culture medium as 1,000× stock solutions in DMSO. Control cultures received vehicle (0.1% DMSO) alone. Total RNA was isolated using TRIzol reagent according to the manufacturer’s instructions. Differentially regulated genes were identified using CuraGen Corp. GeneCalling Technology and RTQ-PCR as described above. Sequences of the primers and probes used for RTQ-PCR are listed in Table 1.
Statistical analysis.
All data were analyzed by one-way ANOVA followed by Duncan’s multiple range test. The 0.05 level of probability was used as the criteria of significance.
Results
FXR activation is hepatoprotective in intrahepatic cholestasis.
FXR-null mice exhibit a phenotype similar to Byler disease, and, moreover, FXR regulates expression of genes involved in bile acid biosynthesis and transport. Therefore, we examined whether the synthetic FXR agonist GW4064 might protect against hepatotoxicity in rodent models of cholestasis. We first tested GW4064 in rats treated with ANIT, which damages biliary epithelial cells and induces intrahepatic cholestasis (23–25). Adult male rats were treated for 4 days with vehicle alone (corn oil), GW4064, or TUDCA. On the second day of treatment the animals received a single dose of ANIT to induce cholestasis. One group of rats received vehicle for 4 days as well as vehicle in place of ANIT to establish baseline values. Serum was collected on day 4 for analysis of clinical chemistry parameters indicative of liver damage. As expected, serum activities of ALT, AST, LDH, and ALP were all significantly increased by ANIT (Figure 1). Serum bilirubin levels were elevated nearly 50-fold following ANIT treatment, and bile acids were increased 25-fold. These data demonstrate that ANIT exposure induced profound cholestasis and hepatocellular damage in the rats and are in line with earlier publications (26, 27).
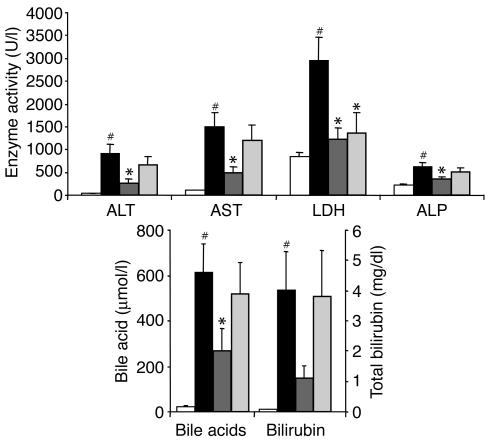
Figure 1.
Protection against ANIT-induced hepatotoxicity by GW4064. Rats (n = 6–8) were treated once daily with vehicle, GW4064, or TUDCA. On the second treatment day the rats received a single dose of ANIT or vehicle. Serum chemistries were measured 4 hours after the final dose. Values are presented as average ± SEM. White bars, vehicle/vehicle; black bars, vehicle/ANIT; dark gray bars, GW4064/ANIT; light gray bars, TUDCA/ANIT. Statistically significant differences between the vehicle/vehicle and vehicle/ANIT groups are indicated (#P < 0.05). Statistically significant differences between the vehicle/ANIT group and either the GW4064/ANIT or the TUDCA/ANIT groups are also indicated (*P < 0.05).
GW4064 treatment resulted in substantial, statistically significant reductions in serum activities of ALT, AST, LDH, and ALP in the ANIT-treated rats (Figure 1). Serum bile acid levels were also significantly reduced by GW4064 treatment. Bilirubin levels were decreased in the GW4064-treated rats, but statistical significance was not achieved. This striking profile demonstrates that GW4064 affords marked hepatoprotection in this established model of intrahepatic cholestasis. Notably, GW4064 was much more effective in decreasing these markers of liver damage than TUDCA, which reduced only LDH levels.
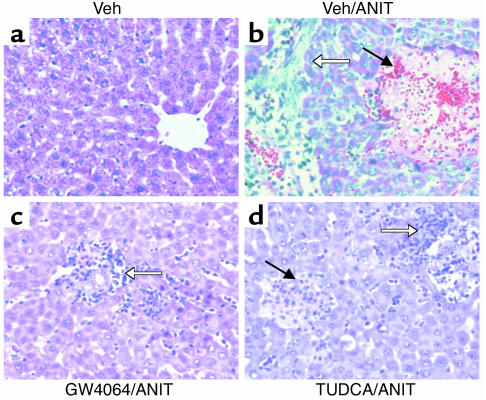
The livers of ANIT-treated rats were examined histologically. Liver sections from vehicle-treated rats showed normal histology (Figure 2a), whereas rats that received ANIT with vehicle showed large areas of hepatic parenchymal necrosis with inflammatory cell infiltration (Figure 2b). GW4064 treatment provided remarkable protection against ANIT-induced cellular damage (Figure 2c). Livers from the GW4064/ANIT–treated rats contained few discernable necrotic foci, and those that were present were substantially smaller than those in the vehicle/ANIT–treated rats. Inflammatory cell infiltration was also less severe in the GW4064/ANIT–treated rats. Consistent with the results of the serum analysis, TUDCA afforded less hepatoprotection than GW4064 (Figure 2d). Small areas of necrosis were seen throughout the sections from the TUDCA-treated animals, and inflammatory cell infiltration was pronounced. Thus, GW4064 is more efficacious than TUDCA at protecting the liver in a standard model of intrahepatic cholestasis.
