Abstract
The plant Withania somnifera (Linn.) (Solanacea) is a well-known herbal medicine used in many parts of the world. It has anti-inflammatory, antioxidant, and antitumor as well as neural protective properties. It seems as if the two most active withanolide components, namely withaferin A and withanolide D, found in methanol (MeOH) extracts, are responsible for the anti-inflammatory and antioxidant properties of the plant. The current research evaluated and compared the cytotoxic potential of water and methanol extracts of W. somnifera using a combined crystal violet MTT and Neutral Red assay. MRC-5 cells, a human embryonic lung-derived diploid fibroblast cell line, were the cells of choice. We found that the three lowest concentrations (0.007, 0.042, 0.250 µg/ml) of the plant material extracted in double distilled H2O and MeOH do not differ significantly in any of the assays. We therefore suggest that low concentrations of MeOH extracts (up to 0.250 µg/ml plant material) do not cause cell damage to the MRC-5 cells, however, higher levels should be avoided as cell viability and cell numbers are negatively influenced.
Keywords: Withania somnifera, anti-inflammatory, water extract, methanol extract
Introduction
Withania somnifera (Linn.) is a member of the plant family Solanaceae and is also known as Ashwagandha, Indian Ginseng or Winter Cherry. It is an important component of the Ayurvedic pharmacopoeia of India, where it has been used for hundreds of years (Henderson and Anderson, 1966; Ahmad et al., 2005; Glaser, 1988). It has a wide distribution in Africa, Southern Europe and Asia, and is considered to be indigenous to South Africa (Henderson and Anderson, 1966). It also grows in the drier tropical regions and is also found from Liberia to Nigeria and the Southeast Islands (Iwu, 1993).
The plant is chemically very complex and more than 80 compounds have been isolated (van Wyk et al., 2000). The withanolides (steroids with an ergostane skeleton), particularly Withaferin A, 3-β-hydroxy-2,3-dihydrowithanolide F as well as Withanolide D are of particular medicinal interest (Chu et al., 1988; van Wyk et al., 2000). Research has shown that W. somnifera has anti-inflammatory, antioxidant anti-tumour and immunomodulatory properties (Anbalagan and Sadique, 1981; Begum and Sadique, 1988; Somasundaram et al., 1983; Kulkarni et al., 1991; Al-Hindawi et al., 1992; Begum and Sadique, 1987; Bhattacharya et al., 1997; Parihar and Hemnani, 2003; Parihar et al., 2004).
Some of the important active compounds reported in the herb include the following: Withaferin A, sitoindosides VII-X, 5-dehydroxywithanolide-R, Withasomniferin-A, 1-oxo-5beta,6beta-epoxy-witha-2-ene-27-ethoxy-olide,4-(1-hydroxy-2,2-methylcyclpropanone) -2,3-dihydrowithaferin A, 2,3-dihydrowithaferin A, 24,25-dihydro-27-desoxywithaferin A, physagulin D (1-6)-beta-D-glucopyranosyl-(1-4)-beta-D-glucopyranoside, 27-O-beta-D-glucopyranosylphysagulin D, physagulin D, Withanoside I–VII, 27-O-beta-D-glucopyranosylviscosalactone B, 4,16-dihydroxy-beta,6beta-epoxyphysagulin D, Viscosalactone B and Diacetylwithaferin A (Uma Devi, 1996; Ganzera et al., 2003; Jayaprakasam et al., 2003., Kaur et al., 2003; Matsuda et al., 2001).
The two most active Withanolide components, Withaferin A and Withanolide D are found in methanol extracts (Kuboyama et al., 2002; Ganzera et al., 2003; Choudhary et al., 2004). Also, the main antioxidant activity of W. somnifera was found in the methanol fraction (Parihar et al., 2004). Although water extracts are also known to have great medicinal potential, the methanol extracts seem to extract the active components the best. The question, however, arises whether the methanol extracts of the herb may be cytotoxic to cells in culture. Therefore, because of the potential use of the herb as an anti-inflammatory product, the current research aimed to evaluate and compare the cytotoxic potential of water and methanol extracts of W. somnifera using a combined Crystal Violet assay, the MTT assay and the Neutral Red assay. The cells of choice were MRC-5 cells, which is a human embryonic lung-derived diploid fibroblast cell line. Statistical analyses were employed to determine whether there is a correlation between the two extract methods, as well as the concentrations used.
Experimental Procedures
Herb material
Root material (WS1PRETORIA) was collected in the Pretoria area (South Africa) and identified by botanists from the Department of Botany, University of Pretoria, South Africa. A herbarium specimen was prepared and compared to an authentic specimen in the HGJW Sweikerdt herbarium at the University of Pretoria.
Preparation of extracts
Leaves, stems and roots were fragmented and dried at 45°C for 48 h and MeOH and water extracts were made. Plant material (140 mg) was added to 1,400 ml double distilled (dd) H2O of water, yielding a final concentration of 100mg plant material per ml. 2.1039 g of MeOH extracted plant material was added to 4.2 ml MeOH, yielding a final concentration of 0.5 g of plant material per ml MeOH. This was diluted 1:4 with dd H2O, yielding a final concentration of 100 mg plant material in 1 ml. Five concentrations of extracts of both ddH2O and MeOH were added to the cells in culture. The concentrations were: 0.007, 0.042, 0.250, 1.151, and 9.090 µg/ml.
MRC-5 Permanent cell line
MRC-5 cells were obtained from Highveld Biological Company, Johannesburg, South Africa. The cells were plated at a cell concentration of 2×104 cells per ml in 24 flat well plates and were kept for 24 h at 37°C and 5% CO2 before conducting the experiments. Solution of 100mg/ml of each of the plant extracts was prepared either in dd H2O or MeOH and exposed to MRC-5 cells for 48 h. The cytotoxicity of MeOH alone was also determined.
Combined Crystal Violet, MTT and Neutral Red assay
Cell number, cell viability and lysosomal membrane integrity, were determined using a combined Crystal Violet, MTT and Neutral Red (CV/MTT/NR) assay. To each well 100µl 0.15% NR prepared in DPBS (A 10× DPBS stock solution was prepared by dissolving 2g/l KCl, 2g KH2PO4, 80g/l NaCl and Na2HPO4 in ddH2O that was diluted 1:9 with ddH2O prior to use) was added and then incubated for 90 min at 37°C in a CO2 incubator. A further 50µl volume of a 0.1 mg/ml 3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution prepared in DPBS was added to each well and the cell culture plates were maintained for a further 60 min at 37°C. The medium was then carefully removed and the plates were blotted dry. The cells were fixed for 10 min with 200µl of a 1% acetic acid and 1% formaldehyde solution in ddH2O. The fixative was removed and the NR was solubilized with 200µl of a 1% acetic acid and 50% ethanol solution prepared in ddH2O. The dissolved NR was transferred into a 96-flat well plate. The MTT formazan crystals were then dissolved with 200µl DMSO by shaking of the plates for 20 min before being transferred into a 96-flat well plate. Each well was washed once with DPBS and was dried in a microwave oven for a few mins at 60% power. The cells attached to the bottom of the plate were stained by adding a volume of 300µl of a 0.1% (weight/ volume (w/v) CV solution prepared in 200mM of formic acid pH 3.5, each well for 60 min. After the microplate was washed with ddH2O and dried, the bound dye was dissolved in 10% acetic acid prepared in ddH2O. The solution was transferred to a 96-well and the absorbance of all bioassays was determined spectrophotometrically at 570 nm using the Microplate Reader (ELx800).
Statistical analysis
The experiment was conducted in a factorial design with main effects the extracts at two levels (plant material extracted in ddH2O and also in MeOH) and concentration at five levels (0.007, 0.042, 0.250, 1.151, and 9.090 µg/ml). For each of the three assays the outcome variable was analysed in an appropriate analysis of variance (ANOVA) with two fixed effects, namely extract and concentration as well as the interaction. Pair-wise comparisons were done using the LSD method of Fisher. Testing was done at the 0.05 level of significance.
Results and Discussion
Combined Crystal Violet, MTT and Neutral Red assay
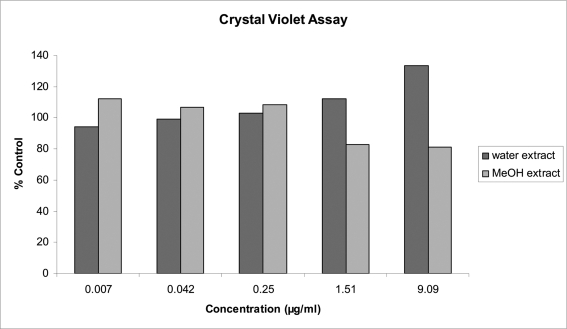
The CV assay measures cell number in the presence of the 5 concentrations of the two extracts. All analyses were performed on the dataset and bar graphs of the results compiled (Figure 1 – 3). From the ANOVA the two extracts differ significantly (p = 0.109) but not the concentrations (p = 0.5676). However, a significant interaction between extracts and concentrations exists (p<0.0001). This interaction is displayed in Figure 1. Note that the first 3 concentrations of the two extracts react similarly but behave very differently over the last two concentrations, causing the significant difference between extracts (p = 0.0109) at these high concentrations, the ddH2O extract increased cell numbers, while the MeOH extract caused a decrease.