Figure 2.
Protection against ANIT-induced necrosis by GW4064. Rats (n = 6–8) were treated once daily with vehicle (Veh), GW4064, or TUDCA. On the second treatment day the rats received a single dose of ANIT or vehicle. Livers were taken for histological analysis 4 hours after the final dose. The panels show representative H&E-stained liver sections from each treatment group at ×400 magnification. (a) Vehicle/vehicle–treated rat showing normal liver histology. (b) Vehicle/ANIT–treated rat showing a large area of parenchymal necrosis (filled arrow) with inflammatory cell infiltration (open arrow). (c) GW4064/ANIT–treated rat showing inflammatory cell infiltration (open arrow) around the bile duct, but no necrosis. (d) TUDCA/ANIT–treated rat showing parenchymal necrosis (filled arrow) with inflammatory cell infiltration (open arrow).
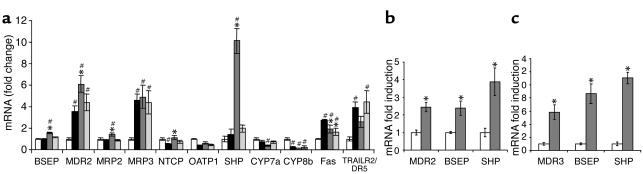
To further understand the molecular basis for the hepatoprotective effects of GW4064 in this model, we examined the expression of key genes involved in bile acid biosynthesis and transport in the livers of these rats. Gene expression was measured by RTQ-PCR. We first examined expression of the canalicular transporters BSEP and MDR2, which have been implicated in the pathogenesis of cholestasis. In agreement with a previous study (28), BSEP levels were unchanged by ANIT treatment but were significantly induced by GW4064 (Figure 3a). ANIT treatment increased MDR2 expression approximately fourfold, which also agrees with a previous study (29). Interestingly, GW4064 treatment further increased the expression of MDR2 above that seen with ANIT treatment (Figure 3a). In normal rats, 4-day GW4064 treatment resulted in increased expression of MDR2 (Figure 3b). In addition, CuraGen GeneCalling analysis and RTQ-PCR show marked induction of MDR3, the human homologue of the rat MDR2, by GW4064 and CDCA (data not shown) in primary human hepatocytes (Figure 3c). Pretreatment of primary human hepatocytes with the protein synthesis inhibitor cycloheximide did not prevent the induction of MDR3 mRNA by GW4064 (data not shown), indicating this induction may be a direct effect of FXR activation, in agreement with the recent report of Huang et al. (30). Thus, GW4064 may protect against hepatotoxicity by inducing MDR2 and BSEP expression and promoting transport of bile acids and phospholipid from hepatocytes. In contrast to GW4064, TUDCA did not affect MDR2 or BSEP expression in the ANIT-treated rats (Figure 3a).
Figure 3.
Liver gene expression profile in ANIT model of cholestasis. Total RNA was isolated from rat liver or primary human hepatocytes, and gene expression was measured using RTQ-PCR. (a) Gene expression profile in the liver of rats with ANIT-induced cholestasis. White bars, vehicle/vehicle; black bars, vehicle/ANIT; dark gray bars, GW4064/ANIT; light gray bars, TUDCA/ANIT. Statistically significant differences from the vehicle/vehicle group are indicated (#P < 0.05). Statistically significant differences from the vehicle/ANIT group are also indicated (*P < 0.05). (c) Induction of MDR2, BSEP, and SHP in the liver of the normal rat treated once daily for 4 days with corn oil vehicle (white bars) or GW4064, 30 mg/kg intraperitoneally (dark gray bars). Statistically significant inductions are indicated (*P < 0.05). (d) Induction of MDR3, BSEP, and SHP in primary human hepatocytes treated 12 hours with 0.1% DMSO as vehicle (white bars) or 1 μM GW4064 (dark gray bars). Statistically significant inductions are indicated (*P < 0.05).
We also analyzed the expression of other transporters, including MRP2, MRP3, NTCP, and OATP1. Expression of the canalicular transporter MRP2 was not changed after ANIT treatment or TUDCA treatment, but was increased in rats given GW4064, as expected (13) (Figure 3a). MRP3 transports bile acids from the hepatocyte into the portal circulation. MRP3 expression is normally low but is induced following ANIT (31, 32). Induction of MRP3 expression during cholestasis may help reduce the concentration of potentially harmful bile acids in the hepatocytes. There was a 4.5-fold induction of MRP3 expression by ANIT but no further increase in response to GW4064 or TUDCA (Figure 3a). Sodium taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptide-1 (OATP1) transport bile acids into hepatocytes from the portal circulation, thereby increasing the bile acid concentration in the hepatocyte. Under cholestatic conditions, repression of these genes may be beneficial. Indeed, ANIT treatment alone caused repression of OATP1 and NTCP. Surprisingly, GW4064 treatment slightly increased OATP1 expression and increased NTCP expression to normal levels (Figure 3a). TUDCA treatment had no effect on OATP1 or NTCP expression in these studies (Figure 3a).