Figure 1.
Effects of 5 concentrations of W. somnifera, extracted in ddH2O and MeOH and prepared in cell culture medium on MRC-5 cell number after 48 h exposure: results from Crystal Violet Assay.
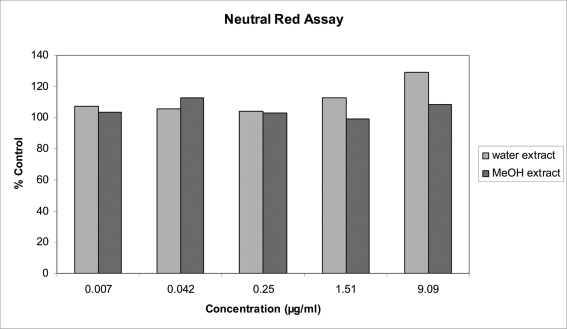
Figure 3.
Effects of 5 concentrations of W. somnifera, extracted in ddH2O and MeOH and prepared in cell culture medium on MRC-5 lysosomal integrity after 48 h exposure: results from Neutral Red Assay.
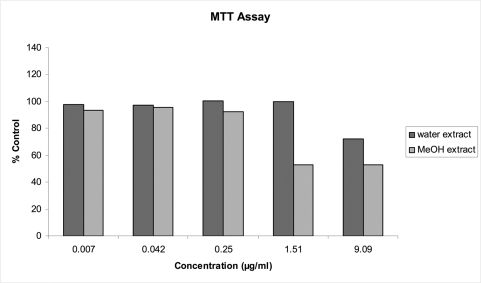
Figure 2 displays the results of the MTT assay, showing cell viability. ANOVA showed that the two extracts differ significantly (p = 0.0005) and that the concentrations also differ significantly (p<0.0001). In particular, following pair wise comparisons, the first three concentrations do not differ significantly but differ from the last two concentrations which also differ from each other. There is also a significant interaction between extract and concentration (p = 0.0110) which is clear from Figure 2, where except for the fourth concentration the extracts behave similarly. However, what can be concluded from the graph is that cell viability decreases when using the two highest concentrations of the MeOH extract and the highest concentration of the ddH2O extract.
Figure 2.
Effects of 5 concentrations of W. somnifera, extracted in ddH2O and MeOH and prepared in cell culture medium on MRC-5 cell viability after 48 h exposure: results from MTT Assay.
The final assay, namely the NR assay, which determines lysosomal membrane integrity, is presented in Figure 3. ANOVA showed that respectively the extracts and the concentrations are significantly different (p = 0.0304 and p = 0.0170). In particular, LSD pair-wise comparisons show that the first four concentrations do not differ significantly; however, these four concentrations differ from the highest concentration. The significant interaction between extract and concentration is also clear in Figure 3, where the two extracts behave similarly over the first three concentrations and quite differently over the last two concentrations.
We therefore conclude that the three lowest concentrations (0.007, 0.042, and 0.250 µg/ml) of the of the plant material extracted in ddH2O and MeOH seems not to be significantly different in any of the assays. At these three concentrations cell numbers, cell viability and lysosomal membrane activity were preserved.
Thus low concentrations of MeOH extracts (up to 0.250 µg/ml plant material) do not cause cell damage to the human embryonic lung-derived diploid fibroblast cell line, however, higher levels should be avoided as particularly cell viability and cell numbers are negatively influenced. The two active components Withaferin A and Withanolide D are most successfully extracted using MeOH (Kuboyama et al., 2002; Ganzera et al., 2003; Choudhary et al., 2004). Research therefore investigating the anti-inflammatory or antioxidant activity of W. somnifera can use MeOH extracts, but care should be taken not to use concentrations that are too high.