Next, we examined the effects of GW4064 and TUDCA on genes involved in bile acid production. SHP is a primary FXR target gene that represses bile acid biosynthesis by inhibiting the transcriptional activity of liver receptor homologue 1 on the CYP7A1 and CYP8B1 promoters (14–16). SHP mRNA levels were not altered by ANIT administration alone, but, as previously described, were markedly induced by GW4064 treatment (Figure 3a) (15). CYP7A1 expression was decreased in animals receiving ANIT and further decreased by GW4064 treatment (Figure 3a). Interestingly, CYP8B1 expression was reduced 81% in rats that received ANIT (Figure 3a) and 88% following GW4064 treatment. In marked contrast, TUDCA treatment did not induce SHP or repress CYP7A1 or CYP8B1 compared with vehicle. Thus, unlike GW4064, TUDCA would not be expected to protect the liver by decreasing bile acid biosynthesis.
Finally, because evidence exists for the involvement of Fas and TNF-related apoptosis-inducing ligand receptor 2/death receptor 5 (TRAILR2/DR5) in mediating cholestatic injury and hepatocyte apoptosis (33, 34), we investigated expression levels of these mRNAs in the ANIT model. Both Fas and TRAILR2/DR5 were significantly induced by ANIT treatment. Both GW4064 and TUDCA treatment significantly reduced the expression of Fas. GW4064 treatment slightly reduced the expression of TRAILR2/DR5, whereas TUDCA had no effect. The reduction in TRAILR2/DR5 by the specific FXR agonist, GW4064, increases the evidence that the induction of TRAILR2/DR5 by bile acids is not FXR mediated (35, 36).
FXR activation is hepatoprotective in extrahepatic cholestasis.
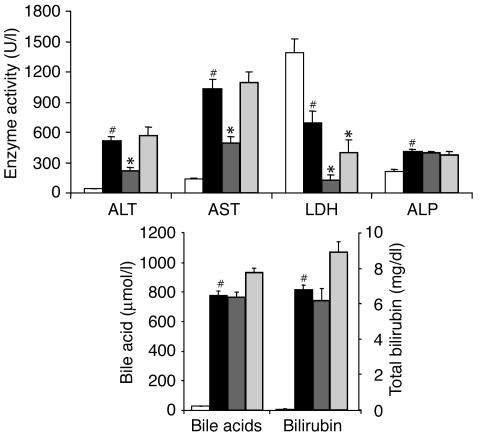
We next tested GW4064 in BDL rats, a particularly severe model of obstructive, extrahepatic cholestasis. As expected, serum activities of ALT, AST, and ALP increased as a consequence of bile duct ligation (Figure 4). Surprisingly, LDH activity decreased in response to bile duct ligation. The basis for this decrease is unclear, but was consistently seen across multiple experiments. Serum levels of bile acids and bilirubin were also strikingly increased in the ligated rats. GW4064 treatment resulted in significant reductions in serum activities of ALT, AST, and LDH in BDL rats (Figure 4). GW4064, however, did not significantly reduce serum levels of ALP, bile acids, or total bilirubin in this severe model of cholestasis. In contrast to GW4064, TUDCA had virtually no effect in this model, decreasing only LDH levels (Figure 4).
Figure 4.
Protection against bile duct ligation–induced hepatotoxicity by GW4064. Rats (n = 6) were subjected to bile duct ligation or laparotomy without bile duct ligation (sham-operated). Beginning 24 hours after bile duct ligation surgery, the rats were treated for 4 days with vehicle, GW4064, or TUDCA. Serum chemistries were measured 4 hours after the final dose. Values are presented as average ± SEM. White bars, sham; black bars, vehicle; dark gray bars, GW4064; light gray bars, TUDCA. Statistically significant differences between the sham-operated and vehicle/BDL groups are indicated (#P < 0.05). Statistically significant differences between the vehicle/BDL group and either the GW4064-treated or the TUDCA-treated groups are also indicated (*P < 0.05).
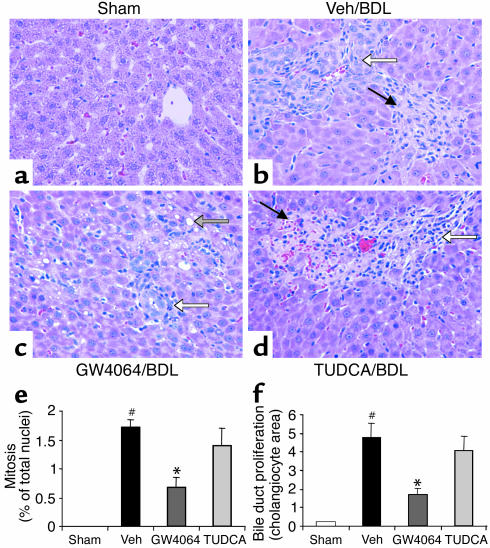
Histological analysis of liver sections from the BDL rats revealed increased levels of bile duct proliferation, parenchymal necrosis, and inflammatory cell infiltration (compare Figure 5a to Figure 5b). In comparison, sections from the GW4064-treated rats had qualitatively fewer and smaller necrotic lesions, decreased fatty cell degeneration, reduced bile duct proliferation, and fewer mitotic nuclei (Figure 5c). The sections from the TUDCA-treated rats were not substantially different from vehicle-treated BDL rats (Figure 5d).