Acknowledgements
We thank the National Research Foundation for funding both E. Pretorius (Indigenous Knowledge Systems: FA2004033100004
References
- 1.Ahmad M, Saleem S, Ahmad AS, Ansari M A, Yousuf S, Hoda N. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24(3):137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hindawi MK, al-Khafaji SH, Abdul-Nabi MH. Anti-granuloma activity of Iraqi Withania somnifera. J Ethnopharmacol. 1992;37:113–116. doi: 10.1016/0378-8741(92)90069-4. [DOI] [PubMed] [Google Scholar]
- 3.Anbalagan K, Sadique J. Influence of an Indian medicine (Ashwagandha) on acute-phase reactants in inflammation. Indian J Exp Biol. 1981;19:245–249. [PubMed] [Google Scholar]
- 4.Begum VH, Sadique J. Effect of Withania somnifera on glycosaminoglycan synthesis in carrageenin-induced air pouch granuloma. Biochem Med Metab Biol. 1987;38:272–277. doi: 10.1016/0885-4505(87)90091-0. [DOI] [PubMed] [Google Scholar]
- 5.Begum VH, Sadique J. Long term effect of herbal drug Withania somnifera on adjuvant induced arthritis in rats. Indian J Exp Biol. 1988;26:877–882. [PubMed] [Google Scholar]
- 6.Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol. 1997;35:236–239. [PubMed] [Google Scholar]
- 7.Choudhary MI, Yousuf S, Nawaz SA, Ahmed S, Atta-ur-Rahman A. Cholinesterase inhibiting withanolides from Withania somnifera. Chem Pharm Bull. 2004;52:1358–1361. doi: 10.1248/cpb.52.1358. [DOI] [PubMed] [Google Scholar]
- 8.Chu DT, Wong W, Mavligit GM. Immunotherapy with Chinese medicinal herbs. I. Immune restoration of local xenogeneic graft-versus-host reaction in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol. 1988;25:119–129. [PubMed] [Google Scholar]
- 9.Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia. 2003;74:68–76. doi: 10.1016/s0367-326x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 10.Glaser JL. Maharishi Ayurveda: an introduction to recent research. Modern Science and Vedic Science. 1988;2(1):89–108. [Google Scholar]
- 11.Henderson M, Anderson JG. Common weeds in South Africa. Memoirs of the Botanical Survey of South Africa. 1966;37:286. [Google Scholar]
- 12.Iwu MM. Handbook of African Medicinal Plants. Florida, USA: CRC Press; 1993. [Google Scholar]
- 13.Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74:125–132. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Kaur P, Sharma M, Mathur S, Tiwari M, Divekar H M, Kumar R, Srivastava K K, Chandra R. Effect of 1-oxo-5beta, 6beta-epoxy-with a-2-ene 27-ethoxy-olide isolated from the roots of Withania somnifera on stress indices in Wistar rats. J Altern Complement Med. 2003;9:897–907. doi: 10.1089/107555303771952244. [DOI] [PubMed] [Google Scholar]
- 15.Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, Komatsu K. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport. 2002;13:1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni RR, Patki PS, Jog VP, Gandage SG, Patwardhan B. Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J Ethnopharmacol. 1991;33:91–95. doi: 10.1016/0378-8741(91)90167-c. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda H, Murakami T, Kishi A, Yoshikawa M. Structures of Withanolides I, II, III, IV, V, VI and VII, new withanolide glycosides, from roots of Indian Withania somnifera DUNAL and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg Med Chem. 2001;9:1499–1507. doi: 10.1016/s0968-0896(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 18.Parihar MS, Chaudhary M, Shetty R, Hemnani T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: prevention by extracts of Withania somnifera and Aloe vera. J Clin Neurosci. 2004;11:397–402. doi: 10.1016/j.jocn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Parihar MS, Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J Biosci. 2003;28:121–128. doi: 10.1007/BF02970142. [DOI] [PubMed] [Google Scholar]
- 20.Schroter HB, Neumann D, Katritzky AR, Swinborne F. Withasomnine, a pyrazole alkaloid from Withania somnifera. Tetrahedron Lett (London) 1966;22:2895–2897. [Google Scholar]
- 21.Somasundaram S, Sadique J, Subramoniam A. In vitro absorption of [14C]leucine during inflammation and the effect of antiinflammatory drugs in the jejunum of rats. Biochem Med. 1983;29:259–264. doi: 10.1016/0006-2944(83)90046-7. [DOI] [PubMed] [Google Scholar]
- 22.Uma Devi P. Withania somnifera dunal (Ashwagandha): potential plant source of promising drug for cancer chemotherapy and radiosensitization. Indian J Exp Biol. 1996;34:927–932. [PubMed] [Google Scholar]
- 23.Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal plants of South Africa. Briza publications; 2000. pp. 38–39. [Google Scholar]