Figure 5.
Protection against bile duct ligation–induced necrosis, mitosis, and bile duct proliferation by GW4064. Rats (n = 6) were subjected to bile duct ligation or laparotomy without bile duct ligation (sham-operated). Beginning 24 hours after bile duct ligation surgery, the rats were treated for 4 days with vehicle, GW4064, or TUDCA. Livers were taken for histological analysis 4 hours after the final dose. The panels show representative H&E-stained liver sections from each treatment group at ×400 magnification. (a) Sham-operated rats showing normal liver histology. (b) Vehicle-treated BDL rat showing bile duct proliferation (open arrow) and parenchymal necrosis (filled arrow) with inflammatory cell infiltration. (c) GW4064-treated BDL rat showing bile duct proliferation (white arrow) and fatty cell degeneration (shaded arrow). (d) TUDCA-treated BDL rat showing parenchymal necrosis (filled arrow) with inflammatory cell infiltration. (e) Mitotic nuclei were counted in samples from all rats. Mitosis was quantified by expressing the number of hepatocytes showing mitotic nuclei as a percentage of the total number of hepatocytes. White bars, sham; black bars, vehicle (Veh); dark gray bars, GW4064; light gray bars, TUDCA. Values are presented as average ± SEM. Statistically significant differences between the sham-operated and vehicle/BDL groups are indicated (#P < 0.05). Statistically significant differences between the vehicle/BDL group and either the GW4064-treated or the TUDCA-treated groups are also indicated (*P < 0.05). (f) Bile duct proliferation was quantified by measuring the area occupied by cholangiocytes in 40–50 randomly selected fields under ×400 magnification. Groups as in e; statistics as in e.
To quantify some of the differences among the treatment groups, further histological analysis was done. The number of mitotic nuclei was significantly increased by bile duct ligation, and this induction was significantly decreased by GW4064 treatment, but not by TUDCA treatment (Figure 5e). Similarly, bile duct proliferation was significantly increased by bile duct ligation, and this increase was significantly reduced by GW4064 treatment, but not by TUDCA treatment (Figure 5f).
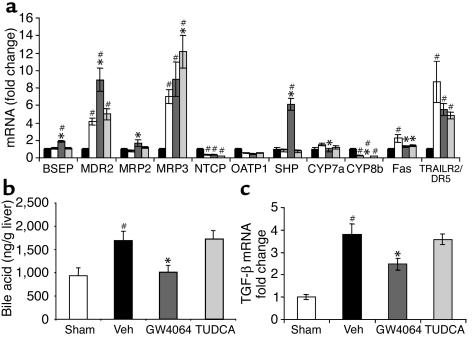
Next, we profiled the expression of key genes in livers from the BDL rats. First, we examined expression of the canalicular transporters BSEP and MDR2. As described previously, BSEP levels were unchanged by bile duct ligation alone (28) (Figure 6a). BSEP expression was induced by GW4064 treatment, as expected, because BSEP is a direct FXR target gene (11, 12) (Figure 6a). Bile duct ligation alone increased MDR2 expression approximately fourfold, in agreement with a previous study (29). GW4064 treatment further increased the expression of MDR2 (Figure 6a), an effect also seen in the ANIT study. Hence, in both the ANIT and BDL models, the hepatoprotection seen following GW4064 treatment may, in part, be due to further induction of MDR2 and BSEP. In contrast to GW4064, TUDCA did not affect MDR2 or BSEP expression (Figure 6a).
Figure 6.
Liver gene expression profile in BDL model of cholestasis; liver bile acid concentration. (a) Gene expression profile in liver of rats with bile duct ligation–induced cholestasis. White bars, sham; black bars, vehicle; dark gray bars, GW4064; light gray bars, TUDCA. Statistically significant differences from sham-treated mice are indicated (#P < 0.05). Statistically significant differences from vehicle are also indicated (*P < 0.05). (b) Liver bile acid concentration in BDL rats. Bile acids were measured using API-LSMS. Groups as in a; statistics as in a. (c) TGF-β1 (TGF-β) expression in liver of rats with bile duct ligation–induced cholestasis. Groups as in a; statistics as in a.
Expression profiling of the canalicular transporter MRP2 showed induction only upon GW4064 treatment (Figure 6a). MRP3 expression was induced sevenfold by bile duct ligation, in agreement with a previous report (35), and only TUDCA treatment resulted in a further increase (Figure 6a). Bile duct ligation alone caused repression of NTCP expression, as reported previously (37, 38), and OATP1 was also repressed by bile duct ligation. There was no further repression of NTCP or OATP1 by either drug treatment (Figure 6a).
Next we examined the expression of genes involved in bile acid biosynthesis. SHP expression was not altered by bile duct ligation but was highly induced by GW4064 treatment, as expected (Figure 6a). Interestingly, we saw an increase in CYP7A1 expression in vehicle-treated BDL rats compared with sham-operated animals, which agrees with previous reports of increased CYP7A1 enzymatic activity in BDL rats (39, 40). CYP7A1 expression was significantly reduced by GW4064 treatment (Figure 6a). As in the ANIT model, CYP8B1 expression was reduced almost 80% by bile duct ligation alone (Figure 6a), and GW4064 treatment resulted in a further significant decrease in CYP8B1. TUDCA had no effect on SHP, CYP7A1, or CYP8B1 expression.
To further investigate whether the GW4064-induced changes in expression of transporters and bile acid biosynthetic genes in the BDL rat might result in changes in liver bile acid concentrations, bile acids, not including UDCA or its conjugates, were measured in liver samples using API-LCMS. As expected, the concentration of bile acids was significantly increased by bile duct ligation alone (Figure 6b). Treatment with GW4064, but not TUDCA, resulted in a statistically significant reduction in the bile acid content of the liver (Figure 6b). Thus it is possible that the hepatoprotection seen with the FXR agonist may be due, in part, to decreased liver bile acid content.
The expression of Fas and TRAILR2/DR5 were also explored in the BDL livers. As in the ANIT study, and as demonstrated previously (33), Fas and TRAILR2/DR5 were induced by bile duct ligation (Figure 6a). Both GW4064 and TUDCA treatment resulted in significant reductions in the expression of Fas, whereas only small reductions were seen in TRAILR2/DR5. Because the repression of Fas and TRAILR2/DR5 by TUDCA does not correlate with reduction in serum markers of damage or histological assessment of damage in these animals, TUNEL staining and caspase assays were performed. Quantitation of TUNEL staining correlated well with the serum and histological indices of hepatic damage, with increased staining in vehicle and TUDCA-treated BDL rats and decreased staining in GW4064-treated rats (data not shown). Only very small changes in caspase 3 activity were seen across the four treatment groups (data not shown). Hence, further investigation is needed to understand the specific effects of TUDCA and FXR agonist treatment in regulation of Fas and TRAILR2/DR5 expression and the downstream effects on apoptotic cell death in the BDL animal.
Recently, it has been suggested that inhibition of Fas-mediated hepatocyte injury might reduce liver fibrogenesis (41). In addition, a reduction in TGF-β1, a key mediator of fibrosis, might be expected to reduce liver fibrogenesis (42). TGF-β1 is produced by activated Kupffer cells secondary to parenchymal necrosis and by cholangiocytes. Since GW4064 treatment of BDL rats leads to reduced necrosis and reduced bile duct proliferation, we investigated whether the FXR agonist might affect fibrosis in the BDL rat. Indeed, bile duct ligation caused a marked increase in TGFβ1 expression (Figure 6c). GW4064 treatment, but not TUDCA, significantly reduced expression of this mediator of fibrosis. At this day-4 time point, increased gene expression and staining of the fibrotic markers, smooth muscle actin and collagen I, were not detected (data not shown) in the vehicle-treated BDL rats, though this might expected at longer time points. Hence, the effect of GW4064 treatment on these fibrotic markers could not be determined in this experiment. Taken together, these data indicate that GW4064 affords substantial protection against hepatocellular damage in the BDL rat and that further investigation into the effects on hepatocyte apoptosis and fibrosis is warranted.
Discussion
The discovery that FXR is activated by physiological concentrations of bile acids (8–10) has led to a tremendous increase in our understanding of the regulation of bile acid synthesis and transport. It has been known for many years that bile acids repress their own biosynthesis and regulate the flow of bile. Furthermore, it has been proposed that bile acids regulate these pathways at the level of gene transcription (43, 44). It is now known that the feedback repression of genes involved in bile acid biosynthesis, notably CYP7A1, is accomplished, in part, by activation of FXR (15–17). In addition to regulating bile acid biosynthesis, FXR has been shown to directly regulate the expression of two genes that encode canalicular bile acid transporters, namely, BSEP and MRP2 (11, 13). These ATP-binding cassette (ABC) proteins transport bile acids and other organic anions, including bilirubin metabolites, across the canalicular membrane of the hepatocyte into the bile ductules. Canalicular transport of bile acids is the rate-limiting step in hepatic excretion of these potentially harmful compounds, and BSEP expression is critical in this process (45, 46). Several mutations in the BSEP gene have been identified in the human population, and inactivating mutations give rise to PFIC2 (2). Induction of BSEP expression following cholic acid feeding is lost in FXR-null mice. These mice have high serum bile acids and hepatocellular necrosis when fed a cholic acid–containing diet, indicative of dysfunctional hepatic bile acid excretion (17). Mutations in MRP2 give rise to Dubin-Johnson syndrome, a disease characterized by high serum bilirubin due to the inability of the liver to excrete this metabolite into bile (47). Hence, these transporters are critical for bile formation and for clearing the hepatocyte of potentially toxic substances. Here we present evidence that FXR agonists induce expression of another canalicular ABC transporter, MDR2/3. Transport of phospholipid into bile by MDR2/3 is required for bile formation (48). MDR2-null mice have inflammatory cholangitis induced by toxic injury by bile devoid of phospholipid (4, 48). Similarly, mutations in the human MDR3 lead to PFIC3 characterized by high γ-glutamyl transferase (GGT) levels, indicative of the damaging effects of unbuffered bile on the cholangiocytes (3). Thus, BSEP, MRP2, and MDR2/3 are key transporters involved in bile formation, and all are induced by GW4064.
During cholestasis, the interruption in bile flow results in liver damage, including necrosis, fibrosis, and cirrhosis. We employed two rodent models of cholestasis to determine if the potent FXR agonist GW4064 could protect against cholestatic liver damage. We compared the effects of GW4064 treatment in these models to those of TUDCA, the drug used clinically for the treatment of cholestasis. TUDCA is a polar bile acid that does not activate FXR (8–10). Although not fully understood, it is thought that UDCA treatment may decrease serum markers of liver damage in cholestatic patients by decreasing the hydrophobicity and toxicity of the bile pool (5). Regrettably, the improvement in serum chemistry in patients taking UDCA does not appear to translate to increased time until liver transplant or death (5, 6).
The ANIT rat model of cholestasis has been used extensively to study cholestatic liver disease. In this intrahepatic rodent model of cholestasis, ANIT is conjugated to glutathione in the hepatocyte and then transported across the canalicular membrane by MRP2, where it damages the cholangiocytes lining the bile ducts (25, 49). Cessation of bile flow is seen within 24 hours (50). The BDL rat model is a severe model of extrahepatic obstructive cholestasis. In this model, the drainage of bile from the liver to the duodenum is blocked, resulting in the accumulation of bile in the liver. In both models we saw compensatory changes in expression of bile transporters and bile acid biosynthetic genes, including MDR2, MRP3, and CYP8B1, as well as NTCP and CYP7A1 in the ANIT study. Yet the serum clinical chemistry parameters and histology indicate these changes are not sufficient to protect the liver from damage. Remarkably, treatment with the FXR agonist yielded large reductions in serum ALT, AST, and LDH activities in both the BDL and ANIT models. ALP activity, serum bile acid, and bilirubin levels were also decreased by GW4064 treatment in the ANIT model. These markers indicate there was a significant improvement in hepatic function in both models following treatment with the FXR agonist. In comparison, only LDH activity was decreased by TUDCA treatment. Histological examination of the livers from both studies revealed profound changes as well. BDL livers and ANIT-treated livers showed large areas of necrosis, inflammatory cell infiltration, as well as bile duct proliferation in the BDL rats. GW4064 treatment led to remarkable decreases in the extent and severity of this damage, whereas TUDCA treatment had much smaller effects.
Because FXR is a ligand-activated transcription factor, it is likely that the hepatoprotection provided by the potent selective agonist, GW4064, is due to transcriptional regulation of target genes. Indeed, GW4064 treatment induced expression of the canalicular transporters BSEP, MDR2, and MRP2 beyond what is seen following administration of ANIT or bile duct ligation alone. This induced expression suggests that the FXR agonist is protecting the liver by providing a mechanism for clearance of the toxic bile constituents from the hepatocyte. Although these transporters are transcriptionally regulated, assessment of the functional activity of the transporter proteins will be necessary to conclusively determine whether GW4064 treatment results in increased transport from the hepatocyte. Vesicular trafficking and cellular compartmentalization may be altered in the cholestatic liver and could affect functional expression of these gene products.
Treatment with GW4064 also gives rise to induction of SHP in both of these models, which leads to further repression of the bile acid biosynthetic genes CYP7A1 and CYP8B1. This suggests a decrease in the synthesis of bile acids may also play a role in the protection afforded by the FXR agonist. Indeed, in the BDL rats GW4064 treatment led to decreased concentrations of bile acids in the liver. Although a reduction in CYP8B1 alone might be expected to increase the hydrophobicity of the bile pool and increase hepatotoxicity, there is no evidence for increased toxicity of the bile pool in the GW4064-treated rats, possibly due to the coordinate downregulation of CYP8B1 and CYP7A1 and reduction in the total bile concentration.
Fas and TRAILR2/DR5 are receptors that mediate cholestatic injury and induce apoptosis (33, 34). ANIT treatment or bile duct ligation induced expression of both receptors. Both TUDCA and GW4064 treatment tended to reduce expression of these receptors, in some cases significantly. Quantitation of the extent of apoptosis in these livers was inconclusive. Because reduction of Fas and TRAILR2/DR5 expression by TUDCA does not correlate with reduction in serum markers of hepatic damage nor with severity of histological damage, it is difficult to gauge its relative importance in these studies. Further investigation in this area will be key to understanding the mechanisms of damage in cholestatic liver disease.
Because GW4064 treatment reduced the amount of parenchymal necrosis and cholangiocyte proliferation in the BDL model, we investigated the effect of the FXR agonist on markers of liver fibrosis. Cholangiocytes and activated Kupffer cells secrete TGF-β1, a key regulator of the liver’s fibrotic response to stress and injury. Reductions in the number of proliferating cholangiocytes and parenchymal necrosis might be expected to correlate with reductions in TGF-β1 and subsequent reduction in the activation of hepatic stellate cells. Indeed, TGFβ1 expression was induced in the vehicle-treated BDL rat liver, and this induction was decreased in response to GW4064 treatment. Unfortunately, the development of fibrosis in this 4-day BDL model had not proceeded to an extent where significant changes in smooth muscle actin, collagen I expression, or staining could be detected. Reductions in Fas have also been suggested as antifibrogenic therapy (41); hence, further investigation into the role of FXR agonists on the development of cholestatic fibrosis is needed.
Due to their ability to repress bile acid biosynthesis and induce canalicular bile acid transporters, it has been proposed that FXR agonists might be useful in the treatment of cholestasis (17, 51). Recently, the modified bile acid, 6-ethyl-chenodeoxycholic acid (6EtCDCA) was shown to be an FXR agonist (52). This potent FXR agonist can reverse the reduction in bile flow caused by infusion of the FXR antagonist, lithocholic acid (LCA) (8, 52, 53). 6EtCDCA also prevented LCA-induced hepatocellular necrosis in an acute setting (52). Since LCA does not accumulate to appreciable levels in the cholestatic rat (39), we believe the protective effects of GW4064 in the chronic BDL and ANIT models are not due to reversal of the effects of LCA antagonism of FXR activity. From our data it appears likely that induction of the canalicular transporters BSEP, MDR2/3, and MRP2 and repression of bile acid biosynthesis by GW4064 in the cholestatic liver provides a mechanism to decrease the concentration of toxic bile acids in the liver. The role of FXR in other liver cell types, such as cholangiocytes, has not yet been elucidated, so it cannot be ruled out that the hepatoprotection by GW4064 may also involve additional mechanisms that are currently undefined.
In conclusion, these data provide substantial in vivo evidence of hepatoprotection by FXR agonists in animal models of cholestatic liver disease. The tremendous improvement in serum liver chemistries and histology supports the hypothesis that FXR agonists may have therapeutic use in cholestatic liver disease.
Acknowledgments
The authors thank Tim Willson, John T. Moore, and Roger Brown for helpful discussions. We also thank Paul Novak and Joe Watson for help with the bile duct ligation studies.
Footnotes
Steven A. Kliewer’s present address is: Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Conflict of interest: Yaping Liu, Jane Binz, Mary Jo Numerick, Steve Dennis, Guizhen Luo, Bhasha Desai, Kathleen I. MacKenzie, Steven A. Kliewer, Bryan Goodwin, and Stacey A. Jones are employees of GlaxoSmithKline.
Nonstandard abbreviations used: primary biliary cirrhosis (PBC); progressive familial intrahepatic cholestasis type I (PFIC1); 2,2,4,4,d4-cholic acid (D4-cholic acid); bile salt export pump (BSEP); ursodeoxycholic acid (UDCA); farnesoid X receptor (FXR); multidrug resistance–related protein 2 (MRP2); sterol 12α-hydroxylase (CYP8B1); cholesterol 7α-hydroxylase (CYP7A1); taurine-conjugated UDCA (TUDCA); alanine aminotransferase (ALT); aspartate aminotransferase (AST); lactate dehydrogenase (LDH); alkaline phosphatase (ALP); α-naphthylisothiocyanate (ANIT); bile duct–ligated (BDL) ; reverse transcription quantitative PCR (RTQ-PCR); atmospheric pressure ionization-liquid chromatography mass spectrometry (API-LCMS); chenodeoxycholic acid (CDCA); lithocholic acid (LCA); organic anion transporting polypeptide-1 (OATP1); TNF-related apoptosis-inducing ligand receptor 2/death receptor 5 (TRAILR2/DR5 or DR5); sodium taurocholate cotransporting polypeptide (NTCP); ATP-binding cassette (ABC); 6-ethyl-chenodeoxycholic acid (6EtCDCA).
References
- 1.Bull LN, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 2.Strautnieks SS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat. Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 3.de Vree JM, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit JJ, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 5.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 6.Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354:1053–1060. doi: 10.1016/S0140-6736(98)11293-X. [DOI] [PubMed] [Google Scholar]
- 7.Forman BM, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 8.Parks DJ, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 9.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 11.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 2001;276:28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 12.Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. Eur. J. Biochem. 2002;269:3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- 13.Kast HR, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 14.del Castillo-Olivares A, Gil G. Suppression of sterol 12alpha-hydroxylase transcription by the short heterodimer partner: insights into the repression mechanism. Nucleic Acids Res. 2001;29:4035–4042. doi: 10.1093/nar/29.19.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin B, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 16.Lu TT, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 17.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Ghishan, F.K. 1996. Inborn errors of metabolism that lead to permanent liver injury. In Hepatology: a textbook of liver disease. D. Zakim and T.D. Boyer, editors. WB Saunders Co. Philadelphia, Pennsylvania, USA. 1574–1630.
- 19.Reichen, J., and Simon, F.R. 1994.Cholestasis. In The liver: biology and pathobiology. I.M. Arias et al., editors. Raven Press Ltd. New York, New York, USA. 1291–1326.
- 20.Bezerra JA, Balistreri WF. Intrahepatic cholestasis: order out of chaos. Gastroenterology. 1999;117:1496–1498. doi: 10.1016/s0016-5085(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 21.Maloney PR, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 22.Muoio DM, et al. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 2002;51:901–909. doi: 10.2337/diabetes.51.4.901. [DOI] [PubMed] [Google Scholar]
- 23.Chisholm JW, Dolphin PJ. Abnormal lipoproteins in the ANIT-treated rat: a transient and reversible animal model of intrahepatic cholestasis. J. Lipid Res. 1996;37:1086–1098. [PubMed] [Google Scholar]
- 24.Orsler DJ, Ahmed-Choudhury J, Chipman JK, Hammond T, Coleman R. ANIT-induced disruption of biliary function in rat hepatocyte couplets. Toxicol. Sci. 1999;47:203–210. doi: 10.1093/toxsci/47.2.203. [DOI] [PubMed] [Google Scholar]
- 25.Kossor DC, et al. Biliary epithelial cell proliferation following alpha-naphthylisothiocyanate (ANIT) treatment: relationship to bile duct obstruction. Fundam. Appl. Toxicol. 1995;26:51–62. doi: 10.1006/faat.1995.1074. [DOI] [PubMed] [Google Scholar]
- 26.Dahm LJ, Roth RA. Protection against alpha-naphthylisothiocyanate-induced liver injury by decreased hepatic non-protein sulfhydryl content. Biochem. Pharmacol. 1991;42:1181–1188. doi: 10.1016/0006-2952(91)90252-z. [DOI] [PubMed] [Google Scholar]
- 27.Di Padova C, Di Padova F, Tritapepe R, Stramentinoli G. S-Adenosyl-L-methionine protection against alpha-naphthylisothiocyanate-induced cholestasis in the rat. Toxicol. Lett. 1985;29:131–136. doi: 10.1016/0378-4274(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, et al. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–172. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- 29.Kagawa T, et al. Differential expression of multidrug resistance (mdr) and canalicular multispecific organic anion transporter (cMOAT) genes following extrahepatic biliary obstruction in rats. Biochem. Mol. Biol. Int. 1998;44:443–452. doi: 10.1080/15216549800201462. [DOI] [PubMed] [Google Scholar]
- 30.Huang, L., et al. 2003. Farnesoid X-receptor activates transcription of the phospholipid pump MDR3. J.Biol. Chem. doi:10.1074/jbc.M308321200. [DOI] [PubMed]
- 31.Ogawa K, et al. Characterization of inducible nature of MRP3 in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- 32.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33:783–791. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi H, Bronk SF, Taniai M, Canbay A, Gores GJ. Cholestasis increases tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity. J. Pharmacol. Exp. Ther. 2002;303:461–467. doi: 10.1124/jpet.102.040030. [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 35.Higuchi H, et al. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. J. Biol. Chem. 2003;278:454–461. doi: 10.1074/jbc.M209387200. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi H, et al. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J. Biol. Chem. 2001;276:38610–38618. doi: 10.1074/jbc.M105300200. [DOI] [PubMed] [Google Scholar]
- 37.Gartung C, Schuele S, Schlosser SF, Boyer JL. Expression of the rat liver Na+/taurocholate cotransporter is regulated in vivo by retention of biliary constituents but not their depletion. Hepatology. 1997;25:284–290. doi: 10.1002/hep.510250205. [DOI] [PubMed] [Google Scholar]
- 38.Gartung C, et al. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- 39.Dueland S, Reichen J, Everson GT, Davis RA. Regulation of cholesterol and bile acid homoeostasis in bile-obstructed rats. Biochem. J. 1991;280:373–377. doi: 10.1042/bj2800373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naito T, Kuroki S, Chijiiwa K, Tanaka M. Bile acid synthesis and biliary hydrophobicity during obstructive jaundice in rats. J. Surgical Research. 1996;65:70–76. doi: 10.1006/jsre.1996.0345. [DOI] [PubMed] [Google Scholar]
- 41.Canbay A, et al. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 42.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J. Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 43.Pandak WM, Heuman DM, Hylemon PB, Chiang JY, Vlahcevic ZR. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108:533–544. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 44.Pandak WM, et al. Regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J. Biol. Chem. 1991;266:3416–3421. [PubMed] [Google Scholar]
- 45.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 46.Suchy FJ, Sippel CJ, Ananthanarayanan M. Bile acid transport across the hepatocyte canalicular membrane. FASEB. J. 1997;11:199–205. doi: 10.1096/fasebj.11.4.9068608. [DOI] [PubMed] [Google Scholar]
- 47.Paulusma CC, et al. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- 48.Mauad TH, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 49.Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 50.Roth RA, Dahm LJ. Neutrophil- and glutathione-mediated hepatotoxicity of alpha-naphthylisothiocyanate. Drug Metab. Rev. 1997;29:153–165. doi: 10.3109/03602539709037578. [DOI] [PubMed] [Google Scholar]
- 51.Arrese M, Karpen SJ. New horizons in the regulation of bile acid and lipid homeostasis: critical role of the nuclear receptor FXR as an intracellular bile acid sensor. Gut. 2001;49:465–466. doi: 10.1136/gut.49.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellicciari R, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, et al. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J. Biol. Chem. 2002;277:31441–31447. doi: 10.1074/jbc.M200474200. [DOI] [PubMed] [Google Scholar